Abstract
Pulmonary embolism (PE) is a relatively common cardiovascular emergency. PE and deep vein thrombosis (DVT) are considered expressions of the same disease, termed as venous thromboembolism (VTE). In the present review, we describe and meta-analyze the efficacy and safety data available with the direct oral anticoagulants (DOAC; dabigatran, rivaroxaban, apixaban, edoxaban) in clinical trials testing these new compounds in the acute/long-term and extended therapy of VTE, providing subgroup analyses in patients with index PE. We analyzed ten studies in 35,019 randomized patients. A total of 14,364 patients (41%) had index PE. In the acute/long-term treatment of VTE, the DOAC showed comparable efficacy in preventing recurrent VTE to standard treatment in patients with index PE (risk ratio [RR]: 0.88; 95% confidence interval [CI]: 0.70–1.11) and index DVT (RR: 0.93; 95% CI: 0.75–1.16) (P for subgroup differences =0.76). VTE recurrence depending on PE anatomical extension and presence/absence of right ventricular dysfunction was only reported in two trials, with results being consistent with those obtained in the overall study populations. In the single trial comparing extended therapy of VTE with DOAC versus warfarin, the point estimate for recurrent VTE tended to disfavor the DOAC in patients with index PE (RR: 2.05; 95% CI: 0.83–5.03) and in patients with index DVT (RR: 1.11; 95% CI: 0.49–2.50) (P for subgroup differences =0.32). In trials that compared DOAC versus placebo for extended therapy, the reduction in recurrent VTE was consistent in patients with PE (RR: 0.15; 95% CI: 0.01–1.82) and in patients with DVT (RR: 0.25; 95% CI: 0.10–0.61) (P for subgroup differences =0.71). The DOAC were associated with a consistently lower risk of clinically relevant bleeding (CRB) than standard treatment of acute VTE and higher risk of CRB than placebo for extended therapy of VTE regardless of index event. In summary, the DOAC were as effective as, and safer than, standard treatment of (hemodynamically stable) PE. Their efficacy in preventing recurrent VTE seemed consistent regardless of anatomical extension of PE (extensive, intermediate, or limit) or presence/absence of right ventricular dysfunction although the data are limited. For extended therapy, the DOAC were more effective than placebo in preventing recurrent VTE but were associated with an increase in CRB regardless of index event.
Introduction
Pulmonary embolism (PE) is a relatively common cardiovascular emergency. PE occurs when clots break off from deep vein walls (deep vein thrombosis [DVT]), usually from the lower limbs, and travel through the right side of the heart to the pulmonary arteries occluding the pulmonary arterial bed. The broader term venous thromboembolism (VTE) refers to DVT, PE, or a combination of both, and has a reported annual incidence of 1–2 cases per 1,000 inhabitants in Western countries.Citation1 PE and DVT are considered as expressions of the same disease, as in different studies, about 70% of patients with documented symptomatic PE had DVT,Citation2 and patients presenting with documented DVT have been shown to have silent PE or perfusion defects on ventilation/perfusion lung scan in 30%–70% of cases.Citation3,Citation4
PE can be broadly classified as either high-risk PE (hemodynamically unstable: presence of shock or hypotension, defined as a systolic blood pressure <90 mmHg or a pressure drop of ≥40 mmHg for 15 minutes if not caused by new-onset arrhythmia, hypovolemia, or sepsis; early mortality risk >15%) or non-high-risk PE (hemodynamically stable; early mortality risk <15%).Citation5 Non-high-risk PE can be further stratified according to the presence of markers of right ventricular dysfunction (RVD) and/or myocardial injury into intermediate- and low-risk PE. Intermediate-risk PE (early mortality risk ≈3%–15%) is diagnosed if at least one of the RVD markers: 1) right ventricular (RV) dilatation, hypokinesis, or pressure overload on echocardiography; 2) RV dilatation on spiral computed tomography; 3) brain natriuretic peptide (BNP) or N-terminal (NT)-pro-BNP elevation; 4) elevated right heart pressure at right heart catheterization; or one myocardial injury marker, cardiac troponin T or I, is positive.Citation5 Low-risk PE is diagnosed when all checked RVD and myocardial injury markers are found negative (early mortality risk <1%). Some clinical scores, such as the simplified PE severity index, may also be useful to identify low-risk PE patients who are potential candidates for outpatient treatment.Citation6 Patients presenting with hemodynamic instability (high-risk PE) are usually treated with either thrombolytic therapy or pulmonary embolectomy followed by anticoagulant therapy, while most hemodynamically stable patients (non-high risk PE) can be treated with anticoagulation alone.
The basic anticoagulant treatment strategy is similar for patients with PE or DVT, and generally includes a parenteral anticoagulant (eg, low-molecular-weight heparin (LMWH), unfractionated heparin, or fondaparinux) for at least 5 days and until the international normalized ratio (INR) is 2.0 or above for at least 24 hours (initial therapy), overlapping with and followed by an oral vitamin K antagonist (VKA) (dose adjusted to keep an INR between 2.0 and 3.0) for at least 3 months (long-term therapy).Citation7 At this point, the decision to continue with anticoagulant treatment depends on the balance between the risk of VTE recurrences if anticoagulation is withdrawn and the risk of bleeding if it is continued. Patients with ongoing risk factors and patients with unprovoked VTE are often prescribed extended therapy, provided that the bleeding risk is not excessive.Citation7
Despite LMWH or fondaparinux being effective and safe, they still require daily parenteral subcutaneous administration. In addition, they are mainly cleared through the kidneys, and their use in patients with severe renal insufficiency may be problematic. On the other hand, the narrow therapeutic window and variability in response of VKA implies that frequent anticoagulant monitoring (using the prothrombin time and its reporting as the INR) is necessary to avoid a subtherapeutic anticoagulation associated with an increased risk of thrombosis or an excessive anticoagulation that increases the risk of bleeding. Such monitoring is inconvenient for patients and medical staff, and costly for health care payers.
In recent years, several direct oral anticoagulants (DOAC) have been developed for the treatment of VTE, among other indications.Citation8 DOAC directly inhibit either thrombin or activated factor X (FXa).Citation9 Among them, the oral direct thrombin inhibitor dabigatran etexilate (Pradaxa®, Boehringer Ingelheim, Ingelheim, Germany)Citation10 and the oral direct FXa inhibitors rivaroxaban (Xarelto®, Bayer AG, Leverkusen, Germany)Citation11 and apixaban (Eliquis®, Bristol-Myers Squibb, New York, NY, USA)Citation12 are currently approved in the European Union, North America, and other regions in several indications related to anticoagulation, including the treatment of VTE, while the oral direct FXa inhibitor edoxaban (Lixiana®, Daiichi-Sankyo, Tokyo, Japan)Citation13 is approved in Japan for thromboprophylaxis after major orthopedic surgery and is currently under regulatory review in the European Union and other regions for the treatment of VTE and for the prevention of stroke and systemic embolism in patients with atrial fibrillation. Their pharmacology is well characterized ().Citation14–Citation30 They are given orally in fixed doses once or twice daily, and they lack validated and available antidotes.Citation10–Citation13
Table 1 Pharmacological characteristics of the old and new anticoagulants used for treatment of VTE
In this review, we describe and meta-analyze the efficacy and safety data available with the DOAC in clinical trials testing these new compounds in VTE, with a focus on patients with index PE, and we discuss their potential advantages and drawbacks over existing therapies in patients with VTE.
Methods
Bibliographic search
A search in Medline database (up to 1 June 2014) and clinical trial registries (ie, clinicaltrials.gov) was conducted using the terms: “dabigatran”, “rivaroxaban”, “apixaban”, “edoxaban”, and “pulmonary embolism”. We also searched regulatory agencies’ websites (European Medicines Agency, US Food and Drug Administration) and relevant conference proceedings related to anticoagulant therapy.
Study selection criteria
We included randomized controlled trials comparing a DOAC (dabigatran, rivaroxaban, apixaban, and edoxaban) with the standard treatment for acute VTE or with warfarin or placebo for extended treatment of VTE.
Data extraction and quality assessment
We collected outcome data on the overall trial populations as well as by index event at baseline: index PE (with or without concomitant DVT) or index DVT (without PE). Primary efficacy outcome was recurrent symptomatic VTE (ie, the composite of recurrent DVT, recurrent non-fatal PE, and VTE-related death) and primary safety outcome was clinically relevant bleeding (CRB) (ie, the composite of major and clinically relevant non-major bleeding). We also extracted data on clinical trials design (eg, superiority, non-inferiority, duration of treatment and follow-up, inclusion and exclusion criteria, and outcomes definitions), treatment characteristics (eg, dosage used in the experimental and control groups), and patients’ characteristics (age and sex; percentage of patients evaluable for efficacy and safety). Study quality was assessed using the Cochrane Collaboration’s tool for assessing risk of bias in randomized studies.Citation31
Data synthesis and analysis
We carried out direct comparisons between the DOAC and standard treatment (initial/long-term therapy), placebo or warfarin (extended therapy) on an intention-to-treat basis.Citation32 We included the randomized population in the overall analysis. Clinical outcomes were analyzed in subgroups of patients with index PE versus those with index DVT when disaggregated data were available. Subgroup analyses by index event (PE or DVT) were conducted in the assessable population for efficacy and safety, as reported in the original publications. A random effects meta-analysis was carried out by conventional methods, as described by DerSimonian and Laird.Citation33 Heterogeneity was assessed using the Cochran Q testCitation34 and the Higgins I2 test.Citation35 A Cochran’s Q P<0.05 and I2>50% were considered to show significant heterogeneity. Calculations were performed using RevMan software, version 5.2 (The Nordic Cochrane Centre, Copenhagen, Denmark) and StatsDirect software, version 2.8.0 (StatsDirect Ltd, Cheshire, UK).
Results
Characteristics of studies and patients
shows the characteristics of the studies and patients. We included ten studies in 35,019 randomized patients. A total of 14,364 patients (41.0%) had index PE.
Table 2 Clinical trials with DOAC in the treatment of VTE
The six studies in the initial/long-term therapy of VTECitation36–Citation41 included 27,127 randomized patients (). Of them, 11,613 patients (42%) had index PE. The four studies in the extended therapy of VTECitation38,Citation42,Citation43 comprised 7,892 randomized patients (). Of them, 2,751 patients (35%) had index PE. Mean age of patients was 55–58 years across trials and 55%–61% were males (). Ethnic group distribution was reported in seven trials.Citation36–Citation39,Citation42 Across studies, the majority of patients included were Caucasians (70%–95%), followed by Asians (2%–21%) and Blacks or African Americans (1%–4%). Extensive PE was present in 24%–46% of patients with index PE (data only available from Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Pulmonary Embolism [EINSTEIN PE], Efficacy and Safety Study of Apixaban for the Treatment of Deep Vein Thrombosis or Pulmonary Embolism [AMPLIFY] and Comparative Investigation of Low Molecular Weight [LMW] Heparin/Edoxaban Tosylate [DU176b] Versus [LMW] Heparin/Warfarin in the Treatment of Symptomatic Deep-Vein Blood Clots and/or Lung Blood Clots [HOKUSAI-VTE]) ().
In the initial/long-term therapy of VTE, a single drug approach was used in rivaroxaban and apixaban trials, while heparin lead-in was used in dabigatran and edoxaban trials. Treatment durations ranged between 3 months and 12 months in clinical trials in the acute/long-term therapy of VTE and between 6 months and 18 months in the extended therapy of VTE (). All studies in the acute/long-term therapy of VTE and Secondary Prevention of Venous Thrombo Embolism (VTE) (RE-MEDY)Citation42 study in extended therapy of VTE used a non-inferiority approach, while placebo-controlled studies in extended therapy of VTE were superiority trials (). In placebo-controlled trials, patients were recruited if there was clinical equipoise about the continuation or cessation of anticoagulant therapy. All studies but two (Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Deep Vein Thrombosis [EINSTEIN DVT] and PE) were double blinded (). Methods for assessment of recurrent VTE were consistent across studies.Citation36–Citation43 DVT diagnosis was established by compression ultrasonography or venography of leg veins, while non-fatal PE was diagnosed by ventilation–perfusion lung scanning, angiography, or spiral computed tomography of pulmonary arteries. Diagnosis of fatal PE was based on objective testing, autopsy, or death that could not be attributed to a documented cause and for which PE could not be ruled out. CRB definition was based on that used by the van Gogh trialistsCitation44 in studies with rivaroxaban, apixaban, and edoxaban, but all dabigatran studies used a different definition.Citation36 Time in therapeutic range (TTR) ranged between 57% and 65% across studies that included warfarin as control treatment ().Citation36–Citation42
Risk of bias was assessed as “low” in the eight double-blinded studies and “uncertain” in the two open-label studies, EINSTEIN DVT and PE ().
Recurrent VTE
Initial and long-term treatment of VTE
In the overall populations, the DOAC were as effective as standard therapy (risk ratio [RR]: 0.91; 95% confidence interval [CI]: 0.79–1.06), with no evidence of heterogeneity (). A total of 11,589 patients (DOAC: 5,794; standard therapy: 5,795) with index PE and 15,434 patients (DOAC: 7,718; standard therapy: 7,716) with index DVT were assessable for recurrent VTE (). The DOAC showed comparable efficacy in preventing recurrent VTE to standard treatment in patients with index PE (RR: 0.88; 95% CI: 0.70–1.11) and index DVT (RR: 0.93; 95% CI: 0.75–1.16) (P for subgroup differences =0.76) ().
Figure 1 Recurrent VTE in clinical trials with DOAC in the treatment of VTE.
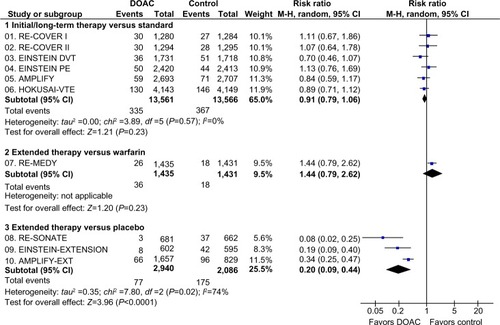
Figure 2 Subgroup analysis of recurrent VTE depending on index event (PE or DVT) in clinical trials with DOAC in the treatment of VTE.
Abbreviations: AMPLIFY, Efficacy and Safety Study of Apixaban for the Treatment of Deep Vein Thrombosis or Pulmonary Embolism; AMPLIFY-EXT, Efficacy and Safety Study of Apixaban for Extended Treatment of Deep Vein; CI, confidence interval; df, degrees of freedom; DOAC, direct oral anticoagulant; DVT, deep vein thrombosis; EINSTEIN DVT, Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Deep Vein Thrombosis; EINSTEIN-EXTENSION, Once-Daily Oral Direct Factor Xa Inhibitor Rivaroxaban In The Long-Term Prevention Of Recurrent Symptomatic Venous Thromboembolism In Patients With Symptomatic Deep-Vein Thrombosis Or Pulmonary Embolism; EINSTEIN PE, Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Pulmonary Embolism; HOKUSAI-VTE, Comparative Investigation of Low Molecular Weight (LMW) Heparin/Edoxaban Tosylate (DU176b) Versus (LMW) Heparin/Warfarin in the Treatment of Symptomatic Deep-Vein Blood Clots and/or Lung Blood Clots; M-H, Mantel–Haenszel; PE, pulmonary embolism; RE-COVER I, Efficacy and Safety of Dabigatran Compared to Warfarin for 6 Month Treatment of Acute Symptomatic Venous Thromboembolism; RE-COVER II, Phase III Study Testing Efficacy and Safety of Oral Dabigatran Etexilate versus Warfarin for 6 Month Treatment for Acute Symptomatic Venous Thromboembolism (VTE); RE-MEDY, Secondary Prevention of Venous Thrombo Embolism (VTE); RE-SONATE, Twice-daily Oral Direct Thrombin Inhibitor Dabigatran Etexilate in the Long Term Prevention of Recurrent Symptomatic VTE; VTE, venous thromboembolism.
VTE recurrence depending on PE anatomical extension and presence/absence of RVD was only reported in two trials.Citation39,Citation41 In EINSTEIN PE, the rates of recurrent VTE in the rivaroxaban versus standard-therapy group were 1.7% (10 of 597) versus 1.4% (8 of 576) among patients with anatomically extensive PE at baseline, respectively; 2.5% (35 of 1,392) versus 2.2% (31 of 1424) in intermediate PE, respectively; and 1.6% (5 of 309 patients) versus 1.3% (4 of 299) in limited PE at baseline, respectively.Citation39 In HOKUSAI-VTE, 28% (n=938) of patients with PE (n=3,319) had RVD (NT-pro-BNP level ≥500 pg/mL). The rate of recurrent VTE in this subgroup was 3.3% in the edoxaban group and 6.2% in the warfarin group (HR: 0.52; 95% CI: 0.28–0.98).Citation41 Similar results were observed among patients with RVD assessed by the presence of RV dilatation on computed tomography (HR: 0.42; 95% CI: 0.15–1.20).Citation41
Extended therapy of VTE
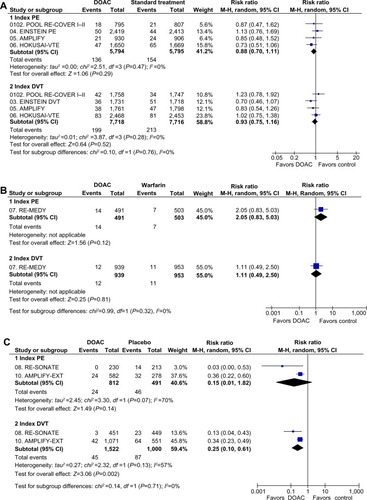
During extended therapy of VTE, the DOAC were as effective as warfarin (RR: 1.44; 95% CI: 0.79–2.62) and more effective than placebo (RR: 0.20; 95% CI: 0.09–0.44) in preventing recurrent VTE. However, there was statistical heterogeneity among placebo-controlled studies (P=0.02), mainly due to a greater effect size in reduction of recurrent VTE in RE-SONATE than in the other studies ().
A total of 2,297 patients (DOAC: 1,303; warfarin: 503; placebo: 491) with index PE and 4,414 patients (DOAC: 2,461; warfarin: 953; placebo: 1,000) with index DVT were assessable for recurrent VTE (). In the single trial that compared a DOAC with warfarin for extended therapy of VTE (RE-MEDY), the point estimate for recurrent VTE tended to favor warfarin without significant differences between patients with index PE (RR: 2.05; 95% CI: 0.83–5.03) or index DVT (RR: 1.11; 95% CI: 0.49–2.50) (P for subgroup differences =0.32) (). In trials that compared the DOAC and placebo for extended therapy, the effect on recurrent VTE was also consistent in patients with PE (RR: 0.15; 95% CI: 0.01–1.82) and in patients with DVT (RR: 0.25; 95% CI: 0.10–0.61) (P for subgroup differences =0.71) ().
Clinically relevant bleeding
Initial and long-term treatment of VTE
The DOAC were associated with a lower risk of CRB than standard treatment in the overall population (RR: 0.72; 95% CI: 0.57–0.91), but there was evidence of statistical heterogeneity across studies (P<0.00001), mainly at the expense of a greater effect size in reduction of CRB in the studies with dabigatran and apixaban than in studies with rivaroxaban and edoxaban ().
Figure 3 Major and clinically relevant nonmajor bleeding in clinical trials with DOAC in the treatment of VTE.
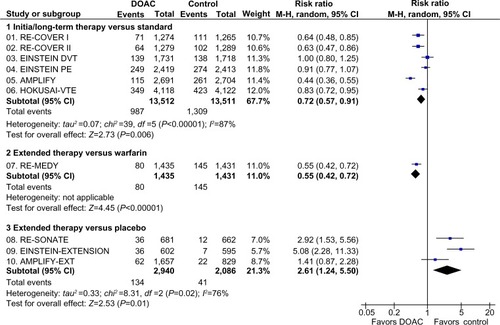
The risk of CRB versus standard treatment was consistent in patients with index PE (RR: 0.90; 95% CI: 0.80–1.02) and index DVT (RR: 0.87; 95% CI: 0.67–1.12) (P for subgroup differences =0.80) ().
Figure 4 Subgroup analysis of major and clinically relevant nonmajor bleeding events depending on index event (PE or DVT) in clinical trials with DOAC in the treatment of VTE.
Abbreviations: AMPLIFY-EXT, Efficacy and Safety Study of Apixaban for Extended Treatment of Deep Vein; CI, confidence interval; df, degrees of freedom; DOAC, direct oral anticoagulant; DVT, deep vein thrombosis; EINSTEIN DVT, Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Deep Vein Thrombosis; EINSTEIN PE, Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients With Acute Symptomatic Pulmonary Embolism; HOKUSAI-VTE, Comparative Investigation of Low Molecular Weight (LMW) Heparin/Edoxaban Tosylate (DU176b) Versus (LMW) Heparin/Warfarin in the Treatment of Symptomatic Deep-Vein Blood Clots and/or Lung Blood Clots; M-H, Mantel–Haenszel; PE, pulmonary embolism; VTE, venous thromboembolism.
Extended therapy of VTE
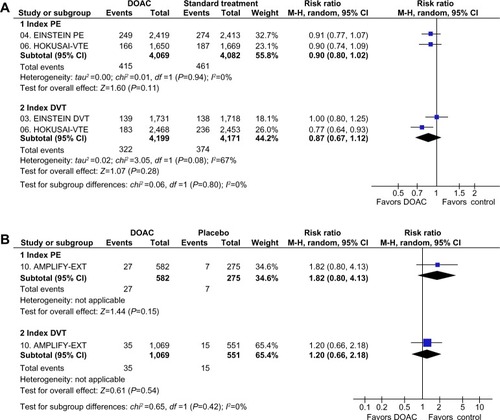
The DOAC were associated with a lower risk of CRB than warfarin (RR: 0.55; 95% CI: 0.42–0.72) and a higher risk of CRB than placebo (RR: 2.61; 95% CI: 1.24–5.50). However, there was evidence of statistical heterogeneity among placebo-controlled studies (P=0.02), mainly at expenses of a higher increase in bleeding in dabigatran and rivaroxaban studies than in the AMPLIFY-EXT study with apixaban ().
Disaggregated safety data of extended therapy in patients with PE or DVT were only available from AMPLIFY-EXT.Citation43 In that trial, the trend toward increase in risk of CRB with apixaban versus placebo was consistent in patients with index PE (RR: 1.82; 95% CI: 0.80–4.13) and index DVT (RR: 1.20; 95% CI: 0.66–2.18) (P for subgroup differences =0.42) (). No disaggregated data of CRB depending on index VTE were reported in the remaining three studies in the extended therapy.Citation38,Citation42
Clinical outcomes in anticoagulated patients with index PE versus index DVT
Recurrent VTE and mortality risk were similar in both clinical presentations (index PE and index DVT). However, patients with index PE had more CRB complications than patients with index DVT during the initial/long-term anticoagulation therapy (RR: 1.29; 95% CI: 1.18–1.42) (). Patients with index PE had similar clinical outcomes than patients with DVT during extended anticoagulation therapy ().
Table 3 Pooled risks of recurrent VTE, CRB, and mortality by index event in patients receiving anticoagulant therapy (DOAC or heparin/warfarin)*
Discussion
Overall, the DOAC seem associated with similar efficacy and lower bleeding risk than standard therapy following acute VTE. Our analysis shows that the efficacy and safety of the DOAC are consistent regardless of the clinical presentation of acute VTE as hemodynamically stable PE or DVT. The analysis is based on six clinical trials in the initial/long-term therapy of VTE (range: 3–12 months of treatment).Citation36–Citation41 These studies included a total of 11,589 randomized patients with index PE and 15,434 randomized patients with index DVT. Across trials, 24%–46% of patients with index PE had anatomically extensive PE,Citation39–Citation41 and 28% of patients with index PE had RVD.Citation41 Available data in these subgroups, although scarce, suggest that the effect in patients with index PE is consistent regardless of anatomical extension of PE,Citation34 or presence/absence of RVD.Citation41
In the extended therapy, we found a reduction in recurrent VTE with the DOAC in comparison with placebo, which was also consistent in patients with PE and in patients with DVT. On the contrary, the point estimate for the RR of recurrent VTE disfavored dabigatran versus warfarin in the RE-MEDY trial, particularly in patients with PE. Moreover, the trend toward a poorer efficacy of dabigatran versus warfarin during extended therapy of VTE was further suggested by the numerical increase in acute coronary syndromes (ACS) (13 versus three patients). The TTR in RE-MEDY was 65%, better than in all the remaining studies with the DOAC in the treatment of VTE. These data may suggest that patients with index PE who remain well controlled while on extended therapy with warfarin should not generally be switched to dabigatran, as it could potentially result in an increased risk of thromboembolism. Since the RE-MEDY trial is so far the only study comparing the DOAC and warfarin in extended anticoagulant therapy in patients with VTE, these results must be interpreted with caution and are not necessarily applicable with other DOAC. Moreover, in the RE-SONATE study (extended therapy with dabigatran or placebo), the rates of recurrent VTE and ACS with dabigatran (0.4% and 0.1%, respectively) were much lower than those in the dabigatran arm of the RE-MEDY study (1.8% and 0.9%, respectively). These differences could be explained by an optimal quality of anticoagulation with warfarin in RE-MEDY and by different baseline risk of treatment groups and follow-up duration between trials. Whether dabigatran increases the risk of ACS or the findings are due to a more protective effect of warfarin than of dabigatran against ACS is still unclear.
In extended therapy, dabigatran showed a significantly lower risk of bleeding than warfarin in RE-MEDY,Citation42 which is consistent with the lower bleeding tendency of all DOAC versus standard initial/long-term therapy. On the contrary, we found an increase in CRB with the DOAC versus placebo, which was heterogeneous across trials. The heterogeneity found may be due to differences in bleeding definitions across trials or might reflect differences in the bleeding profile of each of the DOAC, as they are different compounds with unique pharmacological characteristics. Head-to-head comparisons between the DOAC are needed to solve this issue.
Important differences are apparent between trials regarding the dosing regime (single-drug approach used in rivaroxaban and apixaban trials versus heparin lead-in used in dabigatran and edoxaban trials) that have implications when prescribing the DOAC in patients with VTE (). In addition, there are other issues to bear in mind when prescribing the new compounds, like their potential for drug–drug of food–drug interactions and appropriate use in renal insufficiency ().
Table 4 Summary of prescribing information of the DOAC in the treatment of VTE
A single-drug regimen with rivaroxaban or apixaban may simplify treatment of acute PE, thus allowing for outpatient treatment in low-risk PECitation6 or early discharge in intermediate-risk PE patients and potentially reducing hospital stays. A post hoc analysis of EINSTEIN studies has shown that the proportion of hospitalized patients for PE with a length of stay of 5 days or fewer receiving rivaroxaban was 45% compared with 33% for enoxaparin/VKA.Citation45 Reduction in subsequent hospitalizations after initial discharge has been reported with apixaban in the acute/long-term therapy versus standard treatment (AMPLIFY)Citation46 and during extended therapy versus placebo (AMPLIFY-EXT).Citation47 Limitations of these analyses include their post hoc nature and well-monitored clinical trial setting, in which decisions on admission and discharge could vary from real-world management.
The DOAC may provide an alternative to warfarin for extended therapy of PE, particularly in those patients considered to be at high risk of bleeding. Patients with VTE and cancer are among those patients who have a high risk of both recurrence and bleeding. DOAC have shown promising results in patients with cancer in comparison with warfarin.Citation48,Citation49 However, LMWH instead of VKA is the standard of care in VTE associated with cancer.Citation50 Further head-to-head comparative trials between the DOAC and LMWH are needed to recommend the use of the DOAC in VTE associated with cancer.
Heparin-induced thrombocytopenia (HIT) is a rare but serious complication of heparin treatment.Citation51 DOAC may provide an alternative for anticoagulation in patients with VTE and with history of HIT, because they do not interact with PF4 in vitro.Citation52–Citation54 A clinical study is underway to investigate rivaroxaban for treatment of patients with current suspected or confirmed HIT (n=200) (Clinicaltrials.gov identifier: NCT01598168).Citation55
The lack of validated and currently available antidotes is one of the drawbacks of the DOAC.Citation48 Adequate supportive care and temporary removal of all antithrombotic drugs constitute the basis for management of serious bleeding complications.Citation56 Prohemostatic agents (unactivated or activated prothrombin complex concentrate and activated factor VIIa) have been tried for the DOAC with varying success.Citation56 Hemodialysis can remove 50%–60% of circulating dabigatran,Citation10 while administration of activated charcoal may be useful to reduce absorption of rivaroxaban or apixaban if taken <6 hours after overdose or accidental ingestion.Citation11,Citation12 Highly specific reversal agents for the direct thrombin and FXa inhibitors are under development and might be available during the next years.Citation56
Other drawbacks of the DOAC include their higher costs in comparison with VKA and the uncertainty about the adherence to therapy in day-to-day practice. Since the DOAC have a shorter half-life (<24 hours) than warfarin (36–42 hours), suboptimal adherence may be more dangerous for the DOAC than for warfarin. Furthermore, some DOAC have twice-daily dosing schedules (), which may be more difficult for some patients with DVT or PE to adhere to than a daily regimen, particularly in the long term.Citation57 However, no head-to-head comparisons for checking compliance with once-daily versus twice-daily regimes of DOAC are available, and therefore no firm conclusions can be drawn in this respect.
Conclusion
The DOAC are as effective as, and probably safer than standard treatment of hemodynamically stable PE. The efficacy and safety of the DOAC seem consistent regardless anatomical extension of PE (extensive, intermediate, or limit) or presence/absence of RVD, although the data are limited. For extended therapy, the DOAC are more effective than placebo in preventing recurrent VTE but are associated with an increase in CRB either in patients with PE or DVT.
Disclosure
The contents of this review are solely the responsibility of the authors and do not necessarily represent the official view of their institutions or any other party. No funding/support grant was received for this review. A Gómez-Outes, ML Suárez-Gea, AI Terleira-Fernández, and E Vargas-Castrillón declare no conflicts of interest. R Lecumberri declares personal fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, and Daiichi Sankyo, and grants and personal fees from Rovi Pharmaceuticals, outside the submitted work.
References
- SilversteinMDHeitJAMohrDNPettersonTMO’FallonWMMeltonLJ3rdTrends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based studyArch Intern Med199815865855939521222
- KearonCNatural history of venous thromboembolismCirculation200310723 Suppl 1I22I3012814982
- SteinPDMattaFMusaniMHDiaczokBSilent pulmonary embolism in patients with deep venous thrombosis: a systematic reviewAm J Med2010123542643120399319
- BullerHRLensingAWPrinsMHA dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT Dose-Ranging StudyBlood200811262242224718621928
- TorbickiAPerrierAKonstantinidesSGuidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)Eur Heart J200829182276231518757870
- AujeskyDPerrierARoyPMValidation of a clinical prognostic model to identify low-risk patients with pulmonary embolismJ Intern Med2007261659760417547715
- KearonCAklEAComerotaAJAntithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice GuidelinesChest2012141Suppl 2e419Se494S22315268
- Gómez-OutesASuárez-GeaMLCalvo-RojasGDiscovery of anticoagulant drugs: a historical perspectiveCurr Drug Discov Technol2012928310421838662
- CabralKPPharmacology of the new target-specific oral anticoagulantsJ Thromb Thrombolysis201336213314023645472
- European Medicines AgencyPradaxa® – Summary of Product Characteristics2014 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000829/WC500041059.pdf
- European Medicines AgencyXarelto® – Summary of Product Characteristics2014 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf
- European Medicines AgencyEliquis® – Summary of Product Characteristics2014 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002148/WC500107728.pdf
- LipGYAgnelliGEdoxaban: a focused review of its clinical pharmacologyEur Heart J201435281844185524810388
- MungallDBIBR-1048 Boehringer IngelheimCurr Opin Investig Drugs200236905907
- StangierJClinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilateClin Pharmacokinet200847528529518399711
- TrocónizIFTillmannCLiesenfeldKHSchäferHGStangierJPopulation pharmacokinetic analysis of the new oral thrombin inhibitor dabigatran etexilate (BIBR 1048) in patients undergoing primary elective total hip replacement surgeryJ Clin Pharmacol200747337138217322149
- BlechSEbnerTLudwig-SchwellingerEStangierJRothWThe metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humansDrug Metab Dispos200836238639918006647
- KubitzaDBeckaMVoithBZuehlsdorfMWensingGSafety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitorClin Pharmacol Ther200578441242116198660
- KubitzaDBeckaMWensingGVoithBZuehlsdorfMSafety, pharmacodynamics, and pharmacokinetics of BAY 59-7939 – an oral, direct Factor Xa inhibitor – after multiple dosing in healthy male subjectsEur J Clin Pharmacol2005611287388016328318
- KubitzaDBeckaMRothAMueckWDose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjectsCurr Med Res Opin200824102757276518715524
- LangDFreudenbergerCWeinzCIn vitro metabolism of rivaroxaban, an oral, direct factor Xa inhibitor, in liver microsomes and hepatocytes of rats, dogs, and humansDrug Metab Dispos20093751046105519196846
- WongPCCrainEJXinBApixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studiesJ Thromb Haemost20086582082918315548
- WangLZhangDRaghavanNIn vitro assessment of metabolic drug-drug interaction potential of apixaban through cytochrome P450 phenotyping, inhibition, and induction studiesDrug Metab Dispos201038344845819940026
- RaghavanNFrostCEYuZApixaban metabolism and pharmacokinetics after oral administration to humansDrug Metab Dispos2009371748118832478
- FurugohriTIsobeKHondaYDU-176b, a potent and orally active factor Xa inhibitor: in vitro and in vivo pharmacological profilesJ Thromb Haemost2008691542154918624979
- MorishimaYHondaYKamisatoCComparison of antithrombotic and haemorrhagic effects of edoxaban, an oral direct factor Xa inhibitor, with warfarin and enoxaparin in ratsThromb Res2012130351451922647432
- OgataKMendell-HararyJTachibanaMClinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteersJ Clin Pharmacol201050774375320081065
- BathalaMSMasumotoHOgumaTHeLLowrieCMendellJPharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humansDrug Metab Dispos201240122250225522936313
- MasumotoHYoshigaeYWatanabeKTakakusaHOkazakiOIzumiTIn vitro metabolism of edoxaban and the enzymes involved in the oxidative metabolism of edoxabanAAPS J201012S2W4308 [abstract]
- KawajiHIshiiMTamakiYSasakiKTakagiMEdoxaban for prevention of venous thromboembolism after major orthopedic surgeryOrthop Res Rev201245364
- HigginsJPAltmanDGGøtzschePCThe Cochrane Collaboration’s tool for assessing risk of bias in randomised trialsBMJ2011343d592822008217
- LiberatiAAltmanDGTetzlaffJThe PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health-care interventions: explanation and elaborationBMJ2009339b270019622552
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- CochranWGThe combination of estimates from different experimentsBiometrics1954101101129
- HigginsJPTThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- SchulmanSKearonCKakkarAKDabigatran versus warfarin in the treatment of acute venous thromboembolismN Engl J Med2009361242342235219966341
- SchulmanSKakkarAKGoldhaberSZTreatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysisCirculation2014129776477224344086
- EINSTEIN InvestigatorsBauersachsRBerkowitzSDOral rivaroxaban for symptomatic venous thromboembolismN Engl J Med2010363262499251021128814
- EINSTEIN–PE InvestigatorsBüllerHRPrinsMHOral rivaroxaban for the treatment of symptomatic pulmonary embolismN Engl J Med2012366141287129722449293
- AgnelliGBullerHRCohenAOral apixaban for the treatment of acute venous thromboembolismN Engl J Med2013369979980823808982
- The Hokusai-VTE InvestigatorsEdoxaban versus warfarin for the treatment of symptomatic venous thromboembolismN Engl J Med2013369151406141523991658
- SchulmanSKearonCKakkarAKExtended use of dabigatran, warfarin, or placebo in venous thromboembolismN Engl J Med2013368870971823425163
- AgnelliGBullerHRCohenAApixaban for extended treatment of venous thromboembolismN Engl J Med2013368869970823216615
- van Gogh InvestigatorsBullerHRCohenATIdraparinux versus standard therapy for venous thromboembolic diseaseN Engl J Med20073571094110417855670
- van BellenBBamberLCorrea de CarvalhoFPrinsMWangMLensingAWReduction in the length of stay with rivaroxaban as a single-drug regimen for the treatment of deep vein thrombosis and pulmonary embolismCurr Med Res Opin201430582983724432872
- LiuXJohnsonMMardekianJPhatakHThompsonJCohenAApixaban reduces hospitalizations in patients with VTE: an analysis of the AMPLIFY trialJ Am Coll Cardiol201463Suppl 12A2045 [abstract]
- LiuXThompsonJPhatakHMardekianJPorcariARJohnsonMRApixaban reduces hospitalization in patients with venous thromboembolism: An analysis of the AMPLIFY-EXT TrialBlood20131223638 [abstract]
- Gómez-OutesASuárez-GeaMLLecumberriRTerleira-FernándezAIVargas-CastrillónERochaEPotential role of new anticoagulants for prevention and treatment of venous thromboembolism in cancer patientsVasc Health Risk Manag2013920722823674896
- van der HulleTden ExterPLKooimanJvan der HoevenJJHuismanMVKlokFAMeta-analysis of the efficacy and safety of new oral anticoagulants in patients with cancer-associated acute venous thromboembolismJ Thromb Haemost2014271116112024819040
- FargeDDebourdeauPBeckersMInternational clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancerJ Thromb Haemost2013111567023217107
- LinkinsLADansALMooresLKTreatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice GuidelinesChest2012141Suppl 2e495Se530S22315270
- KrauelKHackbarthCFürllBGreinacherAHeparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodiesBlood201211951248125522049520
- WalengaJMPrechelMJeskeWPRivaroxaban – an oral, direct factor Xa inhibitor – has potential for the management of patients with heparin-induced thrombocytopeniaBr J Haematol20081431929918671707
- WalengaJMPrechelMHoppensteadtDApixaban as an alternate oral anticoagulant for the management of patients with heparin-induced thrombocytopeniaClin Appl Thromb Hemost201319548248723780399
- LinkinsLAWarkentinTEPaiMDesign of the rivaroxaban for heparin-induced thrombocytopenia studyJ Thromb Thrombolysis Epub2192014
- SuryanarayanDSchulmanSPotential antidotes for reversal of old and new oral anticoagulantsThromb Res2014133Suppl 2S158S16624862137
- LalibertéFBookhartBKNelsonWWImpact of once-daily versus twice-daily dosing frequency on adherence to chronic medications among patients with venous thromboembolismPatient20136321322423857628
