Abstract
Purpose
Venous thromboembolism is a common complication after major orthopedic surgery. When prescribing anticoagulant prophylaxis, clinicians weigh the benefits of thromboprophylaxis against bleeding risk and other adverse events. Previous benefit–risk analyses of the REgulation of Coagulation in ORthopaedic surgery to prevent Deep vein thrombosis and pulmonary embolism (RECORD) randomized clinical studies of rivaroxaban versus enoxaparin after total hip (THA) or knee (TKA) arthroplasty generally used pooled THA and TKA results, counted fatal bleeding as both an efficacy and a safety event, and included the active and placebo-controlled portions of RECORD2, which might confound benefit–risk assessments. We conducted a post hoc analysis without these constraints to assess benefit–risk for rivaroxaban versus enoxaparin in the RECORD studies.
Patients and methods
Data from the safety population of the two THA and two TKA studies were pooled separately. The primary analysis compared the temporal course of event rates and rate differences between rivaroxaban and enoxaparin prophylaxis for symptomatic venous thromboembolism plus all-cause mortality (efficacy events) versus nonfatal major bleeding (safety events). Additionally, these rates were used to derive measures of net clinical benefit, number needed to treat (NNT), and number needed to harm (NNH) for these two end points.
Results
After THA or TKA, and compared with enoxaparin, rivaroxaban therapy resulted in more efficacy events prevented than safety events caused, with benefits exceeding harms early and throughout treatment and follow-up. Relative to enoxaparin, rivaroxaban treatment prevented six efficacy events per harm event caused for THA, with NNT =262/NNH =1,711. For TKA, rivaroxaban treatment prevented four to five efficacy events per harm event caused, with NNT =102/NNH =442. Sensitivity analysis that included surgical-site bleeding resulted in NNH =345 for THA and NNH =208 for TKA.
Conclusion
In the RECORD studies, considering death, symptomatic venous thromboembolism, and major bleeding, rivaroxaban resulted in greater benefits than harms compared with enoxaparin. When incorporating surgical-site bleeding, rivaroxaban also results in greater benefit than harm for TKA and is balanced with enoxaparin for THA.
Introduction
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is common after total hip arthroplasty (THA) and total knee arthroplasty (TKA) without thromboprophylaxis. During the 90 days after primary arthroplasty, in patients who receive thromboprophylaxis, hospitalization resulting from symptomatic DVT occurs in 0.7% of THA patients and 0.9% of TKA patients, and hospitalization resulting from PE occurs in 0.3% of patients in both groups.Citation1 VTE is associated with significant morbidity and mortality;Citation2 DVT may lead to recurrent VTE or post-thrombotic syndrome,Citation2–Citation4 and nonfatal PE may result in chronic thromboembolic pulmonary hypertension.Citation5,Citation6
Despite the availability of thromboprophylactic therapies, the prevention of VTE after THA or TKA remains a challenge, in part owing to increased risk of bleeding and the drawbacks of existing chemothromboprophylaxis.Citation1,Citation7–Citation9 Warfarin is a common and important therapeutic option, although it usually takes several days to achieve pharmacologic effect and requires regular coagulation monitoring to ensure that the target international normalized ratio is achieved and maintained.Citation10 Low-molecular-weight heparins (eg, enoxaparin) do not require the same level of careful monitoring but must be administered parenterally. Rivaroxaban is an oral anticoagulant that directly inhibits Factor Xa and does not require routine coagulation monitoring. The REgulation of Coagulation in ORthopaedic surgery to prevent Deep vein thrombosis and pulmonary embolism (RECORD) Phase III clinical trial program compared the efficacy and safety of rivaroxaban with enoxaparin for the prevention of VTE after elective THA and TKA.Citation11–Citation14 Rivaroxaban demonstrated superiority to various enoxaparin regimens in the reduction of postoperative total VTE (composite of any DVT, nonfatal PE, and all-cause mortality) in the individual RECORD trials and pooled analyses;Citation11–Citation15 however, there were nonstatistically significant increases in major bleeding alone and in the composite of major and nonmajor clinically relevant bleeding in the individual studies, and there was a statistically significant increase in the composite of major and nonmajor clinically relevant bleeding in the pooled safety population.
For thromboprophylaxis, clinicians weigh the benefits of reduced rates of DVT and PE against the increased rate of bleeding. Approaches to assess drug benefit–risk have been considered by both industry and regulatory groups,Citation16–Citation21 but there are few regulatory guidelines for conducting benefit–risk analyses. Generally, efficacy and safety results are reported separately, and the benefit–risk balance tends to be characterized qualitatively, as in previous analyses of the RECORD studies.Citation15,Citation22,Citation23 The number of quantitative assessments of benefit–risk profiles of anticoagulants is growing, however.Citation24–Citation32 The purpose of this paper is to quantitatively assess benefit versus risk for rivaroxaban versus enoxaparin in the RECORD studies, in patients who had THA or TKA.
Patients and methods
RECORD studies
Between February 7, 2006 and January 31, 2008, 12,729 patients were randomized in the RECORD program.Citation11–Citation15 Men and women aged 18 years or older scheduled to undergo THA or TKA were eligible. Of the randomized patients, 12,383 were included in the safety analysis.Citation15 The same number of patients in both groups (n=173) were excluded from the safety population because they did not receive any study medication.Citation15 The primary reasons for study discontinuation were similar between the groups and included consent withdrawn, clinical end point reached, noncompliance, protocol violation, adverse events (AEs), and other reason.Citation15 The two groups were well balanced in terms of demographic and surgical characteristics.Citation15
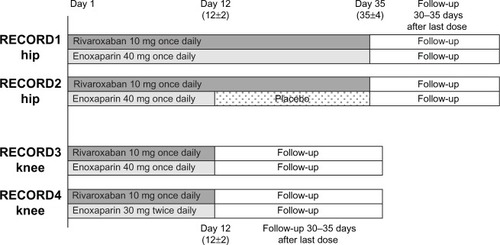
The four studies were of similar design but had important differences (). In RECORD1 and RECORD2 (THA), patients received rivaroxaban until day 35±4, with enoxaparin given until day 35±4 in RECORD1 and until day 12±2 followed by placebo in RECORD2. In RECORD3 and RECORD4 (TKA), prophylaxis with rivaroxaban or enoxaparin was given until day 12±2. Ten milligrams of rivaroxaban (initiated 6–8 hours postoperatively once hemostasis was assured) was administered orally once daily in all four studies. Subcutaneous enoxaparin was dosed at 40 mg once daily beginning preoperatively in RECORD1, RECORD2, and RECORD3, and at 30 mg twice daily beginning postoperatively in RECORD4. Patients underwent mandatory bilateral venography the day after the last dose of study drug. In all four studies, patients were followed for 30–35 days after treatment.
Figure 1 RECORD study designs.
Abbreviation: RECORD, REgulation of Coagulation in ORthopaedic surgery to prevent Deep vein thrombosis and pulmonary embolism.
End points and rates analyzed
Symptomatic VTE, all-cause mortality, bleeding, wound infection, and reoperation-related end points were among the end points analyzed (). Composite end points were analyzed using time-to-first event analysis. End points were assessed in the safety population, which included all patients who had taken at least one dose of study medication and all events from first dose to the end of follow-up. Details on data collection in the RECORD studies are described in previously published papers.Citation11–Citation15
Table 1 Efficacy and safety end points analyzed
Previous benefit–risk analyses of the RECORD studies used pooled data from all four studies to enable more precise estimates of the incidence of uncommon but clinically important events.Citation28,Citation33 Here, the data from the two THA studies and the two TKA studies are pooled separately to enable assessment of the different time course of benefit–risk after both types of arthroplasty. Earlier pooled analyses of the RECORD studies also generally utilized relative effect measures (odds ratio or hazard ratio).Citation15 Our analyses use rate differences between rivaroxaban and active enoxaparin treatment to show more clearly the number of events caused versus those prevented. Finally, to allow for a head-to-head comparison, only the treatment-controlled portion of RECORD2 is included. Therefore, follow-up time is truncated so that events in both arms of RECORD2 are censored (omitted) starting 2 days after the last administration of enoxaparin in the enoxaparin arm or matching treatment in the rivaroxaban arm (treatment duration 12±2 days). All events from first dose to end of the planned follow-up period (active study duration) in the other three studies are included. For RECORD1, this includes events up to day 70 (35 days active treatment + 35 days follow-up). For the TKA studies, this includes events up to day 47 (12 days treatment + 35 days follow-up).
Benefit–risk assessment
The primary analysis compares the temporal course of event rates and rate differences for symptomatic VTE plus all-cause mortality versus nonfatal major bleeding. This efficacy end point was chosen to capture symptomatic events from the primary efficacy end point of the individual trials. The comparison with the risk of major bleeding was used because it was the most severe type of bleeding measured. Because fatal bleeding events are already included in all-cause mortality, the comparison uses nonfatal major bleeding to avoid counting the same event twice.
In addition to comparing Kaplan–Meier rates of symptomatic VTE plus all-cause mortality versus nonfatal major bleeding, this analysis compared number needed to treat (NNT) and number needed to harm (NNH) for these two end points and assessed a measure of net clinical benefit. NNT and NNH were defined as the number of individuals who need to be exposed, on average, to one treatment versus the other to prevent one harmful efficacy event or to induce one additional harmful safety event, respectively. NNT and NNH were calculated as the reciprocal of the respective Kaplan–Meier rate differences. Net clinical benefit was defined as the sum of the rate differences for symptomatic VTE plus all-cause mortality and nonfatal major bleeding, and it can be interpreted as the difference between the numbers of safety events caused and efficacy events prevented by rivaroxaban compared with enoxaparin in a hypothetical cohort of 10,000 patients. Negative net clinical benefit indicates fewer harmful events associated with rivaroxaban than with enoxaparin treatment.
Comparison of clinical severity for efficacy and safety end points
The comparison should ideally be made using end points with similar clinical impact. Across the four RECORD studies, for symptomatic VTE and major bleeding events (), the same percentages of both types of event were classified by investigators as severe (27%) or requiring hospitalization (54%), where severe was defined as preventing normal activities; however, symptomatic venous thromboembolic events were more likely than bleeding events to be considered serious by investigators (99% versus 70%) or to require remedial therapy (86% versus 30%). Symptomatic VTE also had a longer median duration than major bleeding events: 29 days for symptomatic VTE in both THA and TKA patients compared with 6.5 days for major bleeding in THA patients and 2 days in TKA patients, leading to a greater probability of a case of symptomatic VTE being unresolved at discharge from hospital. Finally, the median time to onset of symptomatic VTE (18 days for THA; 5 days for TKA) was longer than that for major bleeding (1 day for THA; 3 days for TKA), leading to greater chance of a case of symptomatic VTE occurring after hospital discharge.
Table 2 Clinical impact comparisons of symptomatic venous thromboembolic events versus major bleeding events pooled over both treatment arms from REgulation of Coagulation in ORthopaedic surgery to prevent Deep vein thrombosis and pulmonary embolism (RECORD)1–4 over the treatment periodCitation15
In all measures used, investigators regarded symptomatic VTE as having a clinical impact similar to or greater than that of major bleeding, and, by extension, similar to or greater than that of nonfatal major bleeding as used in the analyses below. Additionally, only one major bleeding event in the RECORD studies was a hemorrhagic stroke.Citation33 Although these observations suggest that symptomatic VTE plus all-cause mortality events had greater clinical impact than the nonfatal major bleeding events in RECORD,Citation24,Citation30 to be conservative in our assessment, we regarded the two end points as having equal clinical impact.
Results
After THA, rivaroxaban prevented more harmful efficacy events than enoxaparin but was associated with more safety events (). The number of efficacy events that were prevented was greater than the number of harms caused, both early and throughout treatment and follow-up, with rates plateauing at a ratio of six efficacy events prevented per harm caused (). In 10,000 patients, compared with enoxaparin, rivaroxaban would be expected to be associated with 38 (95% confidence interval [CI]: −6 to 82) fewer symptomatic VTE plus all-cause mortality events and six (95% CI: −15 to 26) more nonfatal major bleeding events (). These data correspond to NNT =262 and NNH =1,711; for every 262 patients receiving rivaroxaban versus enoxaparin, one fewer symptomatic VTE or death would be expected to occur. In contrast, 1,711 patients would need to be treated to see one more nonfatal major bleeding event. Assuming these efficacy and safety events have equal clinical impact, summing the rate differences gives a net clinical benefit of 32 fewer symptomatic VTE, all-cause mortality, or nonfatal major bleeding events with rivaroxaban compared with enoxaparin per 10,000 patients. If this analysis were repeated using nonfatal major plus surgical-site bleeding instead of nonfatal major bleeding (a valuable sensitivity analysis, because the RECORD studies did not include surgical-site bleeding under their definition of major bleeding), the net clinical benefit would be nine (95% CI: −67 to 85) fewer events associated with rivaroxaban, and an NNH of 345, or 1.3 efficacy events prevented per harm event caused. Of the rate difference for symptomatic VTE plus all-cause mortality, 20%–25% was attributed to reductions in death, and most of the rate difference for nonfatal major bleeding resulted from major bleeding events leading to reoperation (). Major plus nonmajor clinically relevant bleeding favored enoxaparin (primarily owing to nonmajor clinically relevant bleeding). The combination of nonfatal major bleeding and surgical-site bleeding also favored enoxaparin. Comparison with nonfatal major bleeding demonstrated that most of these events were surgical-site nonmajor bleeding events. Surgical wound infections and those leading to rehospitalization or prolongation of hospitalization, which includes many of the bleeding events listed, favored enoxaparin. The end point of any serious AE, which included all efficacy and safety events in the RECORD studies, favored rivaroxaban.
Figure 2 Temporal course of net clinical benefit is shown for VTE prophylaxis after total hip arthroplasty.
Abbreviations: RECORD, REgulation of Coagulation in ORthopaedic surgery to prevent Deep vein thrombosis and pulmonary embolism; VTE, venous thromboembolism.
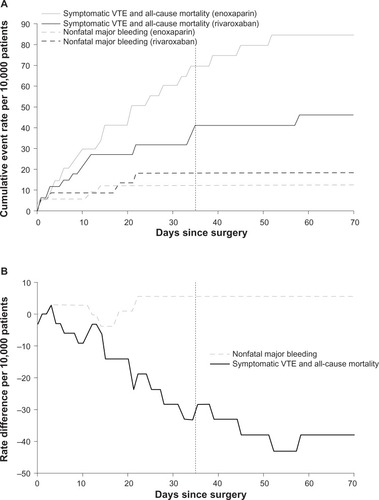
Table 3 Kaplan–Meier event rates, rate difference, number needed to treat/number needed to harm, and net clinical benefit for total hip arthroplasty (RECORD1 and RECORD2) and total knee arthroplasty (RECORD3 and RECORD4) patients
Table 4 Kaplan–Meier event rates and rate difference per 10,000 patients after total hip arthroplasty and total knee arthroplasty over treatment and follow-up period
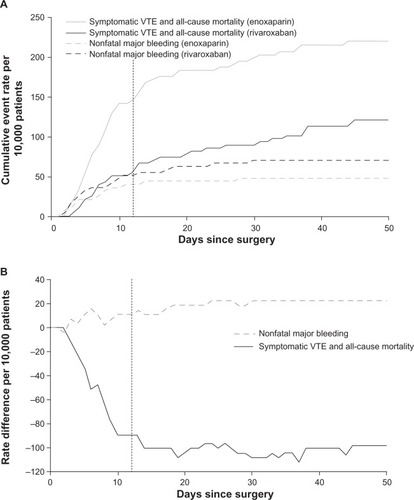
After TKA, rivaroxaban prevented more harmful efficacy events than enoxaparin but was associated with more safety events (). The number of efficacy events that were prevented was greater than the number of harm events caused. This began early in treatment and continued throughout treatment and follow-up, with rates plateauing at four to five efficacy events prevented per harm caused (). In 10,000 patients, compared with enoxaparin, rivaroxaban was associated with 98 (95% CI: 27 to 169) fewer symptomatic VTE plus all-cause mortality events and 23 (95% CI: −19 to 64) more nonfatal major bleeding events. These data correspond to NNT =102 and NNH =442. Net clinical benefit was 76 fewer symptomatic VTE, all-cause mortality, or nonfatal major bleeding events for rivaroxaban versus enoxaparin per 10,000 patients. Sensitivity analysis using nonfatal major plus surgical-site bleeding instead of nonfatal major bleeding yielded a net clinical benefit of 50 (95% CI: −48 to 148) fewer events associated with rivaroxaban, and an NNH of 208, or two efficacy events prevented per harm event caused. The 98 fewer efficacy events per 10,000 patients associated with rivaroxaban treatment exceeded the increase in risk for any bleeding event assessed (). Approximately 20% of these efficacy events were attributable to death. Most of the rate difference for nonfatal major bleeding was attributable to bleeding events leading to reoperation. Surgical wound infections and those leading to rehospitalization or prolongation of hospitalization favored rivaroxaban, as did all serious AEs.
Figure 3 Temporal course of net clinical benefit is shown for VTE prophylaxis after total knee arthroplasty.
Abbreviations: RECORD, REgulation of Coagulation in ORthopaedic surgery to prevent Deep vein thrombosis and pulmonary embolism; VTE, venous thromboembolism.
Discussion
Clinicians make benefit–risk decisions regarding thromboprophylaxis after THA and TKA by weighing the benefit of a reduction in venous thromboembolic events against the increased risk of bleeding. Previous benefit–risk analyses of the RECORD studies generally used pooled THA and TKA results, counted fatal bleeding as both an efficacy and a safety event, and included the placebo-controlled portion of RECORD2.Citation28,Citation31,Citation33,Citation34 We conducted a post hoc analysis without these constraints to quantitatively assess benefit–risk in the RECORD studies.
In the THA RECORD studies, the reduction in symptomatic VTE plus all-cause mortality exceeded the increase in nonfatal (and essentially non-stroke)Citation33 major bleeding. The net benefit was observed early and maintained throughout treatment and follow-up. Net benefit was also obtained when balancing the efficacy end point against nonfatal major plus surgical-site bleeding, of particular importance for postoperative management. The analytic approach was designed specifically for benefit–risk assessment and avoids the constraints in prior benefit–risk analyses for RECORD listed above. In particular, censoring the placebo-controlled portions of RECORD2 avoids potentially overestimating the treatment benefit of rivaroxaban.
In the TKA studies of the RECORD program, the reduction in symptomatic VTE plus all-cause mortality also exceeded the increase in nonfatal (non-stroke) major bleeding. As with the THA studies, the net benefit was observed early, was maintained throughout treatment and follow-up, and was also obtained when balancing the efficacy end point against nonfatal major plus surgical-site bleeding. The absolute risk difference of symptomatic VTE plus all-cause mortality and that for nonfatal major bleeding were larger for TKA compared with those for THA patients (). Similar findings were obtained in the Global Orthopaedic Registry, which showed higher rates of DVT and bleeding complications after TKA.Citation35 Despite these differences, the relative magnitude of benefit of rivaroxaban, in terms of events prevented per harmful event caused, was similar across the indications: in THA patients, approximately six symptomatic VTE plus all-cause mortality events were prevented per nonfatal major bleeding event caused; in TKA patients, the ratio was approximately four to five events prevented per nonfatal major bleeding event caused.
The sensitivity analysis in which nonfatal major plus surgical-site bleeding was used instead of nonfatal major bleeding showed less net clinical benefit for rivaroxaban compared with enoxaparin for TKA and, essentially, a benefit–risk balance between rivaroxaban and enoxaparin for THA. These results are similar to those reported by Gómez-Outes et al in 2012,Citation32 though our analysis defines net clinical benefit as the sum of rate differences for symptomatic VTE plus all-cause mortality and the rate difference for nonfatal major bleeding, whereas the analysis by Gómez-Outes et al defined net clinical benefit as the composite end point of symptomatic VTE, all-cause death, and major bleeding. The results for THA may be due in part to the 2 weeks’ data used in RECORD2 due to censoring the placebo-controlled portion of that study.
A few limitations of the present analysis are noteworthy. First, using Kaplan–Meier rates in these post hoc analyses required choosing a time point for reporting rates. Theoretically, selection of the time point could show treatment in an especially favorable or unfavorable light; however, the plateaus for rate difference in and show that the choice of dates did not influence the results. Second, the RECORD studies were not designed to track long-term consequences of wound infection (beyond 70 days for THA and 47 days for TKA) nor whether such infections led to reoperation.Citation36,Citation37 Hence, the data do not support measurements of the rates of reoperation or implant replacement owing to wound infection or similar measures. Related end points, the between-treatment differences for surgical wound infections overall and those leading to rehospitalization or prolongation of hospitalization, were small but numerically favored enoxaparin for THA (decrease of 0.22% and 0.24%, respectively) and numerically favored rivaroxaban for TKA (decrease of 0.22% and 0.15%, respectively). The 95% CIs for the hip and knee rate differences of these end points overlap considerably; these end points were not centrally adjudicated; and no biologic mechanism that would account for this difference by site (hip versus knee) was apparent. Although a recent analysis supports an assessment of no treatment difference for various complications of surgery after THR or TKR,Citation34 additional studies would clearly be helpful for this topic. A third limitation is that the analysis did not account for patients potentially halting thromboprophylaxis owing to the impact of bleeding, an important issue but one that is outside the scope of the trial data. Finally, the analyses gave the same weight to nonfatal major bleeding as to symptomatic VTE plus all-cause mortality. However, as described, investigator reports from the RECORD trials suggested that symptomatic venous thromboembolic events in RECORD were, on average, of greater clinical impact than the major bleeding events observed, and hence also of greater clinical impact than nonfatal major bleeding.Citation33 The relative clinical impact of bleeding and VTE is a complex topic with multiple perspectives.Citation29,Citation33,Citation38,Citation39 Even if nonfatal major bleeding was regarded as a more severe event on average than symptomatic VTE plus all-cause mortality, a typical nonfatal, non-stroke major bleed would have to be regarded as more than four to five times as severe as symptomatic VTE plus all-cause mortality for the benefit–risk assessment to switch to favoring enoxaparin.
An additional “limitation” of this work is more a consequence of the heterogeneity of the end points used in thromboprophylaxis studies. Symptomatic VTE ranges from DVTs that cause discomfort and swelling to fatal PEs. Major bleeding ranges from transient drops in hemoglobin that require only supportive care through hemorrhagic stroke, fatal bleeding, and intracranial hemorrhage. Considering such a wide range of events as having a similar clinical impact is clearly a rough approximation at best; however, decomposing these end points into separate measurements with a narrower range of impact can result in very low event rates with high uncertainty. An alternative approach is to use a set of weights for each end point that reflect their rough clinical impact, then multiply the rate differences by these weights in the net clinical benefit calculation. The weights would then be varied over a realistic range to provide a sensitivity analysis to the results, an approach used by Singer et al in 2009.Citation29 In our analysis, we assumed equal weighting for symptomatic VTE plus all-cause mortality and for nonfatal major bleeding. Given that the measurements in all suggest that the clinical impact of nonfatal major bleeding is less than that of symptomatic VTE plus all-cause mortality, we regard the current analysis as on the conservative side that would favor enoxaparin, though this is clearly a topic for further research.
The results in this work align with those from prior benefit–risk assessments for the RECORD studiesCitation28,Citation32–Citation34 and address some of the topics raised by other authors;Citation31 however, because these prior assessments pooled all four of the RECORD studies, whereas the current work censored the placebo comparator portion of RECORD2, some 95% CIs for risk differences here are larger than in prior reports. Of interest, in this and the prior analyses, clinicians generally prefer NNT values considerably smaller than the 100–300 observed here; however, these numbers reflect the comparison of a study drug with a highly effective comparator, that is, in the setting of low baseline rates.Citation28,Citation32–Citation34 Thus, the NNT should be interpreted in the context of clinical practice where the alternative is not placebo but other effective treatments. We note, however, that the NNH (to cause an additional bleeding event) is four- to six-fold larger than the NNT.Citation9,Citation15
Clinical practice decisions depend on many factors beyond formal benefit–risk assessments, such as the oral once-daily formulation of rivaroxaban, the absence of need for regular international normalized ratio monitoring with rivaroxaban, and the lack of availability of any antidote for rivaroxaban. This analysis suggests that rivaroxaban is a viable option compared with enoxaparin for thromboprophylaxis after THA or TKA.
Acknowledgments
We thank Li Wan, a medical writer, who provided editorial support during preparation of this manuscript. Funding for editorial support was provided by Bayer HealthCare Pharmaceuticals and Janssen Scientific Affairs, LLC.
Disclosure
BL, ZY, JAB, RBW, and PMD are employees of Janssen Research & Development, LLC; BL and RBW are invested in a portfolio that periodically includes health care and biotechnology companies; MH is an employee of Bayer HealthCare; and SDB is an employee of Bayer HealthCare Pharmaceuticals. AGGT has received consultancy fees from Bayer HealthCare, Janssen Pharmaceutical Research & Development, Astellas, Portola, and Takeda. RJF has received consultancy fees from Janssen, J&J, Exactech, DJO Surgical, and Cadence. The authors report no other conflicts of interest in this work.
References
- Preventing Venous Thromboembolic Disease in Patients Undergoing Elective Hip and Knee Arthroplasty: Evidence-Based Guideline and Evidence ReportRosemont, ILAmerican Academy of Orthopaedic Surgeons2011 Available from: http://www.aaos.org/research/guidelines/VTE/VTE_full_guideline.pdfAccessed October 1, 2013
- KearonCNatural history of venous thromboembolismCirculation200310723 Suppl 1I22I3012814982
- KahnSRHirschAShrierIEffect of postthrombotic syndrome on health-related quality of life after deep venous thrombosisArch Intern Med2002162101144114812020185
- KahnSRGinsbergJSRelationship between deep venous thrombosis and the postthrombotic syndromeArch Intern Med20041641172614718318
- LangIKerrKRisk factors for chronic thromboembolic pulmonary hypertensionProc Am Thorac Soc20063756857016963535
- PengoVLensingAWAPrinsMHThromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolismN Engl J Med2004350222257226415163775
- EikelboomJWKarthikeyanGFagelNHirshJAmerican Association of Orthopedic Surgeons and American College of Chest Physicians guidelines for venous thromboembolism prevention in hip and knee arthroplasty differ: what are the implications for clinicians and patients?Chest2009135251352019201714
- Falck-YtterYFrancisCWJohansonNAAmerican College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice GuidelinesChest2012141Suppl 2e278Se325S22315265
- WarwickDFriedmanRJAgnelliGInsufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events: findings from the Global Orthopaedic RegistryJ Bone Joint Surg Br200789679980717613508
- AgenoWGallusASWittkowskyACrowtherMHylekEMPalaretiGAmerican College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis. 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice GuidelinesChest2012141Suppl 2e44Se88S22315269
- ErikssonBIBorrisLCFriedmanRJRECORD1 Study GroupRivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplastyN Engl J Med2008358262765277518579811
- KakkarAKBrennerBDahlOERECORD2 InvestigatorsExtended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trialLancet20083729632313918582928
- LassenMRAgenoWBorrisLCRECORD3 InvestigatorsRivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplastyN Engl J Med2008358262776278618579812
- TurpieAGGLassenMRDavidsonBLRECORD4 InvestigatorsRivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trialLancet200937396761673168019411100
- TurpieAGGLassenMRErikssonBIRivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studiesThromb Haemost2011105344445321136019
- CoplanPMNoelRALevitanBSFergusonJMussenFDevelopment of a framework for enhancing the transparency, reproducibility and communication of the benefit-risk balance of medicinesClin Pharmacol Ther201189231231521160469
- Benefit-risk methodology [webpage on the Internet]LondonEuropean Medicines Agency2011 Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/document_listing/document_listing_000314.jsp&mid=WC0b01ac0580223ed6&murl=menus/special_topics/special_topics.jsp&jsenabled=trueAccessed July 16, 2013
- LevitanBSAndrewsEBGilsenanAApplication of the BRAT framework to case studies: observations and insightsClin Pharmacol Ther201189221722421178990
- Structured Approach to Benefit-Risk Assessment in drug Regulatory Decision-MakingUS Food and Drug Administration2013 Available from: http://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM329758.pdfAccessed September 2, 2013
- Benefit-risk integration and representation [webpage on the Internet]PROTECT Investigators Innovative Medicines Initiative Available from: http://www.imi-protect.eu/benefitPres.shtmlAccessed July 16, 2013
- WalkerSLibertiLMcAuslaneNLevitanBSRefining the benefit-risk framework for the assessment of medicines: valuing and weighting benefit and risk parametersClin Pharmacol Ther201189217918221252937
- BauerKATurpieAGGLassenMRKakkarAKErikssonBIGentMRECORD 1–4 InvestigatorsEffects of age, weight, gender and renal function in a pooled analysis of four rivaroxaban studiesJ Thromb Haemost20097S2767 PP-WE-421
- ErikssonBIKakkarAKTurpieAGGOral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacementJ Bone Joint Surg Br200991563664419407299
- BeasleyBNUngerEFTempleRAnticoagulant options – why the FDA approved a higher but not a lower dose of dabigatranN Engl J Med2011364191788179021488759
- GibsonCMLevitanBMurphySAA net clinical outcome analysis comparing fatal or irreversible ischemic and bleeding events in ATLAS ACS 2 – TIMI 51Circulation2012126A13152
- HullRDYusenRDBergqvistDState-of-the-art review: assessing the safety profiles of new anticoagulants for major orthopedic surgery thromboprophylaxisClin Appl Thromb Hemost200915437738819608549
- KaulSDiamondGADoes rivaroxaban provide a clinically relevant favorable benefit-risk profile compared with enoxaparin after hip or knee arthroplasty?J Am Coll Cardiol20105510E1616E1618A172
- LevitanBYuanZTurpieAGGQuantitative benefit–risk assessment of rivaroxaban for the prevention of venous thromboembolismBlood (ASH Annual Meeting Abstracts)2009114227576 A169
- SingerDEChangYFangMCThe net clinical benefit of warfarin anticoagulation in atrial fibrillationAnn Intern Med2009151529730519721017
- UngerEFWeighing benefits and risks – the FDA’s review of prasugrelN Engl J Med20093611094294519726770
- Van ThielDKalodikiEWahiRLitinasEHaqueWRaoGInterpretation of benefit-risk of enoxaparin as comparator in the RECORD program: rivaroxaban oral tablets (10 milligrams) for use in prophylaxis in deep vein thrombosis and pulmonary embolism in patients undergoing hip or knee replacement surgeryClin Appl Thromb Hemost200915438939419608550
- Gómez-OutesATerleira-FernándezAISuárez-GeaMLVargas-CastrillónEDabigatran, rivaroxaban, or apixaban versus enoxaparin for thromboprophylaxis after total hip or knee replacement: systematic review, meta-analysis, and indirect treatment comparisonsBMJ2012344e367522700784
- Advisory Committee Briefing Book: Rivaroxaban for the Prophylaxis of Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE) in Patients Undergoing Hip or Knee Replacement SurgeryJohnson & Johnson Pharmaceutical Research and Development, LLC2009 Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM138385.pdfAccessed September 2, 2013
- LassenMRGentMKakkarAKThe effects of rivaroxaban on the complications of surgery after total hip or knee replacement: results from the RECORD programmeJ Bone Joint Surg Br201294111573157823109641
- CushnerFAgnelliGFitzGeraldGWarwickDComplications and functional outcomes after total hip arthroplasty and total knee arthroplasty: results from the Global Orthopaedic Registry (GLORY)Am J Orthop (Belle Mead NJ)201039Suppl 9222821290028
- JamesonSSRymaszewskaMJamesPWound complications following rivaroxaban administration: a multicenter comparison with low-molecular-weight heparins for thromboprophylaxis in lower limb arthroplastyJ Bone Joint Surg Am201294171554155822832942
- JensenCDStevalAPartingtonPFReedMRMullerSDReturn to theatre following total hip and knee replacement, before and after the introduction of rivaroxaban: a retrospective cohort studyJ Bone Joint Surg Br2011931919521196550
- ChanCMZilberbergMDPreferences in traumatic intracranial hemorrhage: bleeding vs. clottingCrit Care201014315320497598
- LipGYHPiotrponikowskiPAndreottiFThromboembolism and antithrombotic therapy for heart failure in sinus rhythm. An Executive Summary of a joint Consensus Document from the ESC Heart Failure Association and the ESC Working Group on ThrombosisThromb Haemost201210861009102223093044
