Abstract
Background
Atrial fibrillation (AF) has become the most common postoperative arrhythmia of thoracic surgery. This study aimed to investigate the risk factors and complications of perioperative atrial fibrillation (PoAF) in elderly patients who underwent video-assisted thoracoscopic surgery (VATS).
Methods
Data were collected from patients who underwent VATS between January 2013 and December 2022 at Peking Union Medical College Hospital (PUMCH). Univariable analyses and multivariable logistic regression analyses were used to determine the factors correlated with PoAF. Receiver operating characteristic (ROC) curve was used to evaluate the discrimination of the indicators to predict PoAF.
Results
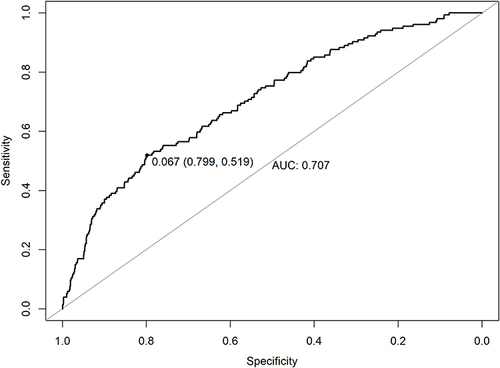
The study enrolled 2920 patients, with a PoAF incidence of 5.2% (95% CI 4.4%-6.0%). In the logistic regression analyses, male sex (OR=1.496, 95% CI 1.056–2.129, P=0.024), left atrial anteroposterior dimension (LAD) ≥40 mm (OR=2.154, 95% CI 1.235–3.578, P=0.004), hypertension (HTN) without regular treatment (OR=2.044, 95% CI 0.961–3.921, P=0.044), a history of hyperthyroidism (OR=4.443, 95% CI 0.947–15.306, P=0.030), surgery of the left upper lobe (compared to other lung lobes) (OR=1.625, 95% CI 1.139–2.297, P=0.007), postoperative high blood glucose (BG) (OR=2.482, 95% CI 0.912–5.688, P=0.048), and the time of chest tube removal (per day postoperatively) (OR=1.116, 95% CI 1.038–1.195, P=0.002) were found to be significantly associated with PoAF. The area under the ROC curve was 0.707 (95% CI 0.519–0.799). 86.9% patients were successfully converted to sinus rhythm. Compared with the non-PoAF group, the PoAF group had significantly greater risks of prolonged air leakage, postoperative acute coronary syndrome, longer ICU stays, and longer hospital stays.
Conclusion
Male sex, LAD≥40 mm, HTN without regular treatment, a history of hyperthyroidism, surgery of the left upper lobe, postoperative BG, and the time of chest tube removal were associated with PoAF. These findings may help clinicians identify high-risk patients and take preventive measures to minimize the incidence and adverse prognosis of PoAF.
Introduction
As the occurrence of lung cancer is increasing and surgical treatment for benign lung diseases is becoming more widespread, the number of people undergoing lung surgery has grown annually across the globe.Citation1,Citation2 Perioperative atrial fibrillation (PoAF) is the most commonly encountered arrhythmia after pulmonary surgery, with an incidence ranging between 6.4 and 12.6%.Citation3,Citation4 Many clinicians regard PoAF as a transient and self-isolated clinical phenomenon.Citation5 However, recent studies have demonstrated that PoAF is associated with prolonged postoperative length of stay (LOS) and increased risk of postoperative stroke, myocardial infarction and even death.Citation6,Citation7 PoAF may develop into a permanent condition, with a similar long-term prognosis and cardiovascular-related and all-cause mortality as other atrial fibrillation (AF) cases not related to surgery.Citation8
Investigating the potential risk factors of PoAF in lung cancer patients can facilitate early intervention for those at greater risk. Several investigators have attempted to evaluate this risk, and elderly age has been recognized as a certain risk factor.Citation9 Male sex, elevated body mass index (BMI), extent of surgical resection, history of chronic heart failure (HF), history of arrhythmias, and brain natriuretic peptide (BNP) have been identified as risk factors of PoAF.Citation10,Citation11 Additionally, chronic obstructive pulmonary disease, diabetes, preoperative hypertension (HTN) history, drinking history, and intraoperative and postoperative blood transfusions have also been suggested to be associated with the risk of PoAF.Citation12,Citation13
Video-assisted thoracoscopic surgery (VATS) is increasingly being used as the preferred option for lung surgery due to its advantage to reduce trauma and reduce surgery time by preventing the use of rib spreaders, cutting intercostal nerves, and dissecting muscle tissue. This approach minimizes intraoperative stress and postoperative pain, decreases the risk of PoAF, and potentially enhances overall recovery.Citation14,Citation15 However, there is a lack of research on the incidence, risk factors, and clinical prognosis of PoAF after VATS. This article aimed to investigate the risk factors of PoAF in elderly patients who underwent lung surgery and to analyze the clinical postoperative complications associated with the occurrence of AF. These findings may help clinicians identify high-risk patients and take preventive measures to minimize the incidence and postoperative complications of PoAF.
Materials and Methods
Study Design and Patients
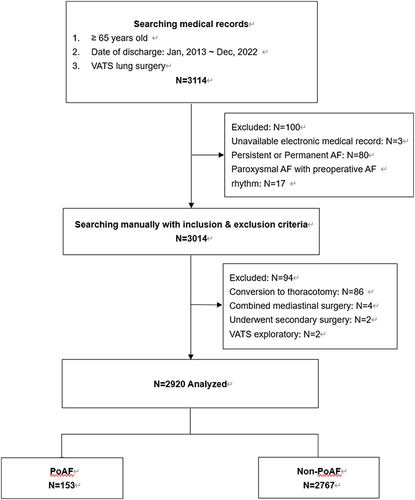
We conducted a single-center retrospective cohort study of patients aged ≥65 years who had undergone VATS lung surgery between January 2013 and December 2022 at Peking Union Medical College Hospital (PUMCH), Beijing, China. Patients with preoperative persistent, long-term persistent or permanent AF were excluded. However, those with a history of paroxysmal AF but who were in sinus rhythm perioperatively were included. Other exclusion criteria included conversion to thoracotomy, VATS combined with simultaneous mediastinal surgery, secondary surgery, simple VATS exploratory and unavailable electronic medical records. The study was conducted in accordance with the Declaration of Helsinki, and ethics board approval for this study was obtained from the PUMCH Institutional Review Board (K3970). We present the following article in accordance with the STROBE reporting checklist. The ethics committee of our hospital agreed to exempt patients from informed consent because of the retrospective nature of the study, and patient data confidentiality was safeguarded.
Data Collection
The medical records of the patients included in the study were reviewed. Perioperative medical data were extracted from the electronic recording system of our institution, including patient demographics, medical history, American Society of Anesthesiologists (ASA) grade, preoperative and postoperative laboratory tests, surgical procedures, intraoperative conditions, pathology results and postoperative complications. Smoking status was defined as a current smoker or someone who had stopped smoking less than 1 month preoperatively. According to the 2023 European Guidelines for hypertension management, preoperative HTN is defined as a blood pressure (BP) of 140/90 mmHg or greater. Patients with HTN who never took any antihypertensive drugs or who did not regularly take antihypertensive drugs were recorded as having HTN without regular treatment. Intraoperative hypotension is indicated by a decrease in systolic blood pressure below 90 mmHg or a 20% decrease in mean arterial pressure from baseline for more than 10 min.Citation16 Postoperative blood glucose (BG) refers to fasting blood glucose levels on the morning of the second day postoperatively.
Anesthesia and Perioperative Management
During surgery, inhalation or total intravenous general anesthesia was administered, and a double-lumen tracheal or bronchial occluder was used for lung isolation. Fentanyl or sufentanil was administered as needed. Multimodal analgesia, including nonsteroidal anti-inflammatory drugs and patient-controlled analgesia, was employed to effectively control postoperative pain. The VATS approach for lobectomy involved a varying number of small incisions (two- to four-port sites) and a 5 to 8cm access incision (utility port). After surgery, continuous monitoring, including monitoring of BP, the saturation of hemoglobin with oxygen (SpO2), and continuous electrocardiogram (ECG) monitoring with rhythm analysis, was performed for at least 24 h for all patients. To conveniently detect the rhythm of the pulse, a pulse oximeter was used as an available and helpful device.Citation17,Citation18 Multiple daily SpO2 monitoring were performed during nursing ward rounds at least three times a day until discharge. If any abnormalities in the SpO2 pulse curve or irregular heart rhythm were detected, as well as if the patient experienced palpitations, the clinical doctor was informed timely. And bedside ECG and myocardial enzyme spectrum examination were performed immediately.
Postoperative complications were evaluated until the patient’s discharge. Information on postoperative complications (prolonged air leakage, pneumonia, heart failure, cerebral infarction), duration of chest tube drainage, duration of postoperative stay, and all-cause mortality was collected. These postoperative complications were evaluated by healthcare providers based on the clinical practice principle at the time of patient admission and noted in the medical records.
Diagnosis and Management of PoAF
Twelve-lead ECG was used to diagnose AF based on the 2014 American Association for Thoracic Surgery (AATS) guidelines.Citation19 The identified AF episodes were reviewed by cardiologists if in doubt to avoid misinterpretation. The main outcome of the study was new AF or atrial flutter with ECG documentation lasting for more than 1 h during surgery and within 30 days. The treatment method and whether it was converted were also recorded. In addition, we reviewed the follow-up outpatient medical records at approximately 30 days after surgery, which may indicate that arrhythmias were diagnosed at other healthcare facilities during the postoperative period.
Upon detection of PoAF, continuous ECG monitoring was carried out until the patient was discharged or returned to sinus rhythm. Initially, oxygen inhalation, electrolyte replacement, or single-dose rate control agents such as beta-blockers or digoxin were employed to treat PoAF. If AF persisted, routine rate control agents or antiarrhythmic drugs such as amiodarone and propaeton were administered. If the patient was unstable, direct cardioversion was taken into account. If AF persisted for more than 48 hours, anticoagulation therapy was initiated.
Statistical Analysis
We estimated that the number of eligible patients who met the inclusion criteria during the study period would be at least 2500. Given that the incidence of PoAF was 5% and the prevalence of a potential risk factor was 20%, this sample size would provide 80% statistical power to detect an odds ratio (OR) less than 0.75 or larger than 1.31 at a two-sided alpha of 0.05. For a continuous risk factor following a standard normal distribution, this sample size would provide 80% statistical power to detect an OR less than 0.77 or larger than 1.29. If the actual incidence of the outcome was higher than the estimated 5%, the statistical power would be higher than the estimated values mentioned above.
Categorical variables were presented as numbers and percentages (%), and continuous variables were presented as the mean ± standard deviation (SD) if the data distribution was normal or else presented as the median and interquartile range (IQR). Two-sample independent t-test or Wilcoxon rank-sum test were used to compare potential differences in continuous variables between PoAF patients and non-PoAF patients, while chi-square or Fisher’s exact test for small samples were used to compare categorical variables. All variables found to be significant at a two-sided alpha of 0.2 in univariable analyses were selected for logistic regression analyses to determine the risk factors for PoAF. Stepwise variable selection with the Akaike information criterion (AIC) was used for optimizing the multivariable prediction models. Receiver operating characteristic (ROC) curve was used to evaluate the discrimination of the indicators to predict PoAF.Citation20 Discrimination (C index) and calibration (Hosmer‒Lemeshow goodness-of-fit test) were adopted to assess the prediction model. A two-sided P value <0.05 was considered statistically significant. Statistical analysis was performed using R (Austria; version 3.5.2) with a graphics user interface (RStudio) and the packages “pROC”,Citation21 “dplyr”,Citation22 “ggplot2”,Citation23 “ResourceSelection”,Citation24 and “rms”.Citation25
Results
Demographics
This study screened 3114 patients aged 65 years and older who underwent VATS, and a total of 2920 patients who met the inclusion criteria were included in the final analysis (). Among them, 153 patients developed PoAF, giving a cumulative incidence risk of 5.2% (95% CI 4.4%-6.0%). The average age of the patients was 70.1 ± 4.3 years, with 1345 male patients (46.1%). Of the 2920 patients, 2041 underwent lobectomy, and the basic information and surgical types of the patients are presented in .
Table 1 Clinical Data of 2920 Patients Undergoing VATS Lung Surgery
Characteristics of PoAF
Among the 153 patients with PoAF, 5 patients experienced intraoperative AF, 2 of whom experienced persistent AF postoperatively. A total of 148 patients developed postoperiative AF, with 132 (89.2%) occurring within 24 hours of surgery. And 148 (96.7%) occurring within the first 3 days after surgery. Among the 148 patients, 121 were identified through asymptomatic monitoring, while 27 were identified based on clinical symptoms such as palpitations and chest tightness.
Risk Factors of PoAF and ROC Analysis
The variables with P<0.2 in the univariable analyses were listed in and included in the logistic regression analyses. Male sex, LAD ≥40 mm, HTN without regular treatment, history of hyperthyroidism, surgery of the left upper lobe (compared to other lung lobes), postoperative BG (per mmol/L increase), and time of chest tube removal (per day postoperatively) were found to be independent risk factors for PoAF (). The area under the ROC curve for the model of risk factors was 0.707 (95% CI 0.519–0.799) ().
Table 2 Comparison of Clinical Data Between PoAF and Non-PoAF Patients
Table 3 Factors Associated with PoAF Found in the Logistic Regression Analyses
Treatment and Postoperative Complications of PoAF
A total of 133 patients were successfully converted to sinus rhythm after treatment prior to discharge. Among them, 7 resolved themselves or only experienced electrolyte replacement, while 126 were treated with drug intervention. Unfortunately, 20 patients were still in persistent AF despite active treatment and had to be given long-term anticoagulation therapy. Postoperative complications between PoAF patients and non-PoAF patients were showed in . Seven patients with PoAF were complicated with acute coronary syndrome (ACS) in duration of hospital stay, including 3 unstable angina and 4 non-ST segment elevation myocardial infarction. However, there was no statistical difference between the two groups in terms of postoperative chylothorax, pulmonary infection, pulmonary embolism, secondary surgery, or postoperative death.
Table 4 Postoperative Complications Between PoAF and Non-PoAF Patients
Discussion
The incidence of PoAF during VATS in elderly patients in this study was 5.2%, which is lower than the previously reported rate of 6.4 to 12.6%.Citation3,Citation4 Age-related factors such as decreased cardiac cell autonomy and conductivity, increased atrial cell apoptosis and fibrosis could increase the risk of AF. However, compared with open chest surgery, VATS is associated with less surgical trauma, stress, inflammation, and postoperative complications.Citation26–28 It is believed that the development of PoAF is associated with reduced cardiovascular reserve, systemic inflammation, heightened sympathetic nervous system activity, and a positive stress response.Citation29
In this study, male sex, LAD≥40 mm, HTN without regular treatment, a history of hyperthyroidism, surgery of the left upper lobe, greater postoperative BG and delayed chest tube removal were associated with PoAF. It has been previously established that males are more likely to suffer from intraoperative AF. However, the exact cause of this phenomenon has yet to be fully elucidated. It is believed that a larger atrial size, increased fibrous or adipose tissue in the sinoatrial node, increased amyloid deposits in the atrial muscle, and a higher pro-inflammatory immune response may be contributing factors.Citation30
It has been demonstrated that left atrial enlargement is a risk factor for PoAF.Citation31,Citation32 The internal diameter of the left atrium is a significant indicator for objectively evaluating atrial structural remodeling and fibrosis. Atrial remodeling is the pathological and physiological basis for the development and persistence of AF.Citation33 It can cause abnormal interatrial or intra-atrial electrical signal conduction, resulting in AF.Citation34 This study presented the potential risk for the generation of PoAF in patients with a preoperative LAD ≥40 mm through multivariable analysis.
Patients with a history of uncontrolled HTN and preoperative hyperthyroidism were more likely to experience PoAF in this study, which might be attributed to the activation of the renin angiotensin system and the release of natriuretic peptide.Citation35 Significantly elevated expression of angiotensin II triggers myocardial cell apoptosis and atrial remodeling, resulting in irreversible changes in atrial structure and function.Citation36,Citation37 Thus, those with a history of HTN and hyperthyroidism should be aware of the potential risk of PoAF.
This study revealed that the location of the tumor in the left upper lobe was associated with a higher incidence of PoAF. It is believed that the pulmonary vein root is a common abnormal atrial pacemaker. When the fibers are cut or ligated, they can disrupt normal atrioventricular conduction or stimulate ectopic pacing points, leading to AF.Citation38,Citation39 The upper lobe of the left lung is closer to the left atrium and the root of the left pulmonary vein, which increases the possibility of stimulating an abnormal atrial pacemaker intraoperatively. Additionally, the unaware stimulation of the autonomic nervous system in the mediastinum during surgery may also contribute to the occurrence of PoAF. Univariable analysis revealed that compared with lung wedge and segmental resection, lobectomy had a greater incidence of PoAF, possibly due to a greater loss of lung function and pulmonary vascular bed, which increased the workload on the heart.
Our data also found a higher postoperative BG was an independent predictor of postoperative AF. Hyperglycemia reflects higher perioperative stress levels with increased levels of catecholamines. Inflammation has been identified as a major contributor to the pathogenesis of AF,Citation40 as the release of inflammatory cytokines leads to ectopic automaticity, triggered activity, myocardial cell apoptosis and fibrosis, and structural and electrical remodeling, leading to an increased risk of AF generation and maintenance.Citation41–43 Inflammation also regulates calcium homeostasis and connexins, which are involved in triggering abnormalities in atrial conduction. Furthermore, long-term physical stimulation, such as thoracic tube placement, can lead to a sustained postoperative inflammatory response, further increasing the risk of PoAF in elderly individuals.
Previous studies have suggested that intraoperative blood transfusion may be associated with an increased risk of PoAF in cardiothoracic surgery, potentially due to the inflammatory response triggered by iron and particles released from damaged red blood cells during processing and storage.Citation44,Citation45 However, in this study, intraoperative blood transfusion was found only to be associated with PoAF in patients who underwent VATS in univariable analysis, which may be explained by the low proportion of blood transfusion in this study. Nevertheless, the potential risks and benefits of transfusion should be evaluated.
The findings of this study indicate that the PoAF group exhibited significantly worse postoperative complications than the non-PoAF group. Therefore, special attention should be given to high-risk elderly patients, and optimization measures should be taken perioperatively. According to the guidelines of the European Society of HTN, the target BP for individuals aged 65–79 is <140/90 mmHg preoperatively, while for those aged 80 and above, it is <150/90 mmHg. For patients with multiple underlying conditions, the target BP should be personalized. Patients who have already taken beta-blockers and calcium channel blockers preoperatively should continue their medication until the morning of surgery.Citation46 For patients with poorly controlled preoperative BP, we recommend early evaluation at the anesthesia clinic during the waiting period for hospital admission. If necessary, patients should also visit the cardiology clinic to adjust their treatment plan.
In addition, we highly recommend strengthening the ERAS management protocol to reduce perioperative stress. Preoperative education can help alleviate patient anxiety. Along with a combination of drugs, paravertebral nerve blocks or intercostal nerve blocks are crucial components of multimodal analgesia. It is important to maintain body temperature, avoid prolonged fasting, and prevent deep vein thrombosis. Chest drainage tubes should be avoided or removed as early as possible.Citation47 Individual glycemic control to minimize the risk of hyperglycemia, hypoglycemia and glucose variability will mitigate the risk of cardiac arrhythmias, including AF.Citation48 Regular monitoring of BG levels every 2 hours postoperatively is advised, with a target range of 7.8–10.0 mmol/L. Insulin therapy should be initiated if BG levels exceed 10.0 mmol/L.Citation49
In this study, the majority of PoAF patients were asymptomatic, as detected by abnormal electrocardiogram within 24 hours after surgery. Therefore, intensive monitoring should be provided for high-risk populations during and after surgery. The duration of postoperative electrocardiogram monitoring should be recommended to extend, especially for male patients with a history of hyperthyroidism, HTN without regular treatment, and increased LAD who are scheduled for left upper lobectomy.
Limitations
In this retrospective study, continuous ECG monitoring lasted for only 24 hours after surgery, followed by intermittent oxygen saturation monitoring, which may have led to a missed diagnosis.Citation50 Prospective studies with continuous ECG monitoring have reported a significantly higher incidence of AF than those without.Citation51 Cases of spontaneously treated AF and those without symptoms or treated at another hospital may have been overlooked, and certain clinical data, such as BNP, thyroid function, and perioperative drug usage, were not collected. In addition, patient characteristics, hospital complexity, and the professional experience of surgeons may influence surgical outcomes as confounding factors. To establish a sound association between AF and adverse postoperative complications, a multivariable regression model adjusting for potential confounders should be built; however, due to the limited number of events in this retrospective cohort study, we did not conduct confounding adjustments, which may have led to biased results when analyzing the association between AF and postoperative complications. As some postoperative factors, such as the duration of drainage tube had uncleared sequential occurrence with PoAF, this study was only able to identify perioperative clinical risk factors associated with PoAF. The significance of predicting PoAF and risk stratification was limited, and a prospective study with prolonged ECG monitoring could be encouraged in the future.
Conclusions
Male sex, LAD≥40 mm, HTN without regular treatment, a history of hyperthyroidism, surgery of the left upper lobe, postoperative BG, and the time of chest tube removal were associated with PoAF in elderly patients who underwent VATS. The presence of PoAF was associated with prolonged ICU and hospital stays. These findings may help clinicians identify high-risk patients and take preventive measures to minimize the incidence and postoperative complications of PoAF.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki, and ethics board approval for this study was obtained from the PUMCH Institutional Review Board (K3970).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Data Sharing Statement
The datasets supporting the Conclusions of this article are available upon request to the corresponding author.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Hoy H, Lynch T, Beck M. Surgical treatment of lung cancer. Crit Care Nurs Clin North Am. 2019;31(3):303–313. doi:10.1016/j.cnc.2019.05.002
- Muranishi Y, Sonobe M, Menju T, et al. Atrial fibrillation after lung cancer surgery: incidence, severity, and risk factors. Surg Today. 2017;47(2):252–258. doi:10.1007/s00595-016-1380-y
- Onaitis M, D’Amico T, Zhao Y, O’Brien S, Harpole D. Risk factors for atrial fibrillation after lung cancer surgery: analysis of the society of thoracic surgeons general thoracic surgery database. Ann Thorac Surg. 2010;90(2):368–374. doi:10.1016/j.athoracsur.2010.03.100
- Bessissow A, Khan J, Devereaux PJ, Alvarez-Garcia J, Alonso-Coello P. Postoperative atrial fibrillation in non-cardiac and cardiac surgery: an overview. J Thromb Haemost. 2015;13(Suppl 1):S304–312. doi:10.1111/jth.12974
- AlTurki A, Marafi M, Proietti R, et al. Major adverse cardiovascular events associated with postoperative atrial fibrillation after noncardiac surgery: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2020;13(1):e007437. doi:10.1161/CIRCEP.119.007437
- Huynh JT, Healey JS, Um KJ, et al. Association between perioperative atrial fibrillation and long-term risks of stroke and death in noncardiac surgery: systematic review and meta-analysis. CJC Open. 2021;3(5):666–674. doi:10.1016/j.cjco.2020.12.025
- Siontis KC, Gersh BJ, Weston SA, et al. Associations of atrial fibrillation after noncardiac surgery with stroke, subsequent arrhythmia, and death: a cohort study. Ann Intern Med. 2022;175(8):1065–1072. doi:10.7326/M22-0434
- Wang H, Wang Z, Zhou M, et al. Postoperative atrial fibrillation in pneumonectomy for primary lung cancer. J Thorac Dis. 2021;13(2):789–802. doi:10.21037/jtd-20-1717
- Amar D, Zhang H, Tan KS, Piening D, Rusch VW, Jones DR. A brain natriuretic peptide-based prediction model for atrial fibrillation after thoracic surgery: development and internal validation. J Thorac Cardiovasc Surg. 2019;157(6):2493–2499. doi:10.1016/j.jtcvs.2019.01.075
- Smith H, Li H, Brandts-Longtin O, et al. External validity of a model to predict postoperative atrial fibrillation after thoracic surgery. Eur J Cardiothorac Surg. 2020;57(5):874–880. doi:10.1093/ejcts/ezz341
- Mita N, Kuroda M, Miyoshi S, Saito S. Association of preoperative right and left ventricular diastolic dysfunction with postoperative atrial fibrillation in patients undergoing lung surgery: a prospective observational study. J Cardiothorac Vasc Anesth. 2017;31(2):464–473. doi:10.1053/j.jvca.2016.09.003
- Imperatori A, Mariscalco G, Riganti G, Rotolo N, Conti V, Dominioni L. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J Cardiothorac Surg. 2012;7(1):1–8. doi:10.1186/1749-8090-7-4
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. doi:10.1016/S0140-6736(16)30958-8
- Jones GS, Baldwin DR. Recent advances in the management of lung cancer. Clin Med Lond. 2018;18(Suppl 2):s41–s46. doi:10.7861/clinmedicine.18-2-s41
- Südfeld S, Brechnitz S, Wagner JY, et al. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119(1):57–64. doi:10.1093/bja/aex127
- Tripathi N, Tripathi M, Pandey M. Pulse oximetry waveform represents an earlier ventricular contraction in relation to the intraarterial blood pressure tracing and electrocardiogram on multichannel monitors: a case series. A a Pract. 2021;15(8):e01505. doi:10.1213/XAA.0000000000001505
- Hess DR. Pulse oximetry: beyond SpO2. Respir Care. 2016;61(12):1671–1680. doi:10.4187/respcare.05208
- Gialdini G, Nearing K, Bhave PD, et al. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA. 2014;312(6):616–622. doi:10.1001/jama.2014.9143
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi:10.2307/2531595
- Turck N, Vutskits L, Sanchez-Pena P, et al. A multiparameter panel method for outcome prediction following aneurysmal subarachnoid hemorrhage. Intensive Care Med. 2010;36(1):107–115. doi:10.1007/s00134-009-1641-y
- R software packages. “dplyr”[homepage on the Internet]. Available from: https://cran.r-project.org/web/packages/dplyr/index.html. Accessed June 28, 2024.
- R software packages. “ggplot2”[homepage on the Internet]. Available from: https://cran.r-project.org/web/packages/ggplot2/index.html. Accessed June 28, 2024.
- R software packages. “ResourceSelection”[homepage on the Internet]. Available from: https://cran.r-project.org/web/packages/ResourceSelection/index.html. Accessed June 28, 2024.
- R software packages. “rms”[homepage on the Internet]. Available from: https://cran.r-project.org/web/packages/rms/index.html. Accessed June 28, 2024.
- Amar D. Postoperative atrial fibrillation: is there a need for prevention? J Thorac Cardiovasc Surg. 2016;151(4):913–915. doi:10.1016/j.jtcvs.2015.09.041
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139(2):366–378. doi:10.1016/j.jtcvs.2009.08.026
- ESTS Database Committee and ESTS Minimally Invasive Interest Group, Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European society of thoracic surgeon database. Eur J Cardiothorac Surg. 2016;49(2):602–609. doi:10.1093/ejcts/ezv154.
- Haverkamp W, Hachenberg T. Post-thoracotomy dysrhythmia. Curr Opin Anaesthesiol. 2016;29(1):26–33. doi:10.1097/ACO.0000000000000285
- Tong C, Zhang Q, Liu Y, Xu M, Wu J, Cao H. Risk factors and outcomes of intraoperative atrial fibrillation in patients undergoing thoracoscopic anatomic lung surgery. Ann Transl Med. 2021;9(7):543. doi:10.21037/atm-20-5035
- Scheggi V, Menale S, Marcucci R, et al. Postoperative atrial fibrillation after thoracic surgery (PoAF): risk factors and outcome. Cardiothorac Surg. 2023;31(1):18. doi:10.1186/s43057-023-00109-7
- Menichelli D, Sciacqua A, Cangemi R, et al. Atrial fibrillation pattern, left atrial diameter and risk of cardiovascular events and mortality, A prospective multicenter cohort study. Int J Clin Pract. 2021;75(3):e13771. doi:10.1111/ijcp.13771
- van de Vegte YJ, Siland JE, Rienstra M, van der Harst P. Atrial fibrillation and left atrial size and function: a Mendelian randomization study. Sci Rep. 2021;11(1):8431. doi:10.1038/s41598-021-87859-8
- Heitmann KA, Løchen ML, Stylidis M, Hopstock LA, Schirmer H, Morseth B. Associations between physical activity, left atrial size and incident atrial fibrillation: the Tromsø Study 1994-2016. Open Heart. 2022;9(1):e001823. doi:10.1136/openhrt-2021-001823
- Reddy V, Taha W, Kundumadam S, Khan M. Atrial fibrillation and hyperthyroidism: a literature review. Indian Heart J. 2017;69(4):545–550. doi:10.1016/j.ihj.2017.07.004
- Cheng Z, Zhang M, Hu J, et al. Cardiac-specific Mst1 deficiency inhibits ROS-mediated JNK signalling to alleviate Ang II-induced cardiomyocyte apoptosis. J Cell Mol Med. 2019;23(1):543–555. doi:10.1111/jcmm.13958
- Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest. 2017;127(5):1600–1612. doi:10.1172/JCI87491
- Xin Y, Hida Y, Kaga K, et al. Left lobectomy might be a risk factor for atrial fibrillation following pulmonary lobectomy. Eur J Cardiothorac Surg. 2014;45(2):247–250. doi:10.1093/ejcts/ezt383
- Iwata T, Nagato K, Nakajima T, Suzuki H, Yoshida S, Yoshino I. Risk factors predictive of atrial fibrillation after lung cancer surgery. Surg Today. 2016;46(8):877–886. doi:10.1007/s00595-015-1258-4
- Gutierrez A, Van Wagoner DR. Oxidant and inflammatory mechanisms and targeted therapy in atrial fibrillation: an update. J Cardiovasc Pharmacol. 2015;66(6):523–529. doi:10.1097/FJC.0000000000000313
- Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1(1):62–73. doi:10.1161/CIRCEP.107.754564
- Garner M, Routledge T, King JE, et al. New-onset atrial fibrillation after anatomic lung resection: predictive factors, treatment and follow-up in a UK thoracic centre. Interact Cardiovasc Thorac Surg. 2017;24(2):260–264. doi:10.1093/icvts/ivw348
- Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79(3):495–502. doi:10.1253/circj.CJ-15-0138
- Sood N, Coleman CI, Kluger J, White CM, Padala A, Baker WL. The association among blood transfusions, white blood cell count, and the frequency of post-cardiothoracic surgery atrial fibrillation: a nested cohort study from the atrial fibrillation suppression trials I, II, and III. J Cardiothorac Vasc Anesth. 2009;23(1):22–72. doi:10.1053/j.jvca.2008.06.009
- Koch CG, Li L, Van Wagoner DR, Duncan AI, Gillinov AM, Blackstone EH. Red cell transfusion is associated with an increased risk for postoperative atrial fibrillation. Ann Thorac Surg. 2006;82(5):1747–1756. doi:10.1016/j.athoracsur.2006.05.045
- Mancia G, Kreutz R, Brunström M, et al. ESH Guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European Society of Hypertension endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023;41(12):1874–2071. doi:10.1097/HJH.0000000000003480
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 2019;55(1):91–115. doi:10.1093/ejcts/ezy301
- Sun DK, Zhang N, Liu Y, et al. Dysglycemia and arrhythmias. World J Diabetes. 2023;14(8):1163–1177. doi:10.4239/wjd.v14.i8.1163
- Wu Z, Liu J, Zhang D, et al. Expert consensus on the glycemic management of critically ill patients. J Intensive Med. 2022;2(3):131–145. doi:10.1016/j.jointm.2022.06.001
- Semeraro GC, Meroni CA, Cipolla CM, Cardinale DM. Atrial fibrillation after lung cancer surgery: prediction, prevention and anticoagulation management. Cancers. 2021;13(16):4012. doi:10.3390/cancers13164012
- McIntyre WF, Vadakken ME, Rai AS, et al. Incidence and recurrence of new-onset atrial fibrillation detected during hospitalization for non-cardiac surgery: a systematic review and meta-analysis. Can J Anaesth. 2021;68(7):1045–1056. doi:10.1007/s12630-021-01944-0