Abstract
Neurogenic orthostatic hypotension (nOH) is due to failure of the autonomic nervous system to regulate blood pressure in response to postural changes due to an inadequate release of norepinephrine, leading to orthostatic hypotension and supine hypertension. nOH is common in Parkinson’s disease (PD). Prevalence varies throughout the course of PD, ranging from 40% to 60%, and resulting in symptomatic nOH in approximately half. Symptomatic nOH, including lightheadedness, can limit daily activities and lead to falls. Symptomatic nOH can also limit therapeutic options for treating PD motor symptoms. Clinical evaluation should routinely include symptom assessment and blood pressure measurement of supine, sitting, and 3-minute standing; 24-hour ambulatory blood pressure monitoring can also be helpful. Non-pharmacological management of symptomatic nOH involves education, physical maneuvers, and adequate hydration. Current pharmacological treatment of symptomatic nOH includes salt supplement, fludrocortisone, midodrine, pyridostigmine, and other empiric medications. Despite these options, treatment of symptomatic nOH remains suboptimal, often limited by severe increases in supine blood pressure. Droxidopa, an oral prodrug converted by decarboxylation to norepinephrine, is a promising therapeutic option for symptomatic nOH in PD, improving symptoms of nOH, daily activities, falls, and standing systolic blood pressure in several recent trials. These trials demonstrated short-term efficacy and tolerability, with comparable increases in standing and supine blood pressures. Longer-term studies are ongoing to confirm durability of treatment effect.
Introduction
Neurogenic orthostatic hypotension (nOH) results from failure of the autonomic nervous system (ANS) to regulate blood pressure in response to postural change, due to an inadequate release of norepinephrine (NE). This leads to both orthostatic hypotension upon standing and supine hypertension when lying. nOH is a hallmark of several neurodegenerative diseases, including multiple systems atrophy, Parkinson’s disease (PD), and primary autonomic failure. PD is the second most common neurodegenerative disease, and nOH is a commonly encountered clinical problem in patients with PD, perhaps affecting up to 40%–60% of patients throughout the multi-decade disease course.Citation1–Citation4 Symptomatic nOH occurs in approximately 20% of patients with PD,Citation1,Citation5 and increases with PD duration, disease severity, age, and levodopa usage.Citation5 However, symptoms such as lightheadedness may be variable though the day, and may abate and recur over time. Thus, there is not always a consistent correlation between orthostatic lightheadedness/dizziness and finding orthostatic hypotension when blood pressure is measured at a single reading. Although several pharmacological and non-pharmacological options are available to help manage this condition, current treatment of symptomatic nOH remains suboptimal.
Normal ANS response to standing
Postural change (ie, standing or lying supine) induces gravitational redistribution of blood volume, leading to changes in blood pressure. Upon standing, pooling of venous blood in the legs is countered by the normal sympathetic ANS to maintain standing blood pressure.Citation6 Lying supine also causes gravitational redistribution of blood volume, and the normal ANS minimizes blood pressure from rising too high. Norepinephrine is the major neurotransmitter in the ANS regulation of blood pressure in response to postural changes.Citation7 Sympathetic activation in response to standing leads to: venoconstriction with increased venous return; an increase in heart rate and myocardial contractility with increased cardiac output; and vasoconstriction with increased blood pressure. Normal activation of the intact ANS, along with sufficient circulating blood volume, prevents the gravity-induced fall in standing systolic blood pressure (s-SBP), maintaining cerebral perfusion and of other vital organs.Citation8
Impaired ANS response to standing in nOH
In patients with PD and nOH, autonomic dysfunction causes blood pressure to fall upon standing, due to an inappropriate NE response to postural change. Orthostatic hypotension has been defined as a drop in SBP of at least 20 mmHg or a drop in diastolic blood pressure of at least 10 mmHg after 3 minutes of standing.Citation9 There is often a loss of the cardioacceleratory response too.Citation10 Non-neurogenic causes of orthostatic hypotension are also common in patients with PD, and contribute to blood pressure drop.Citation11 These non-neurogenic causes of orthostatic hypotension should be identified first, and include dehydration, medications, and cardiac pump failure. A clinical diagnosis of nOH can be made when these non-neurogenic causes of persistent orthostatic hypotension are excluded, and can be confirmed through autonomic testing and plasma NE levels.Citation12
In PD, autonomic dysfunction is mainly a result of cardiac sympathetic denervation with inadequate activation of NE pathwaysCitation7,Citation13–Citation15 and also baroreflex failure.Citation16 This can emerge during the course of PD or can occur early in its course.Citation17–Citation20 Parkinsonism due to multiple system atrophy is also accompanied by prominent autonomic dysfunction, but nOH results from failure of central NE pathways.Citation21,Citation22
Symptomatic nOH
Upon standing, the normal ANS response maintains s-SBP within the range of cerebrovascular autoregulation. In nOH, a fall in blood pressure upon standing may not cause symptoms if s-SBP does not drop too low so that cerebral perfusion is maintained. When blood pressure falls upon standing, compensatory cerebrovascular autoregulation may maintain (or partially maintain) cerebral perfusion, minimizing symptoms.Citation23 Symptomatic nOH occurs when s-SBP falls below the range of cerebrovascular autoregulation, resulting in cerebral hypoperfusion and consequent lightheadedness/dizziness or syncope.
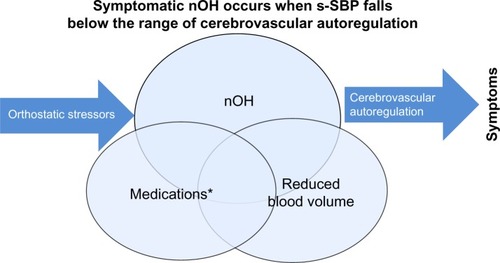
Asymptomatic nOH can become symptomatic in response to worsening autonomic dysfunction or due to orthostatic stress.Citation24 Indeed, in patients with PD, nOH is often compounded by non-neurogenic causes of OH and by orthostatic stressors. Symptomatic nOH may only emerge in response to an orthostatic stressor.Citation25 Mild dehydration can occur due to inadequate fluid intake, dysphagia, and concomitant diuretics. Early morning orthostasis may be problematic due to nocturnal supine fluid shifts.Citation26 Postprandial hypotension can trigger symptomatic nOH,Citation27,Citation28 as can prolonged standing or walking. Increased vasodilation due to heat, alcohol, or other factors may cause symptoms of nOH to emerge.Citation29 Many medications can lower blood pressure, especially during times of orthostatic stress or at their peak effects, including antihypertensive and dopaminergic therapies for PDCitation30–Citation33 ().
Figure 1 Overlapping causes of symptomatic nOH (Venn diagram): 1) autonomic failure of NE pathways upon standing; 2) dopaminergic and other medications causing lower SBP; and 3) suboptimal hydration leading to reduced circulating blood volume.
Abbreviations: nOH, neurogenic orthostatic hypotension; SBP, systolic blood pressure; s-SBP, standing SBP.
Clinical evaluation of nOH
Routine clinical evaluation of patients with PD should always include an assessment of blood pressure (lying, sitting, and 3-minute standing). Although orthostatic hypotension is diagnosed with a reduction of s-SBP of at least 20 mmHg within 3 minutes of standing,Citation9 lesser drops in s-SBP may still be symptomatic, and orthostatic hypotension may not occur until beyond 3 minutes.Citation34 Heart rate should normally rise 4–6 beats per minute upon standing, with a greater increase in response to orthostatic hypotension; with autonomic dysfunction, this cardioacceleratory response is typically blunted.Citation9 Symptomatic nOH may not always correlate with isolated measurements of s-SBP, due to the diurnal circadian rhythm of blood pressure and also to the marked fluctuations in blood pressure that occur throughout the day.Citation35 Twenty-four hour ambulatory blood pressure monitoring can be helpful in the evaluation of patients with symptomatic nOH to better understand temporal fluctuations in blood pressure, especially when clinical symptoms do not regularly correlate with sporadic blood pressure measurements.Citation35
Medical history should query possible offending medications, daily activities, potential orthostatic stressors, and symptoms of nOH. Common symptoms of nOH include lightheadedness, visual disturbances, impaired cognition,Citation36 fatigue, sleepiness (ie, postprandial), and difficulty standing or walking; syncope and falls may also occur. Symptoms of inadequate perfusion of other organs can cause symptoms, such as upper back/neck pain. The Orthostatic Hypotension Questionnaire (OHQ) can be useful to monitor symptoms over time.Citation37 Distinguishing imbalance on standing (due to postural instability) from lightheadedness (due to nOH) is important.Citation38 Other stigmata of autonomic dysfunction may be present, including constipation, gastroparesis, and urinary frequency.Citation39,Citation40
Testing of ANS integrity may be a useful adjunct to clinical evaluation. Testing may include RR-interval variation, spectral analysis of RR interval and blood pressureCitation20, Valsava response, thermoregulatory sweat test, tilt-table, and sampling plasma.Citation11,Citation21,Citation41–Citation43 MIBG scintigraphy has dis tinguished central and peripheral causes of nOH, but is not readily available. In PD, reduced radioisotope uptake reflects the sympathetic cardiac denervation in PD.Citation11,Citation16,Citation44
Management of nOH
Patients with symptomatic nOH should be treated to improve clinical symptoms and to maintain daily activities, such as walking. Treatment will also reduce the risk of injury, falls, and other morbidities associated with nOH. The treatment of symptomatic nOH is aimed at raising s-SBP into the range of compensatory cerebrovascular autoregulation. Treatment is multifaceted. Clinical therapeutic decision making must be based on standing (not sitting) SBP measurements. All possible offending pharmacologic agents should be minimized or stopped. Effective management requires extensive patient education with a combination of non-pharmacological and pharmacological therapiesCitation24 ().
Figure 2 Treatment goals for patients with symptomatic nOH.

Disease education of patients and their caregivers is directed towards an understanding of the role of postural changes on blood pressure, and is a necessary adjunct to clinical management. Rapid changes in posture should be avoided. Arising from supine to standing should be preceded by sitting for several minutes before standing. Standing after prolonged sitting should occur slowly, and several minutes of standing should precede walking. Patients also need to be advised that they should identify places to safely sit and rest in the case of unexpected symptom onset in order to avoid falls.Citation45 Patients and caregivers should especially be educated about the impact of orthostatic stressors on nOH symptoms.Citation24 Extended periods of standing or walking may result in symptoms, as can physical exercise, meals,Citation27 and any rise in core body temperature (fever, hot shower, hot or dry climates).Citation24,Citation29
Polypharmacy should be avoided, and all medications that have potential to lower blood pressure should be discontinued as possible. The use of diuretics and antihypertensives should be discouraged, although shorter-acting antihypertensives prior to bedtime may be needed for supine hypertension.Citation10,Citation24 Other concomitant therapies, including medications for depression (tricyclics), sleep/allergy (antihistamines), and urinary frequency (anticholinergics and alpha-adrenergic antagonists) should be minimized.
Dopaminergic medications also lower blood pressure.Citation30,Citation33 If motor symptoms of parkinsonism permit, modifying the Parkinson’s medication regimen may be one way to minimize blood pressure fluctuations in patients with nOH. Levodopa dose may need to be fractionated if orthostatic symptoms occur at peak dose. Dopamine agonist dose, especially of the immediate-release formulations that have higher peaks, may need to be reduced. Selegiline, anticholinergics, and amantadine also commonly lower blood pressure.
Patient education is often combined with non-pharmacologic measures, and these are sometimes sufficient. Increased hydration and salt intake is essential to maintain sufficient circulating blood volume, although caution must be used in patients with congestive heart failure. In response to lightheadedness or other symptoms of nOH, immediately drinking a 12–16 ounce bolus of water can rapidly raise s-SBP.Citation24,Citation46,Citation47 Instruction on physical maneuvers, such as toe-raises, crossing legs, squatting, and bending at the waist can also improve nOH symptoms when they occur.Citation24,Citation48 Body compression garments, corset or belt, and thigh-high compression stockings are useful,Citation24,Citation49,Citation50 but may be difficult for some patients to routinely wear. Elevation of the head 30° above horizontal will help minimize supine hypertension and nocturnal supine diuresis.Citation26,Citation51
Medication management of nOH is added when patients have persistent symptoms despite these non-pharmacological approaches.Citation10 Fludrocortisone is a synthetic mineralocorticoid that acts to retain sodium and water. This increases circulating blood volume and consequently blood pressure.Citation51,Citation52 Clinical effect of daily dosages of 0.1–0.4 mg should be administered once (or twice) earlier in the day. Onset of effect typically occurs over a period of 3–7 days. Its use is limited by increased supine blood pressure, which must be monitored in patients by sitting and supine blood pressure evaluations. Routine monitoring of electrolytes should also be performed to evaluate for development of hypokalemia. Excessive fluid retention by fludrocortisone can result in pedal edema and congestive heart failure, and may limit its use.
Midodrine is an alpha-adrenergic agonist that can increase blood pressure by increasing peripheral vascular resistance. While some patients may respond to a dosage of 2.5 mg, most will require 5–10 mg dosage.Citation53–Citation55 Clinical response to a dose of midodrine should be assessed before and 1 hour after administration, as onset of action occurs within 30 minutes, peaks at 1 hour, and lasts 3–4 hours.Citation53 Due to its short duration of clinical effect, midodrine is administered several times daily, but should not be given within several hours of bedtime or near other times of lying supine.Citation53 Adverse effects of midodrine include piloerection, pruritus, and increased supine blood pressure, which can be dose limiting.Citation55
Pyridostigmine has also been used to treat nOH. Pyridostigmine is a peripheral inhibitor of acetylcholinesterase, which can cause a mild increase in standing blood pressure without significantly increasing supine blood pressure.Citation56 Adverse effects are cholinergic, including diarrhea, excessive sweating, and siallorhea. When symptomatic nOH persists despite use of fludrocortisone, midodrine, and pyridostigmine, or the use of these medications are limited by adverse effects, several empiric medications have been tried.Citation10,Citation24 Erythropoietin can expand blood volume and increase blood pressure in symptomatic patients, but can increase supine blood pressure.Citation57,Citation58 Desmopressin,Citation59 indomethacin,Citation60 and caffeineCitation61 have been evaluated in small trials. In patients with persistent or severe symptomatic nOH, attempts to augment NE pathways through the use of sympathomimetics or of NE transporter inhibitors (ie, NE-transporter blockers, serotonin-NE reuptake inhibitors) have been tried with variable success.Citation62–Citation65
Droxidopa (L-threo-3-4-dihydroxyphenylserine [L-threo DOPS]), an oral prodrug converted by decarboxylation to NE in both the central and the peripheral nervous systems,Citation66–Citation68 is a promising therapeutic option for symptomatic nOH in PD. Droxidopa improves nOH in PD by replenishing NE.Citation67,Citation69 Over more than two decades, studies in JapanCitation70,Citation71 and in EuropeCitation72 have studied the effects of droxidopa in patients with nOH and PD. In these trials, droxidopa has been demonstrated to increase s-SBP, even in patients taking a decarboxylase inhibitor. Smaller studies in the US have also found similar benefit in nOH.Citation67,Citation73 Droxidopa for nOH has been marketed in Japan since 1989 for the indications of: 1) improvement of frozen gait and dizziness on standing in Parkinson’s disease (Yahr stage III); 2) improvement of orthostatic hypotension, syncope, and dizziness on standing in Shy-Drager syndrome and familial amyloid polyneuropathy; and 3) improvement of orthostatic hypotension symptoms in the hemodialytic patient (dizziness, lightheaded feeling, dizziness on standing up, malaise, and weakness). In the US, a “new drug application” has been filed with the FDA, and is pending regulatory approval.
More recently, droxidopa has been evaluated in several Phase III clinical trials in the US to establish its efficacy and safety in treating symptomatic nOH, including in patients with nOH associated with PD. The results of these trials have been presented in abstract form, but have not yet been fully published. In these trials, efficacy was assessed primarily by the two-part OHQ: Orthostatic Hypotension Symptom Assessment (OHSA; Item 1: dizziness/lightheadedness) and Orthostatic Hypotension Daily Activity Scale.Citation37
An initial trial utilized a withdrawal design and enrolled patients with nOH due to PD, multiple systems atrophy, and other neurological disorders with nOH. After open-label droxidopa titration, droxidopa responders were randomized to treatment with droxidopa or placebo for 2 weeks. While OHQ was significantly improved, the primary endpoint of OHSA Item 1 (dizziness/lightheadedness) was not met.Citation74 In a second trial, patients with nOH were enrolled in an induction design study. After open-label droxidopa titration followed by 1 week washout, droxidopa responders were randomized to treatment with droxidopa or placebo for 1 week. Significant improvement over placebo was found for OHQ, including OHSA Item 1 (dizziness/lightheadedness), and for s-SBP.Citation75 A subsequent 3-month open-label study enrolled patients from these two trials. Overall, patients maintained stable scores on OHQ and OHSA Item 1 (dizziness/lightheadedness) for up to 12 months. However, a randomized 2-week withdrawal after 3 months of open-label treatment did not find significant difference in OHQ between droxidopa and placebo,Citation76 perhaps suggesting a prolonged residual effect of droxidopa. In a safety study, patients were evaluated with 24-hour ambulatory blood pressure monitoring 1 week after droxidopa washout and again after 4 weeks of droxidopa treatment. Droxidopa treated patients had a significantly greater increase in mean daytime SBP without causing sustained elevations in supine SBP overnight.Citation77 Long-term, open-label treatment with droxidopa has also been reported with overall good tolerability and safety.Citation78
Droxidopa has also been evaluated for symptomatic nOH in 225 patients with PD, one of the largest studies conducted of nOH in PD. After 1 week of randomized droxidopa or placebo treatment, there was a significant improvement in OHSA Item 1 (dizziness/lightheadedness), s-SBP, and clinician global improvement.Citation79,Citation80 At week 8, numerical differ ences persisted but were not statistically significant. Some patients had a reduction in falls,Citation81 and treatment effect was seen despite use of decarboxylase inhibitor,Citation82 dopaminergic medications for PD,Citation83 or prior use of midodrine.Citation84
Overall, droxidopa has demonstrated short-term efficacy on symptoms of nOH and on s-SBP, including in patients with PD.Citation85 In long-term open-label treatment, droxidopa had good tolerability and safety, without significant supine hypertension. While some studies found sustained improvements in symptoms and s-SBP, longer-term studies are needed to confirm the durability of droxidopa treatment on nOH symptoms.
Summary
nOH in PD is due to an inadequate release of NE resulting in failure of the ANS to maintain s-SBP. nOH is common and can become symptomatic when compensatory cerebrovascular autoregulation cannot maintain adequate cerebral perfusion upon standing, often in response to orthostatic stress. Clinical evaluation of symptoms and 3-minute s-SBP should routinely be performed, so that treatment can be instituted to improve symptoms, daily activities, and reduce risk of injury, falls, and other morbidities (). Current treatments for nOH are suboptimal and can increase supine blood pressure. Droxidopa is an emerging therapy for symptomatic nOH, and increases s-SBP without greater increases in supine hypertension.
Disclosure
Stuart Isaacson received honoraria for CME, consultancy and research grants, and/or promotional speaking on behalf of Abbvie, Acadia, Adamas, Addex, Allergan, Allon, Astra Zeneca, Biotie, Britannia, Chelsea Therapeutics, Civitas, Eisai, GE, GSK, Impax, Ipsen, Kyowa, Lilly, Merck Schering-Plough, Medtronics, Merz, Michael J Fox Foundation, Novartis, Neurocrine, NIH, Novartis, Orion, Parkinson Study Group, Phytopharm, Purdue, Roche, Santhera, Serono, Shire, Teva, UCB, and US World Meds. Julia Skettini has no conflicts of interests in this work.
References
- SenardJMRaiSLapeyre-MestreMPrevalence of orthostatic hypotension in Parkinson’s diseaseJ Neurol Neurosurg Psychiatry19976355845899408097
- AllcockLMUllyartKKennyRABurnDJFrequency of orthostatic hypotension in a community based cohort of patients with Parkinson’s diseaseJ Neurol Neurosurg Psychiatry200475101470147115377699
- SithinamsuwanPOrrawanhanothaiPThithumKOrthostatic hypotension: a non-motor complication assessment in 82 patients with idiopathic Parkinson’s disease in Phramongkutklao HospitalJ Med Assoc Thai201093Suppl 6S93S9921280520
- JainSGoldsteinDSCardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesisNeurobiol Dis201246357258022094370
- HaADBrownCHYorkMKJankovicJThe prevalence of symptomatic orthostatic hypotension in patients with Parkinson’s disease and atypical parkinsonismParkinsonism Relat Disord201117862562821689962
- MathiasCJTo stand on one’s own legsClin Med20022323724512108475
- SharabiYImrichRHolmesCPechnikSGoldsteinDSGeneralized and neurotransmitter-selective noradrenergic denervation in Parkinson’s disease with orthostatic hypotensionMov Disord200823121725173218661549
- SchatzIJOrthostatic hypotension. I. Functional and neurogenic causesArch Intern Med198414447737776370161
- FreemanRWielingWAxelrodFBConsensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndromeClin Auton Res2011212697221431947
- FreemanRClinical practice. Neurogenic orthostatic hypotensionN Engl J Med2008358661562418256396
- SenardJMPathakANeurogenic orthostatic hypotension of Parkinson’s disease: what exploration for what treatment?Rev Neurol (Paris)20101661077978420817229
- GoldsteinDSSharabiYNeurogenic orthostatic hypotension: a pathophysiological approachCirculation2009119113914619124673
- GoodallMCHarlanWRJrAltonHDecreased noradrenaline (norepinephrine) synthesis in neurogenic orthostatic hypotensionCirculation19683835926035673610
- GoldsteinDSPolinskyRJGartyMPatterns of plasma levels of catechols in neurogenic orthostatic hypotensionAnn Neurol19892645585632510587
- ImrichREldadahBABenthoOFunctional effects of cardiac sympathetic denervation in neurogenic orthostatic hypotensionParkinsonism Relat Disord200915212212718514012
- SharabiYGoldsteinDSMechanisms of orthostatic hypotension and supine hypertension in Parkinson diseaseJ Neurol Sci20113101–212312821762927
- BonuccelliULucettiCDel DottoPOrthostatic hypotension in de novo Parkinson diseaseArch Neurol200360101400140414568810
- MilazzoVDi StefanoCServoSZibettiMLopianoLMauleSNeurogenic orthostatic hypotension as the initial feature of Parkinson diseaseClin Auton Res201222420320622562219
- KaufmannHNahmKPurohitDWolfeDAutonomic failure as the initial presentation of Parkinson disease and dementia with Lewy bodiesNeurology20046361093109515452307
- OkaHToyodaCYogoMMochioSCardiovascular dysautonomia in de novo Parkinson’s disease without orthostatic hypotensionEur J Neurol201118228629220602633
- GoldsteinDSHolmesCSharabiYBrentzelSEisenhoferGPlasma levels of catechols and metanephrines in neurogenic orthostatic hypotensionNeurology20036081327133212707437
- GoldsteinDSHolmesCPatronasNKopinIJCerebrospinal fluid levels of catechols in patients with neurogenic orthostatic hypotensionClin Sci (Lond)2003104664965412540289
- SchatzIJOrthostatic hypotension. II. Clinical diagnosis, testing, and treatmentArch Intern Med19841445103710416143541
- LowPASingerWManagement of neurogenic orthostatic hypotension: an updateLancet Neurol20087545145818420158
- ZiemssenTReichmannHCardiovascular autonomic dysfunction in Parkinson’s diseaseJ Neurol Sci20102891–2748019740484
- OmboniSSmitAAvan LieshoutJJSettelsJJLangewoutersGJWielingWMechanisms underlying the impairment in orthostatic tolerance after nocturnal recumbency in patients with autonomic failureClin Sci (Lond)2001101660961811724647
- LipsitzLARyanSMParkerJAFreemanRWeiJYGoldbergerALHemodynamic and autonomic nervous system responses to mixed meal ingestion in healthy young and old subjects and dysautonomic patients with postprandial hypotensionCirculation19938723914008425288
- LipsitzLAPluchinoFCWeiJYMinakerKLRoweJWCardiovascular and norepinephrine responses after meal consumption in elderly (older than 75 years) persons with postprandial hypotension and syncopeAm J Cardiol19865898108153766423
- PathakALapeyre-MestreMMontastrucJLSenardJMHeat-related morbidity in patients with orthostatic hypotension and primary autonomic failureMov Disord20052091213121915954131
- SenardJMBrefel-CourbonCRascolOMontastrucJLOrthostatic hypotension in patients with Parkinson’s disease: pathophysiology and managementDrugs Aging200118749550511482743
- MecoGPratesiLBonifatiVCardiovascular reflexes and autonomic dysfunction in Parkinson’s diseaseJ Neurol199123841951991895149
- LangAEAcute orthostatic hypotension when starting dopamine agonist therapy in parkinson disease: the role of domperidone therapyArch Neurol200158583511346387
- WoodLDClinical review and treatment of select adverse effects of dopamine receptor agonists in Parkinson’s diseaseDrugs Aging201027429531020359261
- Jamnadas-KhodaJKoshySMathiasCJMuthaneUBRagothamanMDodaballapurSKAre current recommendations to diagnose orthostatic hypotension in Parkinson’s disease satisfactory?Mov Disord200924121747175119562759
- StuebnerEVichayanratELowDAMathiasCJIsenmannSHaenschCATwenty-four hour non-invasive ambulatory blood pressure and heart rate monitoring in Parkinson’s diseaseFront Neurol201344923720648
- PodaRGuaraldiPSolieriLStanding worsens cognitive functions in patients with neurogenic orthostatic hypotensionNeurol Sci201233246947321894556
- KaufmannHMalamutRNorcliffe-KaufmannLRosaKFreemanRThe Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scaleClin Auton Res2012222799022045363
- HillenMEWagnerMLSageJI“Subclinical” orthostatic hypotension is associated with dizziness in elderly patients with Parkinson diseaseArch Phys Med Rehabil19967777107128670000
- CamilleriMMalageladaJRStanghelliniVFealeyRDShepsSGGastrointestinal motility disturbances in patients with orthostatic hypotensionGastroenterology1985886185218593996842
- CersosimoMGBenarrochEEAutonomic involvement in Parkinson’s disease: pathology, pathophysiology, clinical features and possible peripheral biomarkersJ Neurol Sci20123131–2576322001247
- KopinIJPolinskyRJOliverJAOddershedeIREbertMHUrinary catecholamine metabolites distinguish different types of sympathetic neuronal dysfunction in patients with orthostatic hypotensionJ Clin Endocrinol Metab19835736326376874892
- LowPATomaliaVAParkKJAutonomic function tests: some clinical applicationsJ Clin Neurol2013911823346153
- GurevichTYGroozmanGBGiladiNDroryVEHausdorffJMKorczynADR-R interval variation in Parkinson’s disease and multiple system atrophyActa Neurol Scand2004109427627915016010
- HaenschCALerchHJorgJIsenmannSCardiac denervation occurs inde-pendent of orthostatic hypotension and impaired heart rate variability in Parkinson’s diseaseParkinsonism Relat Disord200915213413718515170
- SmitAAWielingWOpfer-GehrkingTLvan Emmerik-LeveltHMLowPAPatients’ choice of portable folding chairs to reduce symptoms of orthostatic hypotensionClin Auton Res19999634134410638808
- JordanJShannonJRBlackBKThe pressor response to water drinking in humans: a sympathetic reflex?Circulation2000101550450910662747
- Z’GraggenWJHessCWHummAMAcute fluid ingestion in the treatment of orthostatic intolerance – important implications for daily practiceEur J Neurol201017111370137620412295
- BouvetteCMMcPheeBROpfer-GehrkingTLLowPARole of physical countermaneuvers in the management of orthostatic hypotension: efficacy and biofeedback augmentationMayo Clin Proc19967198478538790259
- SmitAAWielingWFujimuraJUse of lower abdominal compression to combat orthostatic hypotension in patients with autonomic dysfunctionClin Auton Res200414316717515241645
- PodoleanuCMaggiRBrignoleMLower limb and abdominal compression bandages prevent progressive orthostatic hypotension in elderly persons: a randomized single-blind controlled studyJ Am Coll Cardiol20064871425143217010806
- van LieshoutJJten HarkelADWielingWFludrocortisone and sleeping in the head-up position limit the postural decrease in cardiac output in autonomic failureClin Auton Res2000101354210750642
- HicklerRBThompsonGRFoxLMHamlinJT3rdSuccessful treatment of orthostatic hypotension with 9-alpha-fluorohydrocortisoneN Engl J Med195926178879114401690
- WrightRAKaufmannHCPereraRA double-blind, dose-response study of midodrine in neurogenic orthostatic hypotensionNeurology19985111201249674789
- JankovicJGildenJLHinerBCNeurogenic orthostatic hypotension: a double-blind, placebo-controlled study with midodrineAm J Med199395138487687093
- LowPAGildenJLFreemanRShengKNMcElligottMAEfficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study GroupJAMA199727713104610519091692
- SingerWSandroniPOpfer-GehrkingTLPyridostigmine treatment trial in neurogenic orthostatic hypotensionArch Neurol200663451351816476804
- PereraRIsolaLKaufmannHEffect of recombinant erythropoietin on anemia and orthostatic hypotension in primary autonomic failureClin Auton Res1995542112138520216
- HoeldtkeRDStreetenDHTreatment of orthostatic hypotension with erythropoietinN Engl J Med199332996116158341335
- MathiasCJFosbraeyPda CostaDFThornleyABannisterRThe effect of desmopressin on nocturnal polyuria, overnight weight loss, and morning postural hypotension in patients with autonomic failureBr Med J (Clin Res Ed)19862936543353354
- AbateGPolimeniRMCuccurulloFPudduPLenziSEffects of indomethacin on postural hypotension in ParkinsonismBr Med J19792620314661468393356
- OnrotJGoldbergMRBiaggioniIHollisterASKingaidDRobertsonDHemodynamic and humoral effects of caffeine in autonomic failure. Therapeutic implications for postprandial hypotensionN Engl J Med198531395495543894971
- LahrmannHCortelliPHilzMMathiasCJStruhalWTassinariMEFNS guidelines on the diagnosis and management of orthostatic hypotensionEur J Neurol200613993093616930356
- OnrotJGoldbergMRBiaggioniIWileyRGHollisterASRobertsonDOral yohimbine in human autonomic failureNeurology19873722152203808301
- Fouad-TaraziFMOkabeMGorenHAlpha sympathomimetic treatment of autonomic insufficiency with orthostatic hypotensionAm J Med19959966046107503082
- OkamotoLEShibaoCGamboaASynergistic effect of norepinephrine transporter blockade and alpha-2 antagonism on blood pressure in autonomic failureHypertension201259365065622311903
- FreemanRLandsbergLThe treatment of orthostatic hypotension with dihydroxyphenylserineClin Neuropharmacol19911442963041913697
- KaufmannHSaadiaDVoustianioukANorepinephrine precursor therapy in neurogenic orthostatic hypotensionCirculation2003108672472812885750
- GoldsteinDSL-Dihydroxyphenylserine (L-DOPS): a norepinephrine prodrugCardiovasc Drug Rev2006243–418920317214596
- KaufmannHCould treatment with DOPS do for autonomic failure what DOPA did for Parkinson’s disease?Neurology1996476137013718960711
- NarabayashiHNakanishiTTherapeutic effects of L-DOPS in Parkinson’s disease, double-blind comparative study against placebo as control in patients with long-term levodopa therapyClin Eval198715423457
- YanagisawaNIkedaSHashimotoTEffects of L-threo-Dops on orthostatic hypotension in Parkinson’s diseaseNo To Shinkei1998502157163 Japanese9513205
- MathiasCJSenardJMCortelliPA double-blind, randomized, placebo-controlled study to determine the efficacy and safety of droxidopa in the treatment of orthostatic hypotension associated with multiple system atrophy and Parkinson’s diseaseClin Auton Res200717272
- GoldsteinDSHolmesCKaufmannHFreemanRClinical pharmacokinetics of the norepinephrine precursor L-threo-DOPS in primary chronic autonomic failureClin Auton Res200414636336815666063
- KaufmannHMathiasCFreemanRLowPBiaggioniIDroxidopa Study GroupTreatment with droxidopa – a phase III multinational, placebo-controlled, parallel group, withdrawal-design study in subjects with neurogenic orthostatic hypotension and non-diabetic autonomic neuropathyClin Auton Res200919281
- KaufmannHFreemanRBiaggioniILowPPedderSHewittAMathiasCTreatment of neurogenic orthostatic hypotension: results from a multi-center, double-blind, randomized, placebo-controlled, parallel group, induction design studyNeurology201278PL02.001
- IsaacsonSShillHVerninoSCioffiCHutchmanRDurability of effect with long-term, open-label droxidopa treatment in patients with symptomatic neurogenic orthostatic hypotension (NOH 303)Mov Disord201227S424S425
- KaufmannHKlosKHutchmanRIsaacsonSEffects of droxidopa (Northera™) on 24 hour blood pressure (BP) in patients with symptomatic neurogenic orthostatic hypotension (NOH)Mov Disord201025S405S406
- ShillHVerninoSHutchmanRAdkinsLIsaacsonSA multicenter, open-label study to assess the long-term safety of droxidopa in patients with symptomatic neurogenic orthostatic hypotension (NOH 304)Mov Disord201227S428
- IsaacsonSHHauserRASzakacsCBNCioffiCCDroxidopa treatment impact on orthostatic symptoms and standing systolic blood pressure in patients with Parkinson’s disease and symptomatic neurogenic orthostatic hypotension: Study 306Neurology201380 Emerging Science Abstract 010
- IsaacsonSHauserRASzakacsCBNCioffiCCImpact of droxidopa treatment in patients with Parkinson’s disease and symptomatic neurogenic orthostatic hypotension (study 306)Mov Disord201328Suppl 1468
- HauserRASchwietermanWIsaacsonSImpact of treatment with droxidopa in repeat fallers with Parkinson’s disease and symptomatic neurogenic orthostatic hypotension (NOH 306A)Mov Disord201227S423
- BiaggioniILowPRowseGKaufmannHAnalysis of efficacy in patients with symptomatic neurogenic orthostatic hypotension treated with droxidopa and dopa-decarboxylase inhibitorsMov Disord201227S422S423
- LeWittPGornySAnalysis of efficacy and safety outcomes in patients treated with droxidopa in combination with other drug classesMov Disord201227S425S426
- LowPNelsonJStacyMSafety and efficacy of droxidopa in patients previously treated with midodrineMov Disord201227S426
- MathiasCLowPFreemanRHewittLKaufmannHIntegrated efficacy analysis of droxidopa in 2 double-blind, placebo-controlled Phase 3 studies in patients with neurogenic orthostatic hypotensionMov Disord201227S426

