Abstract
Background
A growing body of evidence links coronary artery atherosclerosis and calcification to osteoporosis in women. The endothelium plays a critical role in maintaining vascular integrity and may play a role in bone metabolism. We aimed to determine whether early coronary atherosclerosis, as detected by coronary microvascular endothelial dysfunction (CMED), predicts the development of osteoporosis in postmenopausal women.
Methods
Coronary vascular reactivity was evaluated in 194 postmenopausal women greater than 50 years of age and with non-obstructive coronary arteries by administration of intracoronary acetylcholine during diagnostic angiography. CMED was defined as ≤50% increase in coronary blood flow from baseline in response to maximal dose. After a median follow-up of 7.0±0.3 years, patients were assessed by a questionnaire for development of osteoporosis.
Results
The average age of the cohort was 60.9±7.4 years. Women with CMED were twice as likely to develop osteoporosis compared with women without endothelial dysfunction after adjustment for potential confounders (relative risk, 2.4; 95% confidence interval [CI], 1.1, 5.6, P=0.02). Epicardial endothelial dysfunction was not associated with development of osteoporosis.
Discussion
Early coronary atherosclerosis with endothelial dysfunction is an independent marker for increased risk of developing osteoporosis in postmenopausal women greater than 50 years of age without obstructive coronary artery disease. The current study supports a link between coronary atherosclerosis and osteoporosis.
Introduction
Cardiovascular disease and osteoporosis are two common age-related conditions that result in significant mortality and morbidity. Growing evidence suggests that there may be an association between coronary artery disease and osteoporosis. Traditionally, both diseases are thought to be concomitantly present due to age-related processes.Citation1,Citation2 However, the link between these two conditions is not entirely understood.
Low bone mineral density has been associated with increased cerebrovascular and cardiovascular morbidity and mortality, especially in women.Citation3–Citation6 The relationship between the early stage of atherosclerosis and osteoporosis is unknown. Endothelial dysfunction is an important early feature of atherosclerosis and coronary artery disease. The endothelium plays a critical role in regulating vascular tone via release of vasodilators such as nitric oxide, which maintain vasodilation, and inhibit platelet aggregation and leukocyte adhesion, thereby impeding the atherosclerotic process. Endothelial dysfunction as a potential link between cardiovascular disease and osteoporosis has previously been suggested by Sanada et al who studied healthy Japanese women and found that postmenopausal women with low bone mineral density have impaired endothelial function in forearm resistance arteries.Citation7 Furthermore, endothelial progenitor cells may also play a role in a link between osteoporosis and coronary artery disease, as they are mobilized from the bone marrow in response to vascular injury.Citation8,Citation9
Due to the association previously described between osteoporosis and coronary artery disease, we aimed to test the hypothesis that women with coronary endothelial dysfunction are more likely to develop osteoporosis than women with normal coronary endothelial function.
Methods
Study design
This prospective single center cohort study was approved by the Institutional Review Board of the Mayo Foundation, and all subjects provided written and informed consent.
Study population
A total of 194 postmenopausal women greater than 50 years of age were enrolled. All patients presented with symptoms of chest pain and underwent cardiac catheterization and comprehensive assessment of coronary vasomotor function, including coronary endothelial function by administration of acetylcholine.Citation8,Citation9
Inclusion criteria included female sex, age greater than 50 years, and postmenopausal status. Exclusion criteria included significant coronary artery stenosis (>50%), ejection fraction <45%, unstable angina, previous myocardial infarction, use of radiographic contrast agents within 12 hours, significant systemic disease, and pregnancy. Medications that may affect cardiovascular hemodynamics were discontinued for at least 48 hours before the study.
Study protocol
All patients underwent diagnostic coronary angiography, and endothelial reactivity was assessed as previously described.Citation8–Citation10 A total of 5,000 units of heparin were infused intravenously, and a Doppler guide wire was positioned in the mid-left anterior descending artery. Acetylcholine was administered in increasing concentrations into the left anterior descending artery. Angiographic, hemodynamic, and coronary Doppler data were recorded as described previously.Citation11–Citation13 Both microvascular and epicardial blood flow were assessed. Microvascular coronary endothelial dysfunction was defined as ≤50% increase in coronary blood flow (CBF) from baseline in response to a maximal dose of acetylcholine. Epicardial dysfunction was defined as a decrease in epicardial coronary artery diameter greater than 20% in response to maximal dosage of acetylcholine.Citation9,Citation13
Follow-up
After a median follow-up of 7.0±0.3 years, patients enrolled in the study answered a standardized questionnaire that inquired about the presence of osteoporosis at the time of catheterization and subsequent development of osteoporosis following cardiac catheterization, either through diagnosis by primary care physician or through bone mineral density testing. The questionnaire also assessed a variety of other parameters including age, sex, medication use, other medical problems, and recurrent chest pain.
Statistical analysis
Statistical analysis was performed using JMP software (SAS Institute Inc., Cary, NC, USA). Continuous variables were presented as mean ± standard deviation, and categorical variables were presented as frequency (%). Student’s t-test was used to compare continuous variables, and categorical data were compared with the chi-square test. Cox proportional hazards regression modeling was used to assess for an independent association between coronary microvascular endothelial dysfunction and epicardial endothelial dysfunction and development of osteoporosis.
Results
A total of 194 postmenopausal women without evidence of osteoporosis were enrolled in the study. lists baseline characteristics of patients with and without osteoporosis. The average age of the cohort was 60.9 years ± 7.4 years. Hypertension was self-reported by 51.2% of the cohort, hyperlipidemia by 62.8%, known vascular disease by 7.7%, and diabetes by 5.6%. The average body mass index of the population was 28.2±6.2.
Table 1 Comparison of baseline characteristics between patients who did and did not develop osteoporosis
Of 194 women, 119 (61.3%) were diagnosed with coronary microvascular endothelial dysfunction on angiography. Thirty-nine patients of the cohort (20.1%) developed osteoporosis during a median follow-up period of 8.4±4.7 years.
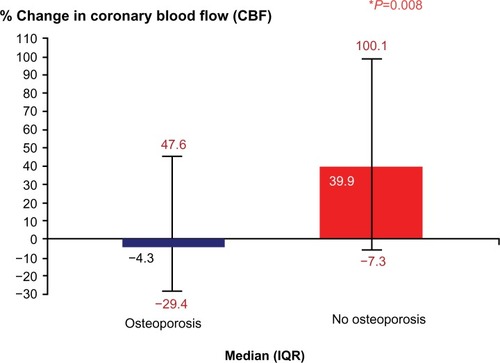
Women who developed osteoporosis had a markedly lower percent change in CBF in response to acetylcholine compared with women who did not develop osteoporosis: median (interquartile range), −4.3 (−29.4, 47.6) versus 39.9 (−7.3, 100.1); P=0.008 (). In univariate analysis, coronary microvascular endothelial dysfunction was significantly associated with the development of osteoporosis (relative risk, 2.1; 95% confidence interval [CI], 1.06, 4.12; P=0.02).
Figure 1 Comparison of percent change in coronary blood flow among patients who developed osteoporosis versus those that did not. Patients who developed osteoporosis had lower percent change in coronary blood flow when compared with patients who did not develop osteoporosis.
Multivariable analysis adjusting for potential confounding variables including age, hyperlipidemia, hypertension, BMI, estrogen use, and steroid use, revealed that microvascular dysfunction remained significantly associated with the development of osteoporosis (relative risk, 2.4; 95% CI, 1.1, 5.6; P=0.02) ().
Table 2 Multivariable analysis depicting microvascular dysfunction as significantly associated with development of osteoporosis
Epicardial microvascular dysfunction was not significantly associated with the development of osteoporosis in this cohort in both univariate and multivariable analyses adjusting for the same potential confounding factors (P>0.05).
Discussion
The current study suggests that coronary endothelial function is an independent predictor for development of self-reported osteoporosis in postmenopausal women after adjustment for potential confounders. Thus, endothelial dysfunction may be a common denominator linking increased risk of cardiovascular disease and mortality to low bone mineral density.
The association between cardiovascular disease and osteoporosis has been described extensively in the literature. Patients with osteoporosis have been shown to be more likely to have cardiovascular disease, and vice versa.Citation2,Citation14–Citation17 Yet, there is a paucity of information regarding the link between the early stage of atherosclerosis and osteoporosis. The current study, supported by these previous observations, demonstrates a link between early atherosclerosis and osteoporosis.
Endothelial dysfunction may be a common precursor to both osteoporosis and coronary artery disease, potentially explaining the link noted between the two conditions. Endothelial dysfunction is an early stage of atherosclerosis, but is also considered to be an important regulator of vascular and bone health.Citation8,Citation9,Citation13 The endothelium plays an important role in mediating vascular processes such as inflammation, vasodilatation/vasoconstriction, thrombosis, and oxidation. Endothelial dysfunction prevents appropriate balance of these processes, playing a key role in initiating atherosclerosis.Citation16
Nitric oxide is a possible explanation for the link seen between atherosclerosis and osteoporosis. Nitric oxide may play a role in bone metabolism via osteoblastic activity.Citation16,Citation18,Citation19 High concentrations of nitric oxide can directly inhibit spreading of osteoclasts and bone resorption, and stimulate or inhibit bone resorption depending on cytokine stimulation.Citation20 Cytokines such as interleukin-1, tumor necrosis factor, and interferon may inhibit osteoblast growth since nitric oxide is produced by osteoblast-like cells in response to pro-inflammatory cytokines, inhibiting osteoblast proliferation and suggesting a role for nitric oxide in bone remodeling.Citation21 Isosorbide mononitrate is associated with reduced urine N-telopeptide levels, which is a marker of bone resorption, and increased levels of alkaline phosphatase, a marker of bone formation, further suggesting nitric oxide as a possible mediator of bone formation.Citation22 When endothelial function is impaired, nitric oxide and its physiologic function can also become impaired, potentially limiting the normally beneficial effects of nitric oxide on bone formation.
Another potential mechanism for this link is based on the premise that endothelial progenitor cells (EPCs) residing in the marrow may be mobilized in response to vascular injury.Citation23 EPCs are mobilized from the bone marrow into circulation and migrate to affected areas to aid in tissue recovery. Traditionally, endothelial and osteoblastic cells have been thought to be derived from two separate progenitor lines; however, increasing evidence suggests that there may be an overlap between these two cell lines. This potential role of EPCs in bone formation is further supported by research that shows these cells can be differentiated into osteoblastic cells,Citation9 supporting the notion that endothelial progenitor cells may have a role in vascular calcification.Citation9 Our group suggested a link between these two seemingly different cell lines when it identified a group of EPCs derived from the bone marrow, which are costained using flow cytometry for osteocalcin (OCN+), an osteoblast marker.Citation9
We have previously reported a greater number of EPCs expressing osteocalcin (OCN+ EPCs) in patients with early coronary atherosclerosis as opposed to patients with normal coronary arteries and no endothelial dysfunction.Citation9 We also found that these EPCs can form mineralized deposits. Patients with CMED retain OCN+ EPCs in coronary circulation when compared with patients without coronary endothelial dysfunction. This further supports the potential relationship between endothelial dysfunction and osteoporosis. Increased numbers of circulating OCN+ EPCs in patients with endothelial dysfunction may contribute to vascular insult and initiation of atherosclerosis, especially with vascular calcification.Citation13 These osteogenic cells may contribute to worsened calcification rather than bone formation. Thus, documented presence of EPCs expressing osteocalcin has been established in patients with early atherosclerosis, endothelial dysfunction, and coronary artery disease.Citation9
There may also be an inflammatory component contributing to the pathophysiologic process as well. Our group has also found that patients with endothelial dysfunction have higher levels of inflammatory cytokines such as interleukin-8.Citation9,Citation13 Such cytokines have been shown to attract EPCs to ischemic areas of the myocardium, further contributing to altered repair of the endothelium.Citation13,Citation24
Our finding that CMED may be associated with the development of osteoporosis is thus plausible. Patients with endothelial dysfunction and early atherosclerosis have impaired bone metabolism, leading to osteoporosis. This in turn leads to increasing levels of OCN+ EPCs, further worsening endothelial function. While many studies have shown a link between coronary artery disease and osteoporosis, there is little clinical data suggesting endothelial dysfunction is associated with osteoporosis. Our findings are consistent with a previous study that showed that postmenopausal women with low bone mineral density have impaired endothelial function in the forearm resistance arteries.Citation7
Our study has several inherent limitations. Our conclusions are based on a standardized questionnaire administered several years after angiography. While bone mineral density studies would have utility in better delineating whether microvascular dysfunction predicts osteoporosis, the strong association we note even with simple questionnaires relying only on self-report and chart review is compelling. Furthermore, not all patients completed the survey, and when there is an incomplete follow-up, there is a possibility for inherent resulting bias. Future studies should be directed at assessing this association with bone mineral density studies.
Conclusion
While the association between cardiovascular disease and osteoporosis has been suggested previously, our findings suggest that endothelial dysfunction may be the underlying mechanism linking these two disease processes. Further research is warranted to determine whether women with low bone mass should undergo more extensive screening and management to delay development of osteoporosis, or whether women with known coronary endothelial dysfunction should be treated in an effort to prevent osteoporosis.
Disclosure
The authors report no conflicts of interest in this work.
References
- FarhatGNCauleyJAThe link between osteoporosis and cardiovascular diseaseClin Cases Miner Bone Metab20085193422460842
- AoyagiKRossPDOrloffJDavisJWKatagiriHWasnichRDLow bone density is not associated with aortic calcificationCalcif Tissue Int200169202411685429
- BrownerWSPressmanARNevittMCCauleyJACummingsSRAssociation between low bone density and stroke in elderly women. The study of osteoporotic fracturesStroke1993249409468322393
- TankóLBChristiansenCCoxDAGeigerMJMcNabbMACummingsSRRelationship between osteoporosis and cardiovascular disease in postmenopausal womenJ Bone Miner Res2005201912192016234963
- von der ReckePHansenMAHassagerCThe association between low bone mass at the menopause and cardiovascular mortalityAm J Med199910627327810190374
- SamelsonEJKielDPBroeKEMetacarpal cortical area and risk of coronary heart disease: the Framingham StudyAm J Epidemiol200415958959515003963
- SanadaMTaguchiAHigashiYForearm endothelial function and bone mineral loss in postmenopausal womenAtherosclerosis200417638739215380463
- GösslMKhoslaSZhangXRole of circulating osteogenic progenitor cells in calcific aortic stenosisJ Am Coll Cardiol2012601945195323062532
- GösslMMödderUIAtkinsonEJLermanAKhoslaSOsteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosisJ Am Coll Cardiol2008521314132518929243
- FlammerAJGösslMLiJPatients with an HbA1c in the prediabetic and diabetic range have higher numbers of circulating cells with osteogenic and endothelial progenitor cell markersJ Clin Endocrinol Metab2012974761476823015657
- HanSHBaeJHHolmesDRJrSex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosisEur Heart J2008291359136918424787
- HasdaiDGibbonsRJHolmesDRJrHiganoSTLermanACoronary endothelial dysfunction in humans is associated with myocardial perfusion defectsCirculation199796339033959396432
- GösslMMödderUIGulatiRCoronary endothelial dysfunction in humans is associated with coronary retention of osteogenic endothelial progenitor cellsEur Heart J2010312909291420935001
- VogtMTCauleyJAKullerLHNevittMCBone mineral density and blood flow to the lower extremities: the study of osteoporotic fracturesJ Bone Miner Res1997122832899041062
- FryeMAMeltonLJIIIBryantSCOsteoporosis and calcification of the aortaBone Miner1992191851941422314
- WarburtonDENicolCWGattoSNBredinSSCardiovascular disease and osteoporosis: balancing risk managementVasc Health Risk Manag2007367368918078019
- SinnottBSyedISevrukovABarengoltsECoronary calcification and osteoporosis in men and postmenopausal women are independent processes associated with agingCalcif Tissue Int20067819520216604285
- EastellRNewmanCCrossmanDCCardiovascular disease and boneArch Biochem Biophys2010503788320542011
- ManolagasSCFrom estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosisEndocr Rev20103126630020051526
- MacIntyreIZaidiMAlamASOsteoclastic inhibition: an action of nitric oxide not mediated by cyclic GMPProc Natl Acad Sci U S A199188293629401849281
- RalstonSHToddDHelfrichMBenjaminNGrabowskiPSHuman osteoblast-like cells produce nitric oxide and express inducible nitric oxide synthaseEndocrinology19941353303367516867
- NabhanAFA randomized clinical trial of the effects of isosorbide mononitrate on bone formation and resorption in post-menopausal women: a pilot studyHum Reprod2006211320132416410328
- UrbichCDimmelerSEndothelial progenitor cells: characterization and role in vascular biologyCirc Res20049534335315321944
- KocherAASchusterMDBonarosNMyocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL-8/Gro CXC chemokinesJ Mol Cell Cardiol20064045546416438981
