Abstract
Kaposiform hemangioendothelioma(KHE) without Kasabach-Merritt phenomenon is a rare tumor primarily observed in pediatric patients; however, its documentation in the literature remains limited. We reported about a 1-year-old boy diagnosed with superficial KHE who received oral propranolol in combination with topical sirolimus and reviewed relevant reports and treatment of superficial KHE.
Introduction
Kaposiform hemangioendothelioma (KHE) is a rare tumour primarily found in pediatric patients and exhibits borderline malignant characteristics with rarely spontaneous resolution.Citation1 Existing reports on superficial KHE are limited, and there is no established standard treatment. In this study, we report about a case involving a pediatric patient who underwent oral propranolol and topical sirolimus therapy for the management of KHE.
Case Presentation
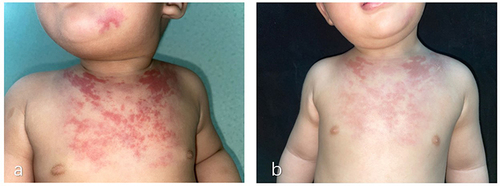
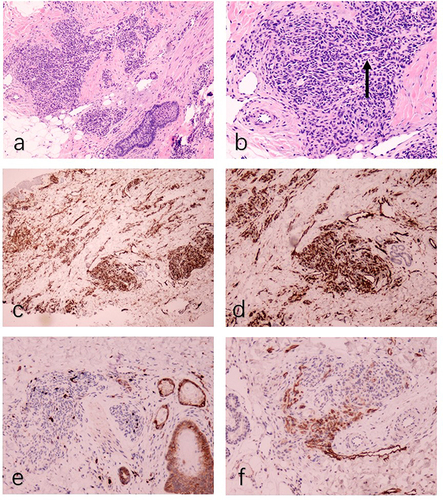
The patient was a 1-year-old boy. At 2 months of age, red plaques appeared on the left mandible and prothorax. Physical examination revealed firm, red plaques, and elevated cutaneous temperature with no pain (). His family history was unremarkable. Laboratory investigations found no obvious abnormalities in routine blood and coagulation tests, and there was no evidence of the Kasabach–Merritt phenomenon (KMP). B-ultrasonography showed a patchy hyperechoic area in the chest wall, approximately 0.6 cm thick, with unclear boundaries abundant blood flow signals, and vascular echoes. The diameter of the thicker blood vessels was approximately 0.16cm (). MRI indicated a thickening of the anterior chest wall epidermis, a slightly longer T2 signal on the fat suppression sequence consistent with T1, and a slightly longer T2 signal cloud shadow in the subcutaneous tissue of the left anterior margin of the mandible. Histological examination of a biopsy sample from the plaques identified blood vessels in a tufted or plexiform distribution, CD31(+), CD34(+), D2-40(-), and GLUT-1(-) (). The diagnosis was confirmed as superficial KHE.
Figure 2 (a) B-ultrasound examination showed hyperechoic area with vascular echo. (b) Follow-up B-ultrasound showed scattered hyperechoic areas with a little blood flow signals.
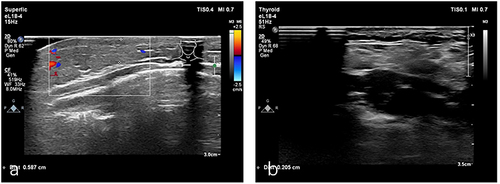
Figure 3 (a) Histopathological showing focal, well-circumscribed nodules comprised of atypical spindle cells (HE, ×50). (b) The spindle-shaped endothelial cells aligned to form a slit-like vascular channels (arrow, HE, ×100). (c) The proliferated vessels were positive for CD31(CD31, ×50). (d) The proliferated vessels were positive for CD34(CD34, ×50). (e) The proliferated vessels were negative for GLUT-1 (GLUT-1, ×50). (f) D2-40 staining was negative in the center of the lesion and positive in the surrounding dilated lymphatic vessels (D2-40, ×50).
Considering the patient’s young age and the less aggressive nature of the tumor, we suggested administering oral propranolol at a daily dose of 2mg/kg and applying topical sirolimus 0.1% gel twice daily. The patient had no significant adverse reactions during the treatment period. One year later, there was a noticeable lightening of lesion color, resolution of the induration, and a decrease in cutaneous temperature (). Follow-up B-ultrasound revealed a reduction in thickness to approximately 0.21 cm with minimal blood flow signals (). Reexamination of MRI showed that the abnormal signal was reduced.
Discussion
KHE tumors are most commonly found in the extremities, trunk, and head/neck. They typically manifest as infiltrative soft tissue masses that invade the skin, adjacent muscles, and bones. Severe cases may involve coagulopathy, known as KMP, which significantly contributes to increased mortality.Citation1 Presently, KHE is often reported with KMP, while cases of superficial KHE, limited to the skin without KMP, are rarely documented. Only five reports, comprising a total of 34 patients, who were predominantly male children, indicated occurrences on the limbs, face and neck, shoulder, and back.
Liu et alCitation2 reported positive responses to topical sirolimus 0.1% gel applied twice daily in 15 patients with superficial KHE, aged 4.6 months (0.2–36 months), over a treatment duration of 6–23 months. Sirolimus, an inhibitor of the mammalian target of rapamycin (mTOR), acts on the mTOR/PI3K/AKT pathway and decreases VEGF production, which can effectively inhibit vascular endothelial cell growth and angiogenesis. It is currently the most commonly used systemic therapy for KHE, demonstrating a high response rate and long-term effectiveness. For superficial KHE, the use of topical sirolimus is preferable, minimizing the potential infection risk of associated with oral administration.
Propranolol is a non-selective β-adrenergic receptor blocker, but its mechanism in treating KHE remains unclear. Studies have shown that propranolol decreased VEGF and NO levels through downregulating the PI3K/AKT/eNOS/VEGF pathway, leading to growth arrest. Long-term effects induce endothelial cell apoptosis, leading to tumor regression.Citation3 Wei et alCitation4 reported successful outcomes with oral administration of propranolol at a dose of 2 mg/kg/day in 11 patients aged 3 months (1–27months) over a treatment duration of 6–50 months, demonstrating good long-term safety with no serious adverse reactions. Recently, a 2-month-old boy with superficial KHE showed a positive response to oral atenolol for 5 months.Citation5
Topical tacrolimus has also been used to treat superficial KHE in seven patients, ranging from 2 weeks to 60-months-old, who were treated with tacrolimus alone for 18–30 months (mean 24 months) and this was combined with bandage for 6 months. No rebound effect was observed, and the mechanism of action may be related to tacrolimus’ inhibitory effect on T cell-derived cytokines and angiogenic factors.Citation6,Citation7
Conclusion
In this case, the combination of oral propranolol and topical sirolimus may synergistically inhibit angiogenesis and may also regulate the immune response to effectively treat KHE. Despite the limitations of this case report, we demonstrate that oral propranolol and topical sirolimus are practical and effective treatments for KHE, with decreased risks of complications. In view of the safety, this treatment should be considered for low-risk patients with superficial KHE without KMP. Our findings need to be validated by clinical trials in the future.
Abbreviations
KHE, Kaposiform hemangioendothelioma; KMP, Kasabach-Merritt phenomenon; MRI, Magnetic resonance imaging; VEGF, Vascular endothelial growth factor.
Ethics Approval and Consent
All treatment plans were approved by the Ethics Research Association of the Children’s Hospital of Zhejiang University School of Medicine. The Ethics Research Association of Children’s Hospital of Zhejiang University School of Medicine granted approval for the publication of the case details.
Consents Statement
Written informed consent was obtained from the minors’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgment
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors or the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
Additional information
Funding
References
- Ji Y, Chen S, Yang K, et al. Kaposiform hemangioendothelioma: current knowledge and future perspectives. Orphanet J Rare Dis. 2020;15(1):39. doi:10.1186/s13023-020-1320-1
- Liu YX, Zhang J, Nie XL, et al. The effect of topical sirolimus on superficial Kaposiform hemangioendothelioma. Australas J Dermatol. 2021;62(2):e329–e331. doi:10.1111/ajd.13499
- Pan WK, Li P, Guo ZT, et al. Propranolol induces regression of hemangioma cells via the down-regulation of the PI3K/Akt/eNOS/VEGF pathway. Pediatric Blood Cancer. 2015;62(8):1414–1420. doi:10.1002/pbc.25453
- Wei L, Li L, Xu Z, et al. Comparison of effectiveness of two different doses of propranolol on kaposiform hemangioendothelioma. Front Pediatric. 2022;10:760401. doi:10.3389/fped.2022.760401
- Yu L, Wei L, Ma L, et al. First report of oral atenolol for treatment of kaposiform hemangioendothelioma, a viable alternative to propranolol. J Dermatol. 2023;50(4):e123–e124. doi:10.1111/1346-8138.16657
- Zhang X, Yang K, Chen S, et al. Tacrolimus ointment for the treatment of superficial kaposiform hemangioendothelioma and tufted angioma. J Dermatol. 2019;46(10):898–901. doi:10.1111/1346-8138.15031
- Nic DE, Sadlier M. A kaposiform haemangioendothelioma successfully treated with topical tacrolimus and compression bandaging. Clin Exp Dermatol. 2022;47(10):1870–1871. doi:10.1111/ced.15292