Abstract
The release of endogenous prostacyclin (PGI2) is depressed in patients with pulmonary arterial hypertension (PAH). PGI2 replacement therapy by epoprostenol infusion is one of the best treatments available for PAH. Here, we provide an overview of the current clinical data for epoprostenol. Epoprostenol treatment improves symptoms, exercise capacity, and hemodynamics, and is the only treatment that has been shown to reduce mortality in patients with idiopathic PAH (IPAH) in randomized clinical trials. We have reported that high-dose epoprostenol therapy (>40 ng/kg/min) also results in marked hemodynamic improvement in some patients with IPAH. High-dose epoprostenol has a pro-apoptotic effect on PAH-PASMCs via the IP receptor and upregulation of Fas ligand (FasL) in vitro. However, long-term intravenous administration of epoprostenol is sometimes associated with catheter-related infections and leads to considerable inconvenience for the patient. In the future, the development of new routes of administration or the development of powerful PGI2 analogs, IP-receptor agonists, and gene and cell-based therapy enhancing PGI2 production with new routes of administration is required.
Background
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by progressive elevation of pulmonary vascular resistance (PVR) and pulmonary artery pressure (PAP) that leads to right heart failure and death. Increased PVR is induced by pulmonary vasoconstriction, vascular remodeling, and thrombosis.Citation1,Citation2 Those factors are associated with many molecules, substrates, and signaling pathways. Three pathways, namely prostacyclin (PGI2), nitric oxide (NO), and endothelin pathways, are critical for the pathogenesis and progression of PAH.Citation3,Citation4 Impaired production of vasodilators, such as PGI2 and NO, along with over-expression of vasoconstrictors, such as endothelin-1, play an important part in the pathogenesis of idiopathic PAH (IPAH).Citation3 Drugs targeting the three pathways are currently available, and have been shown to be useful. In this review, we focus on the efficacy of high-dose epoprostenol sodium, a PGI2, for treatment of PAH.
Deficient endogenous PGI2 in PAH
An increase in the release of the vasoconstrictor thromboxane A2, suggesting the activation of platelets, occurs in patients with the primary form as well as those with the secondary form of pulmonary hypertension (PH). In contrast, the release of PGI2 is depressed in these patients.Citation5 PGI2 synthase expression has been reported to be decreased in lung tissues from patients with severe PH.Citation6 Whether the imbalance in the release of these mediators is a cause or a result of PH is unknown, but this imbalance may play a part in the development and maintenance of both forms of the disorder.Citation5
Protocol of epoprostenol therapy
We start epoprostenol therapy at a low dose (0.25–0.5 ng/kg/min) and increase the dose daily by 0.25–2.0 ng/kg/min. When the dose of epoprostenol exceeds 10 ng/kg/min, we increase the dose weekly as previously described.Citation7 Thereafter, we gradually increase the dose weekly or monthly to the maximal tolerated dose based on clinical symptoms and side effects in individual cases. We adjust the dose to match the change in body weight. Epoprostenol has a short half-life, and the in vivo half-life of epoprostenol in humans is expected to be no greater than 6 minutes. Continuous intravenous administration by means of an infusion pump and a permanent tunneled catheter is needed for long-term treatment.Citation8
When the patient develops right heart failure before the start of epoprostenol therapy, we initiate treatment with dobutamine and/or dopamine in our centers as previously described.Citation9 We initiate dobutamine infusion at a low dose (3 μg/kg/min) when the patient’s mixed venous oxygen saturation (SvO2) is <60% or cardiac index (CI) is <2.0 L/min/m2 or when right ventricular (RV) failure is clinically suspected before the start of epoprostenol therapy. Clinical RV failure is defined as leg edema and jugular venous distention, heart enlargement in a chest radiograph (cardio-thoracic ratio >50%), and a high level of brain natriuretic peptide (>100 pg/mL). If the value of SvO2 does not increase over 60% or CI does not increase over 2.0 L/min/m2 or if RV failure is not improved, the dose of dobutamine is titrated up. We initiate dopamine infusion at a low dose (3 μg/kg/min) when the patient’s systolic blood pressure (BP) is <90 mmHg or urine volume is >20 mL/h before the start of epoprostenol therapy. If systolic BP cannot be kept over 90 mmHg, the dose of dopamine is titrated up.
Efficacy of epoprostenol therapy
The efficacy of continuous intravenous epoprostenol therapy has been tested in three unblinded randomized clinical trials (RCTs) in patients with IPAHCitation10,Citation11 and in patients with PH due to the scleroderma spectrum of disease, WHO-functional class (WHO-FC) III or IV despite optical medical therapy.Citation12 Epoprostenol treatment improves symptoms, exercise capacity, and hemodynamics, and is the only treatment that has been shown to reduce mortality of patients with IPAH in RCTs.Citation11,Citation13 Meta-analysis for total mortality in the three RCTs performed with the Mantel–Haenszel and Peto fixed-effect methods showed relative risk reductions of 70% and 68%, respectively.Citation13 The fifth World Symposium on Pulmonary Hypertension (WSPH) in Nice, France in 2013 recommended continuous intravenous epoprostenol as first-line therapy for WHO-FC IV patients because of the survival benefit in this subset.Citation13 McLaughlin et al reported the long-term efficacy of epoprostenol therapy in 162 patients with primary PH.Citation14 After 17 months, mean pulmonary pressure (mPAP) had decreased from 61±13 mmHg to 53±13 mmHg, and observed survival with epoprostenol therapy at 1–3 years was significantly greater than the expected survival based on historical data. Additionally, combination therapy with epoprostenol and bosentan improved the hemodynamics, functional class, and exercise capacity of IPAH, and anorexigen-associated PAH patients compared with those in matched controls who received epoprostenol monotherapy.Citation15
In patients with PH due to the scleroderma spectrum of disease, continuous intravenous epoprostenol improves exercise capacity, hemodynamics, and survival compared with conventional therapy.Citation12,Citation16 Additionally, epoprostenol might improve the hemodynamics and survival in patients with PAH associated with connective tissue diseases (CTD), including mixed CTDs, CREST syndrome, systemic lupus erythematosus, scleroderma, and Sjogren syndrome.Citation16–Citation19
Rosenzweig et al reported the results of long-term epoprostenol therapy in patients with PH associated with congenital heart diseases (CHD-PAH).Citation20 Twenty patients (eleven patients: previous cardiac surgery, eleven patients: residual systemic to pulmonary shunt, 15±14 years old) were treated with epoprostenol. mPAP had decreased from 77±20 mmHg to 61±15 mmHg one year after the start of treatment with epoprostenol at a dose of 82±37 ng/kg/min. Thomas et al also reported that long-term continuous PGI2 therapy in adult patients with CHD-PAH (37±10.5 years old) resulted in hemodynamic and clinical improvements.Citation21
Epoprostenol therapy in patients with PH associated with portal hypertension improved hemodynamics but did not improve long-term survival.Citation22–Citation24
Long-term epoprostenol therapy in the dose range of 21–40 ng/kg/min reduced mPAP by 12%–22% and reduced PVR by 32%–53% compared with baseline values ().Citation14,Citation25,Citation26 The dosage of epoprostenol is adjusted upward on the basis of symptoms of PAH and side effects of the drug. Since a chronic overdose of epoprostenol could lead to a high cardiac output state,Citation27 the appropriate dose range of epoprostenol is thought to be 25–40 ng/kg/min on the basis of results of previous studies.Citation3,Citation14,Citation25,Citation26,Citation28 However, treatment with epoprostenol at doses less than 40 ng/kg/min sometimes cannot improve hemodynamics in patients with severe PAH. We reported that high-dose epoprostenol therapy (>40 ng/kg/min) caused marked hemodynamic improvement in 14 patients with IPAH.Citation7 Compared with the baseline state, high-dose epoprostenol therapy reduced mPAP by 30% and PVR by 68% (), and increased CI by 89% and SvO2 by 19%. Further studies are needed to clarify the efficacy of high-dose epoprostenol therapy (>40 ng/kg/min) in patients with PAH associated with CTD and CHD.
Table 1 Hemodynamics before and after epoprostenol therapy
Reverse remodeling of pulmonary arteries by high-dose epoprostenol therapy
Increased PVR is induced by pulmonary vasoconstriction, vascular remodeling, including pulmonary vascular intimal and medial thickening, and thrombosis.Citation1,Citation2 Intima and media thickening is largely composed of smooth muscle cells and myofibroblasts.Citation29,Citation30 Most cases of severe PH also exhibit a disorganized growth of primitive endothelial cells that form plexiform lesions.Citation30 Enhanced proliferation and impaired apoptosis of pulmonary artery smooth muscle cells (PASMCs) cause an inappropriate increase in PASMCs and induce pulmonary vascular medial hypertrophy in PAH.Citation31–Citation35
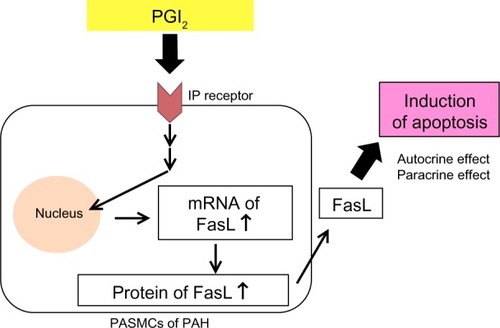
Medical agents that have anti-proliferative and pro-apoptotic effects on PASMCs are required for effective treatment that achieves reverse remodeling.Citation35,Citation36 PGI2 analogs have anti-proliferative effects on PASMCs in vitro.Citation37 We have also reported that high-dose epoprostenol has a pro-apoptotic effect on PAH-PASMCs via the IP receptor and upregulation of Fas ligand (FasL) in vitro ().Citation38 In a case series, we examined the reverse pulmonary vascular remodeling effects of epoprostenol in lung tissues obtained from an IPAH patient treated with high-dose epoprostenol and an IPAH patient not treated with epoprostenol.Citation39 Apoptotic cells were detected in small pulmonary arteries of the IPAH patient treated with high-dose epoprostenol (115 ng/kg/min) but not in those from the IPAH patient not treated with epoprostenol. The media of peripheral pulmonary arteries was thick in the IPAH patient not treated with epoprostenol. On the other hand, the media of peripheral pulmonary arteries was thin in the IPAH patient treated with high-dose epoprostenol. These results indicate that high-dose epoprostenol therapy has the potential for reverse pulmonary vascular remodeling by reduction of medial hypertrophy.
Figure 1 PGI2 induces apoptosis via upregulation of Fas ligand (FasL) in pulmonary artery smooth muscle cells (PASMCs) from patients with pulmonary arterial hypertension (PAH).
However, advanced proliferative vasculopathy after long-term (18 years) and high-dose (60 ng/kg/min) epoprostenol therapy in a patient with IPAH was reported.Citation40 There were frequent plexiform lesions adjacent to arterial branch points. Achcar et al also reported PGI2-treated cases that showed increased perivascular inflammation.Citation41 Of note is the Stacher et al study on the impact of modern treatments of PAH, including treatment with PGI2 and its analogs, endothelin receptor antagonist (ERA), and phosphodiesterase type 5 (PDE-5) inhibitors, on pulmonary vascular pathology.Citation30 Morphometric intima and media fractional thicknesses were significantly larger in the PAH group than in control lungs. Media fractional thickness was largely superimposable with the range of media fractional thickness measured in normal pulmonary arteries. However, the overlap was less in intima fractional thickness, and marked perivascular inflammation was present in a large number of PAH lungs. Furthermore, the number of profiles of plexiform lesions was significantly smaller in the lungs of patients who had never been treated with PGI2 or its analogs. These results indicate that epoprostenol therapy might reverse pulmonary vascular medial thickening but might not ameliorate intima thickening, perivascular inflammation, and plexiform lesions. Further studies to assess the reversal vascular remodeling effects of high-dose epoprostenol therapy are needed.
Acute vasoreactivity testing
Acute vasoreactivity testing is usually performed to predict a good prognosis and identify acute responders who are more likely to have a sustained beneficial response to oral calcium channel blockers (CCBs) and can be treated with these less-expensive drugs. Acute responders have been defined as patients who show a decrease in mPAP of at least 10 mmHg to an absolute level below 40 mmHg with preserved or increased cardiac output.Citation42
Acute vasoreactivity testing is most commonly performed using intravenous epoprostenol,Citation43,Citation44 intravenous adenosine,Citation45 or inhaled nitric oxide.Citation44 Acute testing using intravenous epoprostenol was shown to be useful for identifying patients with good prognosis; however, a good response to epoprostenol does not indicate that all patients will have a long-term response to CCBs.Citation44,Citation46,Citation47 On the other hand, occurrence of life-threatening hemodynamic compromise has often been documented in CCB testing (nifedipine and verapamil).Citation48–Citation51 Therefore, it is accepted that CCBs should not be used for acute testing.Citation3,Citation42 In contrast, intravenous CCB nicardipine might be useful for acute testing,Citation52 because the drug is short-acting compared to nifedipine and more vasoselective than other CCBs.Citation53,Citation54 We have reported that in 65 PAH patients administered low-dose nicardipine, there was no hemodynamic instability requiring additional inotropic agents or death during the testing.Citation52
Storage and stability
Unopened vials of epoprostenol sodium with glycinemannitol excipients (epoprostenol GM) are stable when stored at 15°C–25°C (59°F–77°F), and protected from light in a carton. However, epoprostenol GM in solution has limited stability at room temperature. Prior to use, reconstituted solutions of epoprostenol GM must be protected from light, and must be refrigerated at 2°C–8°C (36°F–46°F) if not used immediately. During use, a single reservoir of reconstituted solution of epoprostenol GM can be administered at room temperature for a total duration of 8 hours or it can be used with a cold pouch and administered up to 24 hours with the use of two frozen 6-oz gel packs in a cold pouch. The need for refrigeration or the use of frozen gel packs during long-term administration leads to considerable inconvenience for the patient. Recently, epoprostenol sodium with arginine excipient has improved thermal stability. It might provide patients with an increased sense of treatment convenience.Citation55,Citation56
Adverse reactions
The most common adverse events (occurring in ≥10% of patients) during dose initiation were flushing, headache, nausea/vomiting, hypotension, anxiety, and chest pain. The most common adverse events (occurring in ≥10% of patients) during chronic administration were dizziness, headache, nausea/vomiting, jaw pain, myalgia, flushing, diarrhea, nonspecific musculoskeletal pain, tachycardia, chills/fever/sepsis/flu-like symptoms, anxiety, and hypesthesia.
Chronic infusion of epoprostenol is delivered using a small, portable infusion pump through an indwelling central venous catheter. It results in limited improvement in quality of life. Catheter-related infections are problematic during long-term treatment. During long-term follow-up, sepsis was reported at a rate of 0.3 infections/patient per year in patients treated with epoprostenol. We have reported that the use of a closed hub system reduces catheter-related infections in patients with PAH receiving continuous therapy with epoprostenol at home.Citation8 The development of new routes of administration is required in the future. Powerful PGI2 analogs, IP-receptor agonists, or gene and cell-based therapy enhancing PGI2 production with new routes of administration are needed.Citation57
Anticoagulation therapy is associated with a survival benefit in patients with IPAH and has been recommended.Citation13,Citation58 Epoprostenol has antiplatelet activity, and has been reported to reduce the levels of plasma tissue-type plasminogen activator and plasminogen activator inhibitor.Citation59 We previously reported that many hemorrhagic complications occur in patients with IPAH who receive both anticoagulation and epoprostenol treatment.Citation60 Among 31 patients, nine had eleven bleeding episodes, nine (81.8%) of which were alveolar hemorrhages. Therefore, we have stopped using anticoagulation in patients receiving high-dose epoprostenol.
Conclusion
PGI2 replacement therapy by epoprostenol infusion is one of the best treatments available for PAH. High-dose epoprostenol therapy (>40 ng/kg/min) causes marked hemodynamic improvement in some patients with PAH.
Disclosure
Drs Nakamura, Akagi, Ogawa, Matsubara, and Ito have received lecturer fees from GlaxoSmithKline and Actelion Pharmaceuticals, Japan. The authors report no other conflicts of interest in this work.
References
- ArcherSRichSPrimary pulmonary hypertension: a vascular biology and translational research “work in progress”Circulation2000102222781279111094047
- MiuraANakamuraKKusanoKFThree-dimensional structure of pulmonary capillary vessels in patients with pulmonary hypertensionCirculation2010121192151215320479166
- HumbertMSitbonOSimonneauGTreatment of pulmonary arterial hypertensionN Engl J Med2004351141425143615459304
- NakamuraKShimizuJKataokaNAltered nano/micro-order elasticity of pulmonary artery smooth muscle cells of patients with idiopathic pulmonary arterial hypertensionInt J Cardiol2010140110210719073348
- ChristmanBWMcPhersonCDNewmanJHAn imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertensionN Engl J Med1992327270751603138
- TuderRMCoolCDGeraciMWProstacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertensionAm J Respir Crit Care Med199915961925193210351941
- AkagiSNakamuraKMiyajiKMarked hemodynamic improvements by high-dose epoprostenol therapy in patients with idiopathic pulmonary arterial hypertensionCirc J201074102200220520697180
- AkagiSMatsubaraHOgawaAPrevention of catheter-related infections using a closed hub system in patients with pulmonary arterial hypertensionCirc J200771455956417384460
- AkagiSOgawaAMiyajiKKusanoKItoHMatsubaraHCatecholamine support at the initiation of epoprostenol therapy in pulmonary arterial hypertensionAnn Am Thorac Soc201411571972724716663
- RubinLJMendozaJHoodMTreatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trialAnn Intern Med199011274854912107780
- BarstRJRubinLJLongWAPrimary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The primary pulmonary hypertension study groupN Engl J Med199633452963028532025
- BadeschDBTapsonVFMcGoonMDContinuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trialAnn Intern Med2000132642543410733441
- GalièNCorrisPAFrostAUpdated treatment algorithm of pulmonary arterial hypertensionJ Am Coll Cardiol20136225 SupplD60D7224355643
- McLaughlinVVShillingtonARichSSurvival in primary pulmonary hypertension: the impact of epoprostenol therapyCirculation2002106121477148212234951
- KempKSavaleLO’CallaghanDSUsefulness of first-line combination therapy with epoprostenol and bosentan in pulmonary arterial hypertension: an observational studyJ Heart Lung Transplant201231215015822138355
- BadeschDBMcGoonMDBarstRJLongterm survival among patients with scleroderma-associated pulmonary arterial hypertension treated with intravenous epoprostenolJ Rheumatol200936102244224919723905
- HumbertMSanchezOFartoukhMShort-term and long-term epoprostenol (prostacyclin) therapy in pulmonary hypertension secondary to connective tissue diseases: results of a pilot studyEur Respir J19991361351135610445611
- KuhnKPByrneDWArbogastPGDoyleTPLoydJERobbinsIMOutcome in 91 consecutive patients with pulmonary arterial hypertension receiving epoprostenolAm J Respir Crit Care Med2003167458058612446266
- ShiraiYYasuokaHTakeuchiTSatohTKuwanaMIntravenous epoprostenol treatment of patients with connective tissue disease and pulmonary arterial hypertension at a single centerMod Rheumatol20132361211122023359006
- RosenzweigEBKersteinDBarstRJLong-term prostacyclin for pulmonary hypertension with associated congenital heart defectsCirculation199999141858186510199883
- ThomasICGlassner-KolminCGomberg-MaitlandMLong-term effects of continuous prostacyclin therapy in adults with pulmonary hypertension associated with congenital heart diseaseInt J Cardiol201316844117412123890862
- KuoPCJohnsonLBPlotkinJSHowellCDBartlettSTRubinLJContinuous intravenous infusion of epoprostenol for the treatment of portopulmonary hypertensionTransplantation19976346046069047158
- KrowkaMJFrantzRPMcGoonMDSeversonCPlevakDJWiesnerRHImprovement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): a study of 15 patients with moderate to severe portopulmonary hypertensionHepatology199930364164810462369
- FixOKBassNMDe MarcoTMerrimanRBLong-term follow-up of portopulmonary hypertension: effect of treatment with epoprostenolLiver Transpl200713687588517539008
- McLaughlinVVGenthnerDEPanellaMMRichSReduction in pulmonary vascular resistance with long-term epoprostenol (prostacyclin) therapy in primary pulmonary hypertensionN Engl J Med199833852732779445406
- SitbonOHumbertMNunesHLong-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survivalJ Am Coll Cardiol200240478078812204511
- RichSMcLaughlinVVThe effects of chronic prostacyclin therapy on cardiac output and symptoms in primary pulmonary hypertensionJ Am Coll Cardiol19993441184118710520810
- McLaughlinVVMcGoonMDPulmonary arterial hypertensionCirculation2006114131417143117000921
- TuderRMPathology of pulmonary arterial hypertensionSemin Respir Crit Care Med200930437638519634077
- StacherEGrahamBBHuntJMModern age pathology of pulmonary arterial hypertensionAm J Respir Crit Care Med2012186326127222679007
- FujioHNakamuraKMatsubaraHCarvedilol inhibits proliferation of cultured pulmonary artery smooth muscle cells of patients with idiopathic pulmonary arterial hypertensionJ Cardiovasc Pharmacol200647225025516495763
- OgawaANakamuraKMatsubaraHPrednisolone inhibits proliferation of cultured pulmonary artery smooth muscle cells of patients with idiopathic pulmonary arterial hypertensionCirculation2005112121806181216157769
- GeraciMWMooreMGesellTGene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysisCirc Res200188655556211282888
- ZhangSFantozziITignoDDBone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cellsAm J Physiol Lung Cell Mol Physiol20032853L740L75412740218
- NakamuraKAkagiSOgawaAPro-apoptotic effects of imatinib on pdgf-stimulated pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertensionInt J Cardiol2012159210010621376411
- SchermulyRTDonyEGhofraniHAReversal of experimental pulmonary hypertension by pdgf inhibitionJ Clin Invest2005115102811282116200212
- ClappLHFinneyPTurcatoSTranSRubinLJTinkerADifferential effects of stable prostacyclin analogs on smooth muscle proliferation and cyclic amp generation in human pulmonary arteryAm J Respir Cell Mol Biol200226219420111804870
- AkagiSNakamuraKMatsubaraHProstaglandin i2 induces apoptosis via upregulation of fas ligand in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertensionInt J Cardiol2013165349950521955608
- AkagiSNakamuraKMatsubaraHReverse remodeling of pulmonary arteries by high-dose prostaglandini2 therapy. A case reportJ Cardiol Cases201395173176
- RichSPogorilerJHusainANTothPTGomberg-MaitlandMArcherSLLong-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertensionChest201013851234123921051399
- AchcarROYungGLSafferHCoolCDVoelkelNFYiESMorphologic changes in explanted lungs after prostacyclin therapy for pulmonary hypertensionEur J Med Res200611520320716723294
- GalieNHoeperMMHumbertMESC Committee for Practice Guidelines (CPG)Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (esc) and the european respiratory society (ers), endorsed by the international society of heart and lung transplantation (ishlt)Eur Heart J200930202493253719713419
- RubinLJGrovesBMReevesJTFrosolonoMHandelFCatoAEProstacyclin-induced acute pulmonary vasodilation in primary pulmonary hypertensionCirculation19826623343387046988
- SitbonOHumbertMJaïsXLong-term response to calcium channel blockers in idiopathic pulmonary arterial hypertensionCirculation2005111233105311115939821
- ZuoXRZhangRJiangXUsefulness of intravenous adenosine in idiopathic pulmonary arterial hypertension as a screening agent for identifying long-term responders to calcium channel blockersAm J Cardiol2012109121801180622459309
- TonelliARAlnuaimatHMubarakKPulmonary vasodilator testing and use of calcium channel blockers in pulmonary arterial hypertensionRespir Med2010104448149620004088
- RaffyOAzarianRBrenotFClinical significance of the pulmonary vasodilator response during short-term infusion of prostacyclin in primary pulmonary hypertensionCirculation19969334844888565165
- CreveyBJDantzkerDRBowerJSPopatKDWalkerSDHemodynamic and gas exchange effects of intravenous diltiazem in patients with pulmonary hypertensionAm J Cardiol19824935785837058767
- YoungTELundquistLJCheslerEWeirEKComparative effects of nifedipine, verapamil, and diltiazem on experimental pulmonary hypertensionAm J Cardiol19835111952006571766
- PackerMMedinaNYushakMAdverse hemodynamic and clinical effects of calcium channel blockade in pulmonary hypertension secondary to obliterative pulmonary vascular diseaseJ Am Coll Cardiol1984458909016491082
- PackerMMedinaNYushakMWienerIDetrimental effects of verapamil in patients with primary pulmonary hypertensionBr Heart J19845211061116743418
- SaitoYNakamuraKMiyajiKAcute vasoreactivity testing with nicardipine in patients with pulmonary arterial hypertensionJ Pharmacol Sci2012120320621223117888
- TeraiMTakenakaTMaenoHInhibition of calcium influx in rabbit aorta by nicardipine hydrochloride (yc-93)Biochem Pharmacol19813043753787213424
- BristowMRGinsburgRLaserJAMcAuleyBJMinobeWTissue response selectivity of calcium antagonists is not due to heterogeneity of [3h]-nitrendipine binding sitesBr J Pharmacol19848223093206329392
- ChinKMBadeschDBRobbinsIMTwo formulations of epoprostenol sodium in the treatment of pulmonary arterial hypertension: Epitome-1 (epoprostenol for injection in pulmonary arterial hypertension), a phase iv, open-label, randomized studyAm Heart J20141672218225 e21124439983
- SitbonODelcroixMBergotEEpitome-2: an open-label study assessing the transition to a new formulation of intravenous epoprostenol in patients with pulmonary arterial hypertensionAm Heart J2014167221021724439982
- RuanCHDixonRAWillersonJTRuanKHProstacyclin therapy for pulmonary arterial hypertensionTex Heart Inst J201037439139920844610
- OlssonKMDelcroixMGhofraniHAAnticoagulation and survival in pulmonary arterial hypertension: results from the comparative, prospective registry of newly initiated therapies for pulmonary hypertension (compera)Circulation20141291576524081973
- Boyer-NeumannCBrenotFWolfMContinuous infusion of prostacyclin decreases plasma levels of t-pa and pai-1 in primary pulmonary hypertensionThromb Haemost19957347357367495094
- OgawaAMatsubaraHFujioHRisk of alveolar hemorrhage in patients with primary pulmonary hypertension – anticoagulation and epoprostenol therapyCirc J200569221622015671616
