Abstract
Identifying the mechanisms that convert a healthy vascular wall to an atherosclerotic wall is of major importance since the consequences may lead to a shortened lifespan. Classical risk factors (age, smoking, obesity, diabetes mellitus, hypertension, and dyslipidemia) may result in the progression of atherosclerotic lesions by processes including inflammation and lipid accumulation. Thus, the evaluation of blood lipids and the full lipid complement produced by cells, organisms, or tissues (lipidomics) is an issue of importance. In this review, we shall describe the recent progress in vascular health research using lipidomic advances. We will begin with an overview of vascular wall biology and lipids, followed by a short analysis of lipidomics. Finally, we shall focus on the clinical implications of lipidomics and studies that have examined lipidomic approaches and vascular health.
Introduction
Identifying the mechanisms that modify a healthy vascular wall to an atherosclerotic wall is of major importance, since the consequences (myocardial infarction, stroke, and others) may threaten human life.Citation1–Citation4 Nowadays, it is well known that during atherogenesis, low-density lipoprotein (LDL) particles enter the arterial wall, either oxidized (ox) or enzymatically degraded, and aggregate. Afterwards, modified LDL particles follow the atherogenic pathway that leads to atherosclerotic plaque formation, instead of the pathway followed by native LDL particles, which supplies cholesterol to peripheral cells. Within the subendothelial space, the modified LDL particles are engaged by scavenger receptors located on macrophages and smooth muscle cells. Then, free cholesterol (FC) and cholesterol ester (CE) from the LDL particles accumulate within the lysosomes,Citation5 where lysosomal acid lipase hydrolyzes CE to FC. FC, after leaving the lysosome, can be re-esterified and located in lipid droplets. In case of the increased accumulation of FC in the lysosome, the inhibition of lysosomal acid lipase activity is observed, leading to the progression of atherosclerosis. Native LDL particles are composed of approximately 3,000 lipid molecules. Lipids can be classified as hydrophobic or amphipathic molecules with carbanion- (fatty acids, polyketides, and others) or carbocation-based (prenols, sterols, and others) units.Citation6 Lipids can also be divided into simple lipids (fatty acids, sterols, and others) or complex lipids (glycerophospholipids and glycosphingolipids), and they can be organized into categories that cover eukaryotic and prokaryotic sources.
Concerning lipidomics, the word entered PubMed in the year 2003.Citation7 Since then, researchers have established a definition for lipidomics, which is derived from several fields with broad deviations. Generally, lipidomics is considered a part of metabolomics (genomics, transcriptomics, proteomics) and is described as the quantitative characterization of the full lipid complement produced by cells, organisms, or tissues.Citation8,Citation9 The term “metabolomics” was first used by Fiehn,Citation10 and was further developed by the Metabolomics Society http://www.metabolomicssociety.org/. Metabolomics is moving metabolic research, which is based on examining single pathways, to focus on complex metabolic networks.Citation11 Metabolomics are closest to the phenotype of the subject (metabolites are the end product of the -omics pathway: genomics; transcriptomics; proteomics; and metabolomics) and allow they us to expand upon disease-causing mechanisms and link them with other -omics. Thus, the term “lipidomics” (the quantitative and molecular determination of lipid molecules) can be referred to as a research field that studies entire cellular lipidomes on a large scale.Citation7 Cellular lipidomes, which were first reported in a journal in 2001,Citation12 represent the the cell. Thanks to recent advances in mass spectrometry (MS) technology, the evaluation of hundred of lipids that make up the lipidome in a single biological specimen is possible.
In this review, we will describe the recent progress in vascular health research using lipidomic advances. We shall begin with an overview of vascular wall biology and lipids, followed by a short analysis of lipidomics and the use of technology to evaluate lipidomics. Finally, we will focus on the clinical implications of lipidomics and studies that have examined lipidomic approaches and vascular health.
Lipids and vascular wall biology
The close relationship between plasma lipids (particularly CEs) and the vascular wall is well documented.Citation13 Inside the vascular wall, CE accumulates in a shape of droplets in the cytosol or in lysosomes (as mentioned earlier).Citation14 Normally, the infiltrating LDL particles, which cross the endothelium, contain a CE-rich core and polyunsaturated linoleic acid.Citation15 The LDL particles enriched with monounsaturated cholesteryl oleate are usually bigger than the LDL particles enriched with polyunsaturated cholesteryl linoleate, and they bind to arterial proteoglycans more vigorously with the consequence of being trapped and forced to follow the atherosclerotic pathway leading to plaque formation.Citation16
Of note is that lipids are able to bind to Toll-like receptors (TLRs) and can initiate intracellular signaling. The TLRs were found to have proatherogenicCitation17 or protective actions.Citation18 Seimon et alCitation19 observed that ox phospholipids, oxLDL particles, and saturated fatty acids activate apoptosis in endoplasmic reticulum-stressed macrophages through a mechanism involving TLRs. Also, lipoprotein (a), a risk factor for cardiovascular disease that has been shown to be genetically linked to myocardial infarction,Citation20 is activated in TLRs.
On the other hand, the removal of lipids from plaques into circulation can be performed by high-density lipoprotein (HDL) particles.Citation21–Citation24 Thus, studying the HDL lipid classes may give more information about the current status of atherosclerotic plaques (as will be discussed).
Lipids
Lipids are involved in many biological mechanisms by acting as membrane barriers; they are an energy source and are involved in signaling events, trafficking, and the sorting of macromolecules, which are the most important functions.Citation25 The methods for lipid evaluation are usually focused on the assessment of total LDL and HDL cholesterol, as well as triglycerides. Although, these measurements still remain a fundamental part of everyday practice, lipidomic evaluation brought about the quantification of additional lipid molecular elements across varying classes (acylglycerols, sterols, sphingolipids, and others).Citation7,Citation8 Moreover, lipidomic evaluation permits a single, untargeted quantitative and qualitative snapshot of lipid concentrations within the whole cell, tissue, or body fluid being examined. Thus, whole-plasma lipidomics take a more global view of lipid metabolism and can provide a detailed picture of the abnormalities in lipid metabolism, which is in contrast to the studies of isolated lipoproteins. For example, Weir et alCitation26 evaluated the lipid species (number [n] =312) in obese subjects and found a correlation with sphingomyelin (SM), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol, CEs, and triglycerides with obesity (1,076 participants of the San Antonio Family Heart Study [SAFHS]). Stübiger et alCitation27 evaluated young subjects with familial hypercholesterolemia or familial combined hyperlipidemia and observed differences of SM/PC and PC/lysoPC ratios and positive correlations between SM versus LDL cholesterol and lysoPC versus very (V)LDL cholesterol between subjects with familial hypercholesterolemia and other study groups.
Plaque composition
Arterial wall dysfunction is caused by atherosclerosis, which consists of a slow lifetime variety of histological changes leading to plaque formation.Citation4,Citation28 The functional anatomy of plaques is dependent on the mechanisms by which lipids may provoke plaque instability and rupture. The intimal thickening is the first and clinically detectable manifestation of atherosclerosis in humans. The lesions present with foam and smooth muscle cells, and as they advance, with lipid pools located in the deeper intima in areas rich in proteoglycans, as well as with calcification.Citation4 The broadest classification of atherosclerotic plaques includes stable and unstable plaques. Unstable plaques are characterized by high lipid load and, particularly, by FC and CEs,Citation29 while stable plaques are characterized by phospholipids and triglyceride content.Citation30 Stübiger et al,Citation27 using the matrix-assisted laser desorption/ionization quadrupole ion trap time-of-flight (MALDI-QIT-TOF)-MS/MS method, reported a positive correlation between oxPC levels and intima media thickness.
The major challenge in the evaluation of atherogenesis is the assessment of new risk factors when detecting the early formation of atherogenesis at a clinical level, in addition to the classic ones such as diet, exercise, LDL and VLDL cholesterol, and others. This is because the morphological tests (organ perfusion measurements or angiography) used for the diagnosis and classification of atherosclerosis are possible only in late-stage disease (they detect only established plaques) and they are not easily undertaken in large population studies. Recent advances in lipidomics make it possible to identify and quantify species from the plaque lipidome, and they have already revealed, that normal vessels in comparison with atherosclerosis-affected locations, have different lipid composition and particularly lysophospholipids and CEs. In the particular case of macrophages, higher levels of FC are related to foam cell formation.Citation31 Furthermore, an increased FC/CE ratio has been described in unstable atheromatous plaques in humans.Citation32 Jové et alCitation33 found that a high-fat diet provoked more changes in the aortic wall than in the plasma lipidome, and they suggested that vessels exhibit a “high-fat molecular memory”. Ox and enzymatically-modified (e)LDL particles play a crucial role in the early stages of atherogenesis. Their uptake by recruited macrophages leads to endolysosomal phospholipidosis (oxLDL) or foam cell formation (eLDL) (). Hinterwirth et alCitation34 used the antioxLDL antibodies conjugated to gold nanoparticles for the extraction and enrichment of oxPCs via the selective trapping of oxLDLs from plasma, combined with their detection by liquid chromatography (LC)-MS/MS, and found that both techniques can offer new possibilities for targeted lipidomics in lipoproteins, as well as for oxidative stress lipid biomarker screening.
Figure 1 Atherosclerotic changes of various cells, as detected by NMRS or mass spectrometry/liquid chromatography methods.
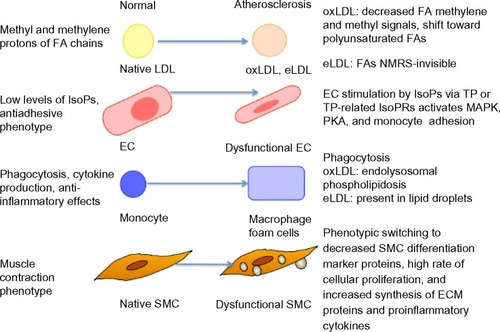
Ramm Sander et alCitation35 reported structural changes in LDL particles as a consequence of atherogenic modifications, as detected by nuclear magnetic resonance spectroscopy (NMRS). Also, monitoring of the differential uptake of these LDL particles by macrophages could be followed. Particularly, eLDL-induced cytosolic lipid droplet formation could be detected. Moreover, eLDL-induced mobile lipids exhibited a greater proportion of polyunsaturated fatty acid chains (which correlated to apoptosis in vivo) when compared with lipids that were already present in macrophages prior to loading. This finding underlines the benefit of NMRS as a lipidomics tool to specifically monitor the mobile lipid pool, which is clearly different from the total lipid pool. Furthermore, the evaluation of isoprostanes by LC/MS methods offers the possibility to study the extent of oxidative stress in humans in various physiological and pathophysiological situations, such as atherosclerosis ().Citation36 Stegemann et alCitation37 analyzed lipids in tissue sections and extracts from human endarterectomy specimens by shotgun lipidomics (in stable and unstable regions) from the same individual. Carotid plaque samples were enriched in CEs, PCs, lysoPCs, and SM species, as compared to radial arteries (control).
With respect to HDL particles, Camont et alCitation38 revealed heterogeneity in the phosphosphingolipidome across human plasma HDL subpopulations using the LC-MS/MS approach, which paralleled the heterogeneity in key atheroprotective HDL functions. Furthermore, they observed that the HDL phosphosphingolipidome components were correlated with the cholesterol efflux capability of HDL from macrophages, antioxidative activity toward LDL, antithrombotic activity toward platelets, and antiapoptotic activity in endothelial cells. Thus, there is space for improvement of the classic lipid markers that are used in the estimation of future risk for major cardiovascular events caused by stable/unstable vascular disease.
Lipidomics
Lipidomics is still a relatively young discipline, which is rapidly developing and is considered to be a part of metabolomics.Citation8 Lipidomics involves detailed documentation of particular cellular lipid species, including the type and number of atoms in each lipid species, as well as their stereo-electronic interactions with neighboring lipids and proteins. A number of methods have been introduced for the evaluation of the lipidome, such as “shotgun lipidomics” (which explores chemical and physical properties of each lipid class and involves direct infusion of the sample),Citation39,Citation40 targeted lipidomics (which obtains one or more of the lipid classes or subclasses, or molecular species of interest),Citation41,Citation42 and untargeted lipidomics (that identifies unknown lipid species).Citation43
It was observed that every lipoprotein class was associated with a particular arrangement of lipids. For example, LDL particles are enriched with ceramide and SM, while HDL particles are enriched with PC, PE, and PE-based plasmalogens. The International Lipid Classification and Nomenclature Committee (ILCNC), the LIPID Metabolites and Pathways Strategy (LIPID MAPS Consortium), and the European Lipidomics Initiative (ELIfe) have classified the lipidomic nomenclature.Citation44 This classification separates fatty acyls from other polyketides, glycerophospholipids from other glycerolipids, and sterol lipids from other prenols.
Current studies in lipidomics are focused on four main areas such as: 1) structural characterization of known and novel lipid species; 2) the development of methods for lipidomics analysis; 3) the evaluation of metabolic factors in a healthy and diseased settings; and 4) therapeutic response and side effects of drugs, which are analyzed in the “Lipidomics in clinical use” section.
Structural characterization of known and novel lipid species
In 1976, the International Union of Pure and Applied Chemistry and the International Union of Biochemistry and Molecular Biology (IUPAC-IUBMB) Commission on Biochemical NomenclatureCitation45 published the lipid nomenclature. Since then, a number of novel lipid classes have been discovered. The LIPID MAPS Consortium established a “Comprehensive Classification System for Lipids” based on well-defined chemical and biochemical principles, together with ontology, and classified lipids into eight categories (fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, sterols, prenol lipids, saccharolipids, and polyketides).Citation6
Lipidomics – technology evaluation
MS techniques and NMRS technology are the main techniques used for metabolomics evaluation (). MS techniques are largely used in the characterization of numerous lipid structures and their structure-specific functions.Citation46 The use of MS techniques began in the 20th century and was indicated in organic, nuclear, geographical, and atomic chemistry, and only later was it applied to biological and medical research. Thus, the MS technique may examine certain areas of research, such as lipidomics, proteomics, metabolomics, gas-phase ion chemistry, proscribed chemicals, pharmacokinetics, protein/peptide chemistry, and others. The use of NMRS technology began circa 1970. NMRS technology, by measuring the magnetic spin of nuclei (1H, 13C, and 31P) contained in the study’s metabolites, can identify structural and quantitative information of metabolites.Citation11 Today, with NMRS technology, it is possible to identify up to 300 metabolites,Citation46 and this is based on two molecular windows: lipoprotein lipid and low-molecular-mass/weight metabolite.Citation47
Table 1 Various technologies and techniques at the forefront of lipidomics evaluation
Fast protein LC technology has been introduced to distinct human serum lipoproteins. Electrospray ionization (ESI) techniques (soft ionization technology used to form either positive or negative ions without derivatization and decomposition) are performed to identify the major species of ceramide, SM, PC, lysoPC, PE, and its derivatives, such as FC and CEs.Citation48 LC tandem MS technology is a method that is used for the analysis of mono- and polyhydroxylated fatty acids.Citation49 The analysis of mono- and polyhydroxylated fatty acids is helpful in establishing the role of lipid mediators in certain biological and/or pathological conditions. Moreover, other technical approaches for the identification of other lipid species are still being developed. For example, Blanksby and MitchellCitation50 have used ozone-induced dissociation to recognize the positions of double bonds in unsaturated fatty acids.
MS technology is still a developing method for evaluating lipidomics; however, one of the essential concerns of this method is the standardized data analysis.Citation51 Lately, this has been improved.Citation52 Almeida et alCitation53 have reported a novel shotgun lipidomics platform containing an Orbitrap Fusion MS equipped with an automated nanoelectrospray ion source. This analysis verified varied lipidome quantification covering more than 300 lipid species, 20 lipid classes, and more than 200 molecular glycerophospholipid species. Also, Stübiger et alCitation27 introduced the MALDI-QIT-TOF-MS/MS and LC-ESI-SRM approaches to investigate atherogenic dyslipidemia in young patients with familial hyperlipidemia.
Evaluation of metabolic factors in a healthy and diseased setting
Lipid homeostasis is essential in health protection and its evaluation is fundamental. Lipidomics in a healthy setting is used to describe the specific lipids involved in dynamic physiological lipid changes, providing further insight into lipid control in cellular biology, such as the structural integrity of cellular membranes, signal transduction, and the regulation of membrane trafficking. On the other hand, any abnormalities in lipid metabolism play a significant role in many diseases, particularly in metabolic syndrome, diabetes mellitus, lipodystrophies, neurological disorders (Alzheimer’s disease, Parkinson’s disease, Niemann–Pick disease, multiple sclerosis, and others), central nervous system injury (stroke, traumatic brain injury, and spinal cord injury), infections, and others.
Thus, lipidomic investigation leading to the discovery of new biomarkers is crucial for preventing these diseases in their very early stages. For example, impaired mitochondrial function – particularly, fatty acyl compositions – were proposed as the potential cause of insulin resistance and/or diabetes progression.Citation65 Also, by using lipidomic methodologies, the cell lipid composition of some infective pathogens is being progressively determined.Citation66 A recent shotgun lipidomics study examined the effect of sulfatide supplementation on neuroblastoma cells. Lipidomic methodologies were also used to map lipid disorders in human muscles of a patient suffering from mulscular dystrophy.Citation67
Lipidomics in clinical use
One of the main clinical uses of lipidomics is the monitoring of therapeutic responses and the potential side effects of existing drugs, as well as the evaluation of newly developed ones. In terms of lipid-lowering (LL) drugs such as statins (3-hydroxy-3-methylglutaryl-coenzyme A inhibitors), which are first-line LL drugs for the treatment of hypercholesterolemia, the evaluation of their efficacy was based on blood lipid concentration measurements.Citation68 Lately, with the introduction of lipidomics, the LL effectiveness and side effects of statins were also extended to lipidomic evaluation. Laaksonen et alCitation69 evaluated 132 plasma lipidomic analyses of subjects treated with simvastatin, atorvastatin, or placebo, and they found that the plasma lipidomic changes in the simvastatin group were associated with the muscle expression of the arachidonate 5-lipoxygenase-activating protein. They concluded that the plasma lipidomic profile might serve as a highly sensitive biomarker of statin-induced metabolic changes in muscle, and it might help clinicians to distinguish between subjects who should be treated with a lower statin dose to prevent possible toxicity. In the plasma lipidomics analysis of men with combined lipidemia, Chen et alCitation70 found that the simvastatin treatment group exhibited a significant reduction in nine out of 33 species. Thus, significant reductions were observed in FC, CEs, triglycerides, PEs, and lysoPCs (which were the primary constituents in CEs), as well as in triglycerides in intermediate-density lipoprotein/VLDL and LDL particles.Citation45 Kaddurah-Daouk et al,Citation71 using a targeted lipidomics platform, reported lipid modifications in blood samples of upper vs lower subgroup (defined by LDL cholesterol response to simvastatin LL effect). Particularly, baseline CEs and phospholipid metabolites were correlated with the LDL cholesterol response to LL treatment. C-reactive protein (CRP) response to therapy correlated with baseline plasmalogens, which are lipids involved in inflammation. Another group of LL drugs includes fibrates. Fibrates act as peroxisome proliferator-activated receptor α agonists. Yetukuri et alCitation72 reported that fenofibrate induced HDL compositional changes that included increased apolipoprotein A-II and SM, as well as reduced lysoPC. The ethanolamine plasmalogens were reduced only in the subgroup of patients with elevated homocysteine levels.
Also, considerable progress was made according to newly developed drugs and lipidomics. Aerts et alCitation73 developed an inhibitor of glucosylceramide synthase (an enzyme that catalyzes the conversion of ceramide to glycosphingolipids), which was administered to high-fat-fed or ob/ob mice and Zucker diabetic fatty rats. The authors found lowered circulating glucose levels, improved oral glucose tolerance, improved insulin sensitivity in the muscle and liver, and a reduction of glycosphingolipid concentration in various tissues. Holland et alCitation74 also found that the inhibition of ceramide synthesis improved glucose tolerance and prevented the onset of diabetes in obese rodents. Moreover, Zhao et alCitation75 and Bijl et alCitation76 found similar results in Zucker diabetic fatty rats and ob/ob rats, respectively. These findings indicate that modifications in sphingolipids can serve as theoretically applicable modulators of insulin action, and they show the new treatment approaches that have arisen due to lipidomics analysis.
Lipidomics and the vascular wall
Plasma lipidomic biomarkers may identify global atherosclerotic risk or the condition of the vascular wall (see “Plaque composition”).Citation77–Citation80 Lipid classes are responsible for the main differentiations between a healthy and a diseased vascular wall. More than 300 lipid classes were already recognized in atherosclerotic plaques, particularly CEs, SMs, PC/lysoPC, and PCs.Citation6,Citation81 Significant modifications of CEs with linoleic acid and other polyunsaturated fatty acids were observed between healthy and diseased arteries.Citation77 Thus, low levels of polyunsaturated fatty acids and high levels of linoleic acid in human atherosclerotic plaques expose them to inflammatory mediatorsCitation78 and lead to the progression of atherosclerosis.
Meikle et alCitation43 evaluated plasma lipid profiles containing 305 lipids from 220 individuals (matched healthy controls, n=80; those with stable angina, n=60; and those with unstable coronary syndrome, n=80) using ESI tandem MS technology. They found that ceramide, phosphatidylinositol, and PE species were positively associated with stable coronary artery disease, while lysoPC, ether-linked, and plasmalogen species of PC were negatively associated with stable disease ().
The most desirable effect of antiatherosclerotic treatment is the regression of already-formed atherosclerotic plaques. In many statin treatment studies, a regression of atherosclerotic plaques has been reported.Citation82–Citation85 This clinical benefit of statin treatment is mainly correlated with the lowering of LDL cholesterol; however, other factors beyond LDL cholesterol lowering may be found. In two studies of statin treatment (the Study of Coronary Atheroma by InTravascular Ultrasound: Effect of Rosuvastatin Versus AtorvastatiN [SATURN] trial,Citation83 and the Rosuvastatin and Atorvastatin in different Dosages and Reverse cholesterol transport [RADAR] trialCitation86), prospective lipidomics analysis was evaluated. Atorvastatin and rosuvastatin decreased plasma SM concentrations. Rosuvastatin increased the plasma concentration of PCs, while atorvastatin reduced the plasma concentrations of PCs. The higher reduction in the SM/SM+PC ratio was observed with rosuvastatin treatment when compared with atorvastatin.Citation86 It is noteworthy to mention that nearly five decades ago, SmithCitation87 found the accumulation of SM in human atheromatous plaques, while Schissel et alCitation88 found that the ratio of SM/SM+PC correlates with atherogenesis. Also, Noël et alCitation89 observed the elevation of SM levels in subjects with familial hypercholesterolemia, a disease that frequently leads to premature cardiovascular disease.
Improvement in the SM/PC ratio by statins may suggest the reduced susceptibility of SM to hydrolysis by sphingomyelinase within the vessel wall.Citation88,Citation90 It worth mentioning that the inhibition of SM synthesis has been shown to reduce plasma total cholesterol and triglyceride concentration levels, and to increase HDL cholesterol concentrations in apolipoprotein E-knockout (apoE−/−) mice, which is a useful animal model for experimental atherosclerosis research with inactivated gene coding for the apolipoprotein E protein.Citation91 These lipid changes were associated with increased plaque regression.Citation92,Citation93
However, more studies have to be designed to determine the correlations between lipidomics and atherosclerosis. Voros et alCitation94 have designed a prospective multicenter study (Genetic Loci and the Burden of Atherosclerotic Lesions) that will examine the biological associations between genomic, proteomic, metabolomic, lipidomic, and phenotypic factors of atherosclerosis from a large (7,500) number of biological factors. Patients will undergo noncontrast-enhanced coronary calcium scanning by computed tomography (CT), coronary artery CT angiography, whole-genome sequencing, DNA methylation, whole blood-based transcriptome sequencing, unbiased proteomics based on MS, metabolomics, and lipidomics.Citation37 Thus, the conclusions concerning panomics and atherosclerosis can be safely drawn.
Conclusion
Lipidomics can provide an unbiased field for the investigation of lipids within atherosclerosis. The introduction of lipidomics analysis will allow us to develop patterns that could identify cardiovascular risk factors beyond classical plasma lipids. Additionally, the combinations of lipidomic analysis and classical risk factors may lead us to form better judgments on whom to treat. Moreover, lipidomics analysis can lead us to new therapeutic targets and to novel therapeutic agents. Also, the side effects of the drugs may be better understood and prevented. Thus, lipidomics is a discipline that is rapidly developing and can offer several new strategies.
Disclosure
The authors report no conflicts of interest in this work.
References
- KolovouGDKolovouVMavrogeniSWe are ageingBiomed Res Int2014201480830725045704
- VasiliadisIKolovouGMavrogeniSNairDRMikhailidisDPSudden cardiac death and diabetes mellitusJ Diabetes Complications201428457357924666923
- AveryPBarzilaiNBenetosAAgeing, longevity, exceptional longevity and related genetic and non genetics markers: panel statementCurr Vasc Pharmacol201412565966124350929
- Silvestre-RoigCde WintherMPWeberCDaemenMJLutgensESoehnleinOAtherosclerotic plaque destabilization: mechanisms, models, and therapeutic strategiesCirc Res2014114121422624385514
- JeromeWGYanceyPGThe role of microscopy in understanding atherosclerotic lysosomal lipid metabolismMicrosc Microanal200391546712597787
- FahyESubramaniamSBrownHAA comprehensive classification system for lipidsJ Lipid Res200546583986115722563
- HanXGrossRWGlobal analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomicsJ Lipid Res20034461071107912671038
- WenkMRThe emerging field of lipidomicsNat Rev Drug Discov20054759461016052242
- DennisEALipidomics joins the omics evolutionProc Natl Acad Sci U S A200910672089209019211786
- FiehnOCombining genomics, metabolome analysis, and biochemical modelling to understand metabolic networksComp Funct Genomics20012315516818628911
- GriffinJLAthertonHShockcorJAtzoriLMetabolomics as a tool for cardiac researchNat Rev Cardiol201181163064321931361
- KishimotoKUradeROgawaTMoriyamaTNondestructive quantification of neutral lipids by thin-layer chromatography and laser-fluorescent scanning: suitable methods for “lipidome” analysisBiochem Biophys Res Commun2001281365766211237708
- BrownAJLeongSLDeanRTJessupW7-Hydroperoxycholesterol and its products in oxidized low density lipoprotein and human atherosclerotic plaqueJ Lipid Res1997389173017459323583
- ShioHHaleyNJFowlerSCharacterization of lipid-laden aortic cells from cholesterol-fed rabbits. III. Intracellular localization of cholesterol and cholesteryl esterLab Invest1979412160167459432
- MallatZNakamuraTOhanJThe relationship of hydroxyei-cosatetraenoic acids and F2-isoprostanes to plaque instability in human carotid atherosclerosisJ Clin Invest199910334214279927504
- DegirolamoCShelnessGSRudelLLLDL cholesteryl oleate as a predictor for atherosclerosis: evidence from human and animal studies on dietary fatJ Lipid Res200950SupplS434S43919029117
- MonacoCGreganSMNavinTJFoxwellBMDaviesAHFeldmannMToll-like receptor-2 mediates inflammation and matrix degradation in human atherosclerosisCirculation2009120242462246919948979
- ColeJENavinTJCrossAJUnexpected protective role for Toll-like receptor 3 in the arterial wallProc Natl Acad Sci U S A201110862372237721220319
- SeimonTANadolskiMJLiaoXAtherogenic lipids and lipo-proteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stressCell Metab201012546748221035758
- HolmerSRHengstenbergCKraftHGAssociation of polymorphisms of the apolipoprotein(a) gene with lipoprotein(a) levels and myocardial infarctionCirculation2003107569670112578871
- MeiklePJWongGBarlowCKKingwellBALipidomics: potential role in risk prediction and therapeutic monitoring for diabetes and cardiovascular diseasePharmacol Ther20141431122324509229
- KontushAChantepieSChapmanMJSmall, dense HDL particles exert potent protection of atherogenic LDL against oxidative stressArterioscler Thromb Vasc Biol200323101881188812920049
- Zerrad-SaadiATherondPChantepieSHDL3-mediated inactivation of LDL-associated phospholipid hydroperoxides is determined by the redox status of apolipoprotein A-I and HDL particle surface lipid rigidity: relevance to inflammation and atherogenesisArterioscler Thromb Vasc Biol200929122169217519762782
- KolovouGDCokkinosDVLow serum levels of high-density lipoprotein cholesterol and hypolipidaemic treatmentCurr Med Res Opin200218526526812240788
- PhillipsMCMolecular mechanisms of cellular cholesterol effluxJ Biol Chem201428935240202402925074931
- WeirJMWongGBarlowCKPlasma lipid profiling in a large population-based cohortJ Lipid Res201354102898290823868910
- StübigerGAldover-MacasaetEBickerWTargeted profiling of atherogenic phospholipids in human plasma and lipoproteins of hyper-lipidemic patients using MALDI-QIT-TOF-MS/MSAtherosclerosis2012224117718622795978
- HalvorsenBOtterdalKDahlTBAtherosclerotic plaque stability – what determines the fate of a plaque?Prog Cardiovasc Dis200851318319419026853
- DaviesMJStability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995Circulation1996948201320208873680
- FeltonCVCrookDDaviesMJOliverMFRelation of plaque lipid composition and morphology to the stability of human aortic plaquesArterioscler Thromb Vasc Biol1997177133713459261265
- TabasIConsequences of cellular cholesterol accumulation: basic concepts and physiological implicationsJ Clin Invest2002110790591112370266
- ChenZIchetovkinMKurtzMCholesterol in human athero-sclerotic plaque is a marker for underlying disease state and plaque vulnerabilityLipids Health Dis201096120540749
- JovéMAyalaVRamírez-NúñezOLipidomic and metabolomic analyses reveal potential plasma biomarkers of early atheromatous plaque formation in hamstersCardiovasc Res201397464265223241314
- HinterwirthHStübigerGLindnerWLämmerhoferMGold nanoparticle-conjugated anti-oxidized low-density lipoprotein antibodies for targeted lipidomics of oxidative stress biomarkersAnal Chem201385178376838423895666
- Ramm SanderPPeerMGrandlMBogdahnUSchmitzGKalbitzerHRNMR spectroscopy of macrophages loaded with native, oxidized or enzymatically degraded lipoproteinsPLoS One201382e5636023457556
- MinuzPFavaCLechiALipid peroxidation, isoprostanes and vascular damagePharmacol Rep200658Suppl576817332673
- StegemannCDrozdovIShalhoubJComparative lipidomics profiling of human atherosclerotic plaquesCirc Cardiovasc Genet20114323224221511877
- CamontLLhommeMRachedFSmall, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalitiesArterioscler Thromb Vasc Biol201333122715272324092747
- YangKChengHGrossRWHanXAutomated lipid identification and quantification by multidimensional mass spectrometry-based shotgun lipidomicsAnal Chem200981114356436819408941
- HanXGrossRWShotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samplesMass Spectrom Rev200524336741215389848
- QuehenbergerOArmandoAMBrownAHLipidomics reveals a remarkable diversity of lipids in human plasmaJ Lipid Res201051113299330520671299
- DennisEADeemsRAHarkewiczRA mouse macrophage lipidomeJ Biol Chem201028551399763998520923771
- MeiklePJWongGTsorotesDPlasma lipidomic analysis of stable and unstable coronary artery diseaseArterioscler Thromb Vasc Biol201131112723273221903946
- WenkMRLipidomics: new tools and applicationsCell2010143688889521145456
- The nomenclature of lipidsRecommendations (1976) IUPAC-IUB Commission on Biochemical NomenclatureLipids1977126455468881947
- RasmienaAANgTWMeiklePJMetabolomics and ischaemic heart diseaseClin Sci (Lond)2013124528930623157406
- SoininenPKangasAJWürtzPHigh-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolismAnalyst200913491781178519684899
- WiesnerPLeidlKBoettcherASchmitzGLiebischGLipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometryJ Lipid Res200950357458518832345
- IsobeYAritaMMatsuedaSIdentification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acidJ Biol Chem201228713105251053422275352
- BlanksbySJMitchellTWAdvances in mass spectrometry for lipidomicsAnnu Rev Anal Chem (Palo Alto Calif)2010343346520636050
- EjsingCSDuchoslavESampaioJAutomated identification and quantification of glycerophospholipid molecular species by multiple precursor ion scanningAnal Chem200678176202621416944903
- EjsingCSSampaioJLSurendranathVGlobal analysis of the yeast lipidome by quantitative shotgun mass spectrometryProc Natl Acad Sci U S A200910672136214119174513
- AlmeidaRPaulingJKSokolEHannibal-BachHKEjsingCSComprehensive lipidome analysis by shotgun lipidomics on a hybrid quadrupole-orbitrap-linear ion trap mass spectrometerJ Am Soc Mass Spectrom201526113314825391725
- Di GirolamoFLanteIMuracaMPutignaniLThe role of mass spectrometry in the “omics” eraCurr Org Chem201317232891290524376367
- FengXLiuXLuoQLiuBFMass spectrometry in systems biology: an overviewMass Spectrom Rev200827663566018636545
- HanXYangKGrossRWMulti-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analysesMass Spectrom Rev201231113417821755525
- FennJBMannMMengCKWongSFWhitehouseCMElectrospray ionization for mass spectrometry of large biomoleculesScience1989246492664712675315
- GriffithsRLSarsbyJGuggenheimEJFormal lithium fixation improves direct analysis of lipids in tissue by mass spectrometryAnal Chem201385157146715323879734
- LonguespéeRBoyonCDesmonsASpectroimmunohistochemistry: a novel form of MALDI mass spectrometry imaging coupled to immunohistochemistry for tracking antibodiesOMICS201418213214124351082
- GawrischKEldhoNVPolozovIVNovel NMR tools to study structure and dynamics of biomembranesChem Phys Lipids20021161–213515112093539
- HornPJChapmanKDLipidomics in situ: insights into plant lipid metabolism from high resolution spatial maps of metabolitesProg Lipid Res201454325224480404
- SezginESchwillePFluorescence techniques to study lipid dynamicsCold Spring Harb Perspect Biol2011311a00980321669985
- GaoXZhangQMengDA reversed-phase capillary ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) method for comprehensive top-down/bottom-up lipid profilingAnal Bioanal Chem201240292923293322354571
- SandraKSandraPLipidomics from an analytical perspectiveCurr Opin Chem Biol201317584785323830914
- LiJRomestaingCHanXCardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesityCell Metab201012215416520674860
- ChanRUchilPDJinJRetroviruses human immunodeficiency virus and murine leukemia virus are enriched in phosphoinositidesJ Virol20088222112281123818799574
- BrunelleALaprévoteOLipid imaging with cluster time-of-flight secondary ion mass spectrometryAnal Bioanal Chem20093931313518777109
- KolovouGDKaterinaAIoannisVCokkinosDVSimvastatin: two decades in a circleCardiovasc Ther200826216617818485137
- LaaksonenRJänisMTOresicMLipidomics-based safety biomarkers for lipid-lowering treatmentsAngiology2008592 Suppl65S68S18632766
- ChenFMaridakisVO’NeillEAThe effects of simvastatin treatment on plasma lipid-related biomarkers in men with dyslipidaemiaBiomarkers201116432133321417623
- Kaddurah-DaoukRBaillieRAZhuHLipidomic analysis of variation in response to simvastatin in the Cholesterol and Pharmacogenetics StudyMetabolomics20106219120120445760
- YetukuriLHuopaniemiIKoivuniemiAHigh density lipoprotein structural changes and drug response in lipidomic profiles following the long-term fenofibrate therapy in the FIELD substudyPLoS One201168e2358921887280
- AertsJMOttenhoffRPowlsonASPharmacological inhibition of glucosylceramide synthase enhances insulin sensitivityDiabetes20075651341134917287460
- HollandWLBrozinickJTWangLPInhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistanceCell Metab20075316717917339025
- ZhaoHPrzybylskaMWuIHInhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetesDiabetes20075651210121817470562
- BijlNSokolovićMVrinsCModulation of glycosphingolipid metabolism significantly improves hepatic insulin sensitivity and reverses hepatic steatosis in miceHepatology20095051431144119731235
- DidangelosAStegemannCMayrMThe -omics era: proteomics and lipidomics in vascular researchAtherosclerosis20122211121722024275
- EkroosKJänisMTarasovKHurmeRLaaksonenRLipidomics: a tool for studies of atherosclerosisCurr Atheroscler Rep201012427328120425241
- AritaMMediator lipidomics in acute inflammation and resolutionJ Biochem2012152431331922923733
- LamSMShuiGLipidomics as a principal tool for advancing biomedical researchJ Genet Genomics201340837539023969247
- Bou KhalilMHouWZhouHLipidomics era: accomplishments and challengesMass Spectrom Rev201029687792920931646
- FeigJEFeigJLKiniASStatins, atherosclerosis regression and HDL: Insights from within the plaqueInt J Cardiol201518916817125897898
- NichollsSJBorgmanMNissenSEImpact of statins on progression of atherosclerosis: rationale and design of SATURN (Study of Coronary Atheroma by InTravascular Ultrasound: effect of Rosuvastatin versus AtorvastatiN)Curr Med Res Opin20112761119112921446892
- PuriRLibbyPNissenSELong-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURNEur Heart J Cardiovasc Imaging201415438038824448227
- AlbertMAGlynnRJFonsecaFARace, ethnicity, and the efficacy of rosuvastatin in primary prevention: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trialAm Heart J20111621106114.e221742096
- BergheanuSCReijmersTZwindermanAHLipidomic approach to evaluate rosuvastatin and atorvastatin at various dosages: investigating differential effects among statinsCurr Med Res Opin20082492477248718655752
- SmithEBIntimal and medial lipids in human aortasLancet19601712879980313831944
- SchisselSLJiangXTweedie-HardmanJSecretory sphingomy-elinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion developmentJ Biol Chem19982735273827469446580
- NoëlCMarcelYLDavignonJPlasma phospholipids in the different types of primary hyperlipoproteinemiaJ Lab Clin Med19727946116215021301
- JeongTsSchisselSLTabasIPownallHJTallARJiangXIncreased sphingomyelin content of plasma lipoproteins in apolipoprotein E knockout mice reflects combined production and catabolic defects and enhances reactivity with mammalian sphingomyelinaseJ Clin Invest199810149059129466986
- KolovouGAnagnostopoulouKMikhailidisDPCokkinosDVApolipoprotein E knockout modelsCurr Pharm Des200814433835118289060
- ParkTSPanekRLRekhterMDModulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in ApoE knockout miceAtherosclerosis2006189226427216458317
- OestvangJBonnefont-RousselotDNinioEHakalaJKJohansenBAnthonsenMWModification of LDL with human secretory phospholipase A(2) or sphingomyelinase promotes its arachidonic acid-releasing propensityJ Lipid Res200445583183814754906
- VorosSMaurovich-HorvatPMarvastyIBPrecision phenotyping, panomics, and system-level bioinformatics to delineate complex biologies of atherosclerosis: rationale and design of the “Genetic Loci and the Burden of Atherosclerotic Lesions” studyJ Cardiovasc Comput Tomogr20148644245125439791
