Abstract
Preeclampsia (PE) is a pregnancy-specific syndrome and one of the leading causes of preterm birth, neonatal and maternal morbidity and mortality. This disease is characterized by new onset hypertension usually in the third trimester of pregnancy and is sometimes associated with proteinuria, although proteinuria is not a requirement for the diagnosis of PE. In developing countries, women have a higher risk of death due to PE than more affluent countries and one of the most frequent causes of death is high blood pressure and stroke. Although PE only affects approximately 2%–8% of pregnancies worldwide it is associated with severe complications such as eclampsia, hemorrhagic stroke, hemolysis, elevated liver enzymes and low platelets (HELLP syndrome), renal failure and pulmonary edema. Importantly, there is no “cure” for the disease except for early delivery of the baby and placenta, leaving PE a health care risk for babies born from PE moms. In addition, PE is linked to the development of cardiovascular disease and stroke in women after reproductive age, leaving PE a risk factor for long-term health in women. This review will highlight factors implicated in the pathophysiology of PE that may contribute to long-term effects in women with preeclamptic pregnancies.
Introduction
The underlying pathophysiology of preeclampsia (PE) is not completely understood, but it is currently believed that the initiating event in PE is reduced placental perfusion, which develops from shallow cytotrophoblast migration toward the uterine spiral arterioles which leads to inappropriate vascular remodeling and a hypoperfused placenta.Citation1 This placenta becomes ischemic as the pregnancy continues which leads to the release of factors that cause maternal vascular endothelial dysfunction.Citation1–Citation8 Endothelial dysfunction results in generalized vasoconstriction, reduced blood to multiple organs and it has been a major phenotype of PE.Citation1,Citation4,Citation5,Citation9–Citation13 Furthermore, pre-existing conditions such as poor nutrition, diabetes, and obesity, are all risk factors for PE, and could exacerbate the maternal response to factors released from the ischemic placenta.Citation9 While 800 women die from pregnancy complications around the world every day and 3 million of preterm births reported each year are related to PE,Citation14,Citation15 there is no effective treatment for this pregnancy disease except for early delivery of the fetus.
The maternal cardiovascular and renal system goes through several important adaptations during a normal pregnancy. The cardiac output, heart rate, and stroke volume increases during pregnancy due to an increase in plasma volume expansion and systemic vascular vasodilation during pregnancy.Citation16 A lack of these changes during pregnancy is often associated with pregnancy complications and an increased risk of developing cardiovascular events (such as myocardial infarctions, venous thromboembolisms, and stroke) in the mother later in life.Citation17 Multiple clinical studies of women with PE show an increased risk of developing cardiovascular diseases later in life.Citation18 For example a Norwegian study from 1967 to 1992, with over 600,000 pregnant women, showed that women with PE have an 8-fold increase in death resulting from a cardiovascular event versus women who have a normal pregnancy.Citation18 A study of ∼30,000 women in the state of Washington from 1967 to 1998 showed that women with mild and severe PE had a 2-fold and 3-fold greater risk of cardiovascular events later in life respectively.Citation18 Furthermore, a 30 year follow-up study of ∼14,000 women in California noted that women with PE have a 2-fold increase in death due to cardiovascular events, and women who had the onset of PE before 34 weeks of gestation had a 9-fold increase of cardiovascular events later in life.Citation18 Finally, a Taiwanese study of 1 million women shows that women with PE have a 12-fold increase in having a cardiovascular event.Citation18 Thus all of these clinical studies taken together show that women with PE have an increased risk of having a cardiovascular event later in life no matter the location, thereby indicating the importance of the common mediators of this disease shared among these women to help identify the link between PE and later cardiovascular events.
Renal insufficiency is a common thread that could contribute to long-term pathology of previously preeclamptic women
Despite an increase in cardiac output during pregnancy, there is a decrease in maternal blood pressure due to a decrease in total peripheral resistance caused by maternal vasodilation during pregnancy. In addition, in the kidneys, the glomerular filtration rate (GFR) and renal blood flow increase due to a decrease in renal vascular resistance.Citation16 Women with PE often have altered renal function, glomerular endotheliosis, and proteinuria.Citation19 Early studies in the 1970s by Gibson, showed that the lack of rise in GFR during early pregnancy is associated with women who have an increased risk of unexplained stillbirths, abortions, or small for gestational age babies.Citation20 A population study consisting of pregnant Norwegian patients from 1967 to 1991, showed that 477 patients out of 570,433 pregnant women developed end-stage renal disease (ESRD).Citation21 Out of those 477 women who developed ESRD, the authors found that women who had been pregnant one or more times and developed PE during the first pregnancy are four times more likely to develop ESRD.Citation21 They also showed that as the number of reoccurring preeclamptic pregnancies in the mother increased, the greater her risk of developing ESRD.Citation21
Importantly, there is a decrease in GFR and proteinuria in preeclamptic patients.Citation19 Several studies show that podocyturia, which is the urinary excretion of podocytes, are elevated in women with PE.Citation22–Citation24 The amount of podocyturia, podocytes in the urine, can be determined by quantifying podocalyxin, podocin, nephrin, and synaptopodin protein structures in the urine.Citation22–Citation24 A more recent study by Garovic et al,Citation22 showed that podocyturia had a greater predictive value for the diagnosis of PE versus other angiogenic factors, such as soluble fms-like tyrosine kinase-1 (sFLT-1), endoglin, and placental growth factor (PlGF). It is important to note that the loss of podocytes, which have a very limited regenerative capacity, from the glomerulus may lead to an increase in proteinuria and glomerulosclerosis due to a disruption of the glomerular filtration barrier. Therefore, podocyturia may serve as a sensitive biomarker for the development of PE and the degree of renal injury damage observed in PE.
Endothelial dysfunction is a common thread that could contribute to long-term pathology of previously preeclamptic women Nitric oxide (NO)
Endothelial dysfunction is characterized by an increase in ET-1, a potent vasoconstrictor, secretion from endothelial cells and a lack of appropriate vascular responses to endothelial mediated vasodilators. While the mechanisms responsible for systemic maternal vascular dysfunction are unknown, mediators of endothelial dysfunction such as decreased NO have been shown to play a role in the development of hypertension in preeclamptic women.Citation25–Citation30 Previous studies have shown that inhibition of NO synthase in pregnant rats is associated with intrauterine growth retardation (IUGR)Citation31 and L-arginine supplementation prevents fetal growth restriction in animal models of IUGR.Citation32 In addition, the supplementation with L-arginine in IUGR pregnant women improves vasorelaxation indicating this pathway could be an important role in the pathophysiology of IUGR in human pregnancies.Citation33
NO, originally identified as the endothelium derived relaxing factor, is an important vasodilator synthetized in response to mechanical and chemical stimuli from L-arginine by a family of calcium–calmodulin-dependent enzymes, called nitric oxide synthases (NOS).Citation34 NO induces vascular smooth muscle relaxation via soluble guanylate cyclase (sGC)/cyclic guanosine monophosphate (cGMP)-dependent and independent mechanisms. In addition, NO inhibits leukocytes adhesion, and has antithrombotic and anti-apoptotic effects.Citation35
NO derived from endothelial nitric oxide synthase (eNOS) is an important mediator of vascular homeostasis.Citation35 An up-regulation of eNOS, resulting in increased NO production has been shown to contribute to increases in uteroplacental blood flow via changes in vascular tone.Citation36 In addition, there is evidence that genetic eNOS polymorphisms may affect the susceptibility to hypertensive disorders of pregnancy.Citation37,Citation38
Alterations during normal pregnancy such as increased blood volume is accommodated within the cardiovascular system by systemic vasodilatation associated with NO production,Citation39 thereby suggesting that NO deficiency could be playing an important role in hypertensive disorders of pregnancy. In fact, several studies in human and animal models have shown that impaired vascular relaxation in PE has been attributed to reduced bioavailability of NO produced via eNOS.Citation14,Citation40,Citation41 Previous studies have demonstrated elevated eNOS expression during PE,Citation42 however both increased arginase expression (regulator of NOS)Citation43 and elevated levels of a natural NOS inhibitor, asymmetric dimethylarginineCitation44 were found which in turn, could impair the balance between vasodilator and vasoconstrictor effects on the vascular smooth muscle thereby contributing to rise in blood pressure in PE. Furthermore, Mutlu-Turkoglu et al have published that circulating NOx levels were decreased in preeclamptic women compared to normal pregnant women before delivery. After 24 hours, post-delivery, these levels did not change in the normal pregnant group, however, nitrate-nitrite (NOx) levels increased in the preeclamptic group supporting the idea that alterations in NO regulation may play a key role in PE.Citation45
Experimental animal models of placental ischemia including reduced uterine perfusion pressure (RUPP) have been shown to exhibit impaired vasorelaxation in conduit vessels and activity of NO has been investigated.Citation28,Citation39,Citation46,Citation47 Moreover, previous investigators have demonstrated a loss or absence of NO-mediated vasorelaxation in RUPP plasma-treated vessels.Citation48 In our recent study we have shown that circulating NOx levels and vascular eNOS expression were decreased in RUPP rats.Citation49
Although extensive research is needed to further investigate the benefits of NO, drugs that target components of NO pathway could increase NO bioavailability contributing to improve the maternal outcomes such as hypertension and endothelial dysfunction. Furthermore, markers of endothelial dysfunction such as ET-1, soluble vascular adhesion molecule and IL-8, or endothelial leukocyte adhesion molecule 1 (ELAM-1), which could be elevated weeks prior the clinical manifestation of PE.Citation50 Indeed, detection of endothelial dysfunction or vasoconstriction may serve as predictor of this disease and may be helpful in order to understand the pathways associated with PE. Peripheral arterial tone and other methods which rely on flow mediated dilatation have been used to assess the endothelial function during pregnancy and PE.Citation51 Although the peripheral arterial tone did not identify women who will develop PE, this technique could be associated with a relative peripheral vasoconstriction in preeclamptic women after delivery and might be used to detect abnormalities that persisted after pregnancy. Furthermore, peripheral pulse pressure waveforms determined by method of applanation tonometry demonstrate the changes in radial artery pulse waveform, and its correlation with central aortic pressure and pulse wave during pregnancy. In fact, tonometry has showed elevated augmentation index in preeclamptic women compared with normal pregnant women.Citation52 Non-invasive measurements of vasoconstriction during pregnancy in future studies may help predict PE and the risks of cardiovascular disease post-delivery.
sFlt-1
Abnormalities in the placenta are associated with increased anti-angiogenic factors such as sFlt-1 or soluble vascular endothelial growth factor receptor 1 which contribute to decreased renal function and hypertension during pregnancy. Preeclamptic women have increased circulating sFlt-1 levels and placental sFlt-1 mRNA compared to women who have normal pregnancies.Citation42 Furthermore, the effects of pro-angiogenic factors VEGF and PlGF which are important to maintain the vascular endothelium are antagonized by sFlt-1 which is a possible cause of endothelial dysfunction during this disease.Citation53 Previous studies have shown that decreased levels of VEGF and PIGF were correlated with increased levels of sFlt-1 during PE.Citation42 In addition, VEGF is important to induce and maintain the integrity of fenestrated endothelium in various tissues, including the renal glomerulus, and VEGF blockade by sFlt-1 may be a cause for renal damage and a decrease in renal function.
The role for sFlt-1 in the pathogenesis of this hypertensive disorder during pregnancy has been supported by animal data. When administered to pregnant and non-pregnant rats using an adenoviral vector, sFlt-1 induces PE-like syndrome resulting in the classical signs such as hypertension, proteinuria, and glomerular endotheliosis.Citation42 Recent studies by Murphy et al demonstrate that infusion of sFlt-1 into normal pregnant rats caused hypertension which was associated with endothelial dysfunction characterized by increased ET-1 and decreased NO.Citation54 Importantly, L-arginine improved hypertension and ET-1 expression.Citation28 These data further support the hypothesis that increased NO availability may be an innovative pathway to target improved treatment of PE.
Importantly, a recent paper has shown that sFlt-1 levels normalized after 2 months post-delivery in PE-exposed compared with control mice. In the same study, an enhanced vascular response to injury in vessels exposed to PE was observed after delivery.Citation55 Additionally, women who develop PE did not show differences in serum sFlt-1 levels at 12 weeks postpartum compared with their third trimester.Citation56 Therefore, high sFlt-1 levels during and after PE could play a persistent role in endothelial dysfunction and damage after PE. Furthermore, altered expression of angiogenesis-proteins was presented in women who had prior PE more than 1 year post-delivery.Citation57 Although PE is resolved by delivery of the placenta, and altered angiogenesis and vascular damage have been associated with long-term cardiovascular risk, larger studies are necessary to verify whether postpartum measures of sFlt-1 levels have a role in predicting future cardiovascular disease.
Chronic inflammation is a common thread that could contribute to long-term pathology of previously preeclamptic women
During normal pregnancy, the fetus must overcome immune rejection by the maternal immune system. Therefore, tolerance for the semi-allogenic fetal antigens must be established, particularly at the maternal-fetal interface, while still maintaining immune protection against pathogens. At the maternal-fetal interface, leukocytes make up 30%–40% of the decidual cells and are comprised of mainly NK cells, CD14+ myelomonocytic cells, and T lymphocytes.Citation58 Normal pregnancy proceeds with mild inflammation, however, women with PE exhibit chronic immune activation and have an exaggerated innate inflammatory response. Multiple studies implicate a number of immune factors in mediating hypertension and endothelial dysfunction during pregnancy.Citation59–Citation69 Clinical studies have demonstrated an increase in pro-inflammatory cytokine production in PE compared to normal pregnancy, and these data are also observed in animal models of PE.Citation5,Citation46,Citation70–Citation76 T lymphocytes are instrumental to immunological memory and could play a role in the long-term sequelae of PE such as stroke or other cardiovascular events.
CD4+ T helper cells
CD4+ T-cells are a heterogeneous group of cells made up of several different subsets to include TH1, TH2, TH17, and CD4+ TReg cells. Clinical studies have shown that the populations of all subsets of CD4+ T-cells are not increased, but rather the TH1 and the TH17 subsets are increased while the TH2 and TReg subsets are decreased in women with PE compared to women with normal pregnancies.Citation77–Citation86 These alterations in the CD4+ populations of cells are described as a CD4+ T-cell imbalance, and recent studies have partially determined how changes in these specific populations contribute to pathophysiology during PE.
TH1 cells produce IL-2 and IFN-γ, are pro-inflammatory, and are involved in cellular immunity, while TH2 cells produce IL-4, IL-5, and IL-13, are anti-inflammatory, and are involved in humoral immunity. Normal pregnancy is associated with a predominant TH2 profile and suppressed TH1-type immunity, whereas an increased ratio of TH1:TH2 cells is observed in PE.Citation77,Citation80,Citation86,Citation87 Zenclussen et al performed adoptive transfer of activated BALB/c TH1-like splenocytes into allogeneically pregnant BALB/c female mice during late gestation.Citation76 This resulted in the development of a PE-like model presenting with increased blood pressure, glomerulonephritis, and proteinuria. Furthermore, in response to adoptive transfer of activated TH1-like cells, uterine cytokine production and fetal reabsorptions were also increased. However, adoptive transfer of these cells into non-pregnant mice did not elicit any pathological response.Citation76 This study demonstrates that increased activation of the TH1 population of cells causes pathophysiological symptoms exclusively during pregnancy.
In our studies with the RUPP rat model of PE, the T-cell profile is similar to that seen in PE patients.Citation66,Citation88 RUPP rats have an increase in CD4+ T helper cells characterized by increased TH17 and decreased TRegs. We demonstrate that adoptive transfer of RUPP CD4+ T-cells into normal pregnant rats increases blood pressure and reduces renal function.Citation64 Furthermore, RUPP CD4+ T-cells cause placental and renal oxidative stress and increased ET-1.Citation64,Citation66,Citation67,Citation88 We believe one mechanism stimulated by T-cells is to facilitate B-cell production of AT1-AA, as discussed in greater detail below and seen in .
Figure 1 Placental ischemia is a stimulus for chronic inflammation that leads to vasoactive factors that could play a role in later CVD in previously preeclamptic women.
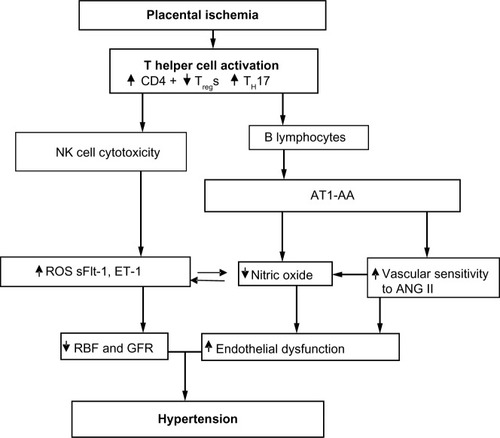
A number of clinical studies have reported that the population of TH17 cells is significantly increased in PE compared to normal pregnancy.Citation66,Citation79,Citation83,Citation89 Recent preclinical studies have begun to elucidate a role for TH17s and IL-17 to cause much of the pathophysiology observed in PE.Citation66,Citation90–Citation92 TH17 cells are distinguished from other populations of CD4+ T-cells by their secretion of IL-17, surface expression of the IL-23 receptor, and intracellular expression of the lineage-specific transcription factor retinoic acid receptor-related organ receptor (RORγ).Citation93
Studies have demonstrated that the increased inflammation observed during PE persists. Vitoratos et al demonstrated that women with PE remained under inflammatory stress up to 12–14 weeks postpartum.Citation94 A later study by Kvehaugen et al showed evidence of chronic systemic inflammation and persistent endothelial dysfunction 5–8 years postpartum in women and their offspring after PE.Citation95 These data suggest that persistent inflammation, postpartum, may play a role in future cardiovascular disease (CVD) risk in women with PE. Furthermore, a preclinical study by Pruthi et al demonstrated that exposure to experimental PE led to increased vascular damage after injury compared with normal pregnancy in mice.Citation55 It is possible that the vascular dysfunction observed in preeclamptic women postpartum is due to the persistent inflammation after delivery. However, more studies are still needed to determine this definitively. Additionally, studies to determine if inflammatory CD4+ helper T-cell populations remain elevated postpartum in preeclamptic women should be performed.
A number of clinical studies demonstrate that in contrast to the increased population of inflammatory cells, such as TH17s, a decrease in the number of CD4+ TRegs occurs in preeclamptic women compared with their normal pregnant counterparts.Citation83,Citation96–Citation99 CD4+ TRegs are classically identified by surface expression of CD4 and CD25, as well as intracellular expression of the transcription factor forkhead box P3 (FoxP3).Citation100,Citation101 TRegs are responsible for maintenance of maternal immune tolerance during pregnancy.Citation102–Citation105 Maternal immune tolerance is dependent on TRegs and uterine NK cells recognizing and accepting the fetal antigens and facilitating placental growth. Breakdown in maternal-fetal tolerance is thought to be a mediating factor in PE development and pathology. Previous studies have shown that depletion of TRegs prior to conception or in early pregnancy resulted in implantation failure and increased resorptions.Citation102,Citation105 Therefore, the importance of TRegs in the maintenance of early pregnancy has previously been established. However, TReg maintenance of proper immune function may be important in mid to late pregnancy, as well.
Because of the roles of the over-stimulated immune response in causing the pathophysiology of PE, proper immune regulation by TRegs may be important to blunt the inflammatory mediators leading to vasoactive pathways that can cause hypertension and PE symptoms. CD4+ TRegs may inhibit endogenous activation of the pro-inflammatory TH1 and TH17 cells leading to decreased inflammation and oxidative stress. Without T-cell activation, inflammatory cytokine production would be inhibited, resulting in fewer inflammatory cells and less production of reactive oxygen species.
To date, no studies have investigated the possibility of a full long-term autoimmune response after PE involving CD4+ T-cells or B-cells. Furthermore, very few studies have investigated the risk of PE leading to other autoimmune diseases later in life. To date, two such studies have determined a slightly increased risk for rheumatoid arthritis (RA) in women who were previously diagnosed with PE. A national cohort study in Denmark examined the possible impact of live births, pregnancy loss, and pregnancy complications on the risk for the development of RA later in life. These studies in Danish women found the rate ratio of RA to be 1.42 in women previously diagnosed with PE compared to women with normal pregnancies, while a recent update reported the hazard ratio of RA in women diagnosed with PE to be 1.96 compared to 1.18 in women diagnosed with gestational hypertension, without PE.Citation106,Citation107 Although these hazard ratios are relatively small, the dysregulation of the preeclamptic woman’s immune system during pregnancy that leads to PE may be related to the subsequent risk for RA. Similarly there remains a need for studies to investigate an association between PE and increased risk for other autoimmune disorders, especially those that disproportionally affect women and are associated with cardiovascular disease, such as systemic lupus erythematosus and psoriasis.
NK cells
NK cells make up 10% of lymphocytes in human peripheral blood and are identified by their expression of CD56 in the absence of CD3. NK cells can differentiate into two distinct subsets, type 1 (NK1) or type 2 (NK2). The NK1 subset is characterized by its release of IFN-γ and potent cytolytic activity upon activation.Citation58,Citation108 IL-2 and IL-12 signaling promote differentiation of NK cells to the NK1 subset and are secreted by CD4+ T helper cells. CD4+ TRegs can inhibit IL-2 mediated differentiation by decreasing availability of the cytokine.Citation109,Citation110 It has also been demonstrated that IL-17 may enhance cytolytic activity of NK cells, suggesting that TH17 cells may play a role in mediating differentiation into the NK1 population subset.Citation111 PE is associated with a shift in the NK cell population from NK2 to NK1 (type 1 shift). A prominence of NK1 cells secreting tumor necrosis factor alpha (TNF-α) has previously been shown in preeclamptic women.Citation112 NK-mediated killing of fetal trophoblasts can contribute to the shallow trophoblast invasion that results in insufficient spiral artery remodeling. Thereby promoting development of the ischemic placenta, leading to the release of vasoactive factors mediating maternal vascular dysfunction. Recent studies in mice suggest a role for cytotoxic NK cells in Angiotensin II (ANGII) mediated vascular dysfunction and artherosclerosis.Citation113–Citation115 Additionally, a study in humans demonstrated an association with NK cells and increased plaque rupture.Citation113,Citation116 The role of the cytolytic NK1 cell population in the development and pathophysiology of PE and other cardiovascular disease is poorly understood and warrants further investigation.
Neutrophils
Neutrophils are activated in response to the placental hypoxia and inflammation that occurs during PE. These cells can also be recruited and activated by increased IL-17.Citation89 These cells may contribute to the increased vascular resistance and fetal morbidity of PE through production of oxidative stress and the release of neutrophil extracellular traps (NETs).Citation89,Citation117 NETs are extracellular structures released from neutrophils that kill extracellular bacteria and fungi, but are implicated in pathogenesis of inflammatory disorders and autoimmunity.Citation89,Citation117 Oxidative stress-induced inflammation and vascular damage and dysfunction may be attributable in part to the activation of neutrophils and subsequent release of NETs in response to hypoxia and IL-17 signaling during PE and requires further study.Citation89,Citation117
AT1-AA
AT1-AAs are elevated in women with PE.Citation118,Citation119 AT1-AAs bind with a high affinity to the 7 amino acid sequence on the second extracellular loop of the AT1R. Binding of the AT1-AA to the AT1R, increases AT1 receptor activity, intracellular calcium levels, and activation of intracellular mitogen activated protein kinase/extracellular signal regulated kinases (MAPK/ERK) pathways.Citation120–Citation122
AT1-AAs are hypothesized to be generated from an immunological loss of self-tolerance toward the AT1R, which results in the accumulation of antibodies against the AT1R. AT1-AAs are also elevated in normotensive pregnancies with uterine growth-restricted fetuses, kidney transplant recipients, and patients with systemic sclerosis, vasculopathy, tissue fibrosis, hypertension, renovascular disease, and pregnant women with hemolysis, elevated liver enzymes and low platelets (HELLP syndrome).Citation123–Citation127
In 1999, Wallukat et al was the first to identify AT1-AAs in women with PE and not in healthy pregnant women or in women with pre-existing essential hypertension and pregnancy.Citation118 In this study, the AT1-AAs were isolated by affinity column antibody purification techniques and used to stimulate cultured neonatal rat cardiomyocytes. However, in the presence of losartan, an AT1R blocker, purified AT1-AAs from preeclamptic patients were not able to stimulate cultured cardiomyocytes. Thus, this study was the first to identify that the actions of the AT1-AA on cardiomyocytes are facilitated through AT1-AA binding to the AT1R.
In vitro experiments with the AT1-AA on vascular smooth muscle cells and trophoblast cells increased reactive oxygen species production, nicotinamide adenine dinucleotide phosphate oxidase components, and activation of nuclear factor kappa-light-chain-enhancer of activated B cells.Citation128 Human coronary artery vascular smooth muscle cells incubated with AT1-AAs increased tissue factor levels, which are elevated in the placenta of preeclamptic patients.Citation122 AT1-AAs administered to human trophoblast cells, increased the secretion of plasminogen activator inhibitor-1 and decreased trophoblast invasiveness.Citation129 Together these effects on the trophoblast cells suggest a mechanism of poor placental placentation, a possible cause of PE. Human mesangial cells incubated with the AT1-AA increased plasminogen activator inhibitor-1 and IL-6 secretions.Citation130 AT1-AAs cultured with cardiomyocytes, increased the beating rate of cardiomyocytes and apoptosis of cardiomyocytes via a TNF-α pathway.Citation131,Citation132 Furthermore, we have shown that human umbilical vein endothelial cells (HUVECs) incubated 6 hours with ANGII (10−7 M) and AT1-AAs drastically increased ET-1 protein expression.Citation133 This ∼100 fold increase in ET-1 expression was absent in HUVECs incubated with ANGII or AT1-AAs alone, which suggest a possible enhancement of ANGII signaling when AT1-AAs are present.Citation133 Therefore the increase in ET-1 expression from HUVECs may play a significant role in hypertension and enhanced ANGII sensitivity during PE. The results from the HUVEC study further emphasize the importance of ET-1 expression in PE, where there is increased AT1-AA production and hypertension. In an earlier study we showed that blockade of ETa receptor in rats administered the AT1-AA and ANGII displayed a decrease in blood pressure.Citation63 Thus this in vitro HUVEC data taken along with the in vivo data strongly supports the notion that ET-1 is a major mediator of hypertension during pregnancy.
Animal models of PE, such as the RUPP model, the transgenic human angiotensinogen and renin gene pregnant rat model, the adoptive transfer of CD4+ T lymphocytes from placental ischemic rats to normal pregnant rats, and the cytokine (TNF-α, IL-6, and IL-17) administration during pregnancy model, display elevations in AT1-AAs during pregnancy.Citation64,Citation90,Citation119,Citation134–Citation138 Isolated human and rodent AT1-AAs administered to rats during pregnancy increase several circulating factors associated with PE, such as sFlt-1, sEng, oxidative stress, ET-1, and endothelial microparticles (EMPs).Citation63,Citation134,Citation136,Citation139,Citation140 EMPs are small endothelial cell debris fragments that are elevated in women with PE and serve as a marker of endothelial cell dysfunction. The mechanism by which AT1-AA increases EMPs is through the p38 MAP kinase signaling transduction pathway.Citation120 Furthermore AT1-AAs administered to pregnant rats on day 12 or 13 of pregnancy exhibited a marked increase in blood pressure on day 19 of pregnancy.Citation63,Citation134,Citation136,Citation140 The rats also display and increase in placental oxidative stress, and ET-1 expression in the kidney cortex, aorta, and placenta.Citation63,Citation90,Citation134,Citation140
The mechanism of increased ANGII sensitivity in PE is unknown. However, we hypothesize that AT1-AAs play an important role in increasing ANGII sensitivity in PE. Studies from our lab have shown that acute ANGII along with AT1-AA infusion during a rat pregnancy increased the blood pressure ∼40 mmHg above AT1-AA administered alone.Citation133,Citation134 In addition, chronic ANGII and AT-AAs together increase blood pressure, oxidative stress, and ET-1 secretion and increases the renal artery resistive index above ANGII or AT1-AA administered separately.Citation133,Citation134
One mechanism by which the AT1-AA can increase ANGII sensitivity may be by altering the binding affinity of ANGII to the AT1R. Preliminary studies from our lab have shown that AT1-AAs increase (∼15-fold) the binding affinity of ANGII to the AT1Rs on HUVECs incubated with fluorescent labeled ANGII and human AT1-AAs isolated from preeclamptic patients for 1 hour. This increase in ANGII binding was also correlated with an increase in ET-1 secretion. Another proposed mechanism by which AT1-AAs increase ANGII affinity to AT1R is via increasing the dimerization of the AT1 receptors.Citation141 Multiple studies have shown an increase in AT1R responsiveness to ANGII, when the AT1R heterodimerizes with the vasodepressor bradykinin receptor (B2).Citation141–Citation143 AbdAlla et al showed that women with PE have increased B2 protein amounts and AT1/B2 heterodimerization in platelets and the omental vessels of preeclamptic patients.Citation141–Citation143 Therefore it can be postulated, that the process by which the AT1-AAs increase ANGII sensitivity in PE is mediated by AT1R receptor dimerization, however, more studies are needed to verify this hypothesis.
Studies by Hubel et al show that ∼18% of postpartum preeclamptic women have elevated circulating AT1-AAs 1 year after delivery.Citation144 These women with elevated AT1-AAs have elevated sFlt-1 levels, decreased VEGF, and increased insulin sensitivity.Citation144 The correlation between increased circulating AT1-AAs and these variables may suggest a mechanism by which women with PE have an increased risk of cardiovascular events later in life. Of caution, this study only consisted of 64 women (35 controls and 29 preeclamp-tic women), in which ∼18% (five out of 29) of postpartum preeclamptic women had elevated circulating AT1-AAs thereby indicating the importance of studies determining the significance of AT1-AAs with larger populations of postpartum preeclamptic women.
Rats administered AT1-AA during pregnancy displayed no differences in cardiac function as compared with normal pregnant rats 16 weeks postpartum in a study conducted by Wang et al.Citation145 However in this study, there were several morphological changes in cardiomyocytes, increased collagen content, and changes in the myocardium structure of rats administered the AT1-AA during pregnancy.Citation145 These changes were prevented when losartan was administered along with AT1-AA during pregnancy.Citation145 These data taken together suggest that the AT1-AA may play a pivotal role in the development or pathogenesis of cardiovascular dysfunction and CVDs in the mother.
PE has long-term effects on the offspring
Not only does PE have a long-term effect on the cardiovascular system of the mother later in life, but it also has an effect on the cardiovascular system of the offspring later in life. PE is one the major contributors to preterm and low birth weight babies. It has been known for several years, especially with the development of the Barker hypothesis, that fetal adaptions during pregnancy, such as malnutrition caused by hypertension and/or placental ischemia during pregnancy, increase the offspring’s risk of developing hypertension, stroke, diabetes, and CVDs later in life.Citation146,Citation147 Furthermore, several recent meta-analysis studies have shown that for each 1 kg increase in birth-weight, there is a 9%–15% decrease in cardiovascular mortality and 2–4 mmHg decrease in systolic blood pressure in adulthood.Citation147 The offspring of women with PE have elevated blood pressure, body mass index, and increased triglycerides and cholesterol content in both adolescence and adulthood.Citation147,Citation148 A meta-analysis study conducted by Davis et al in 2011, examined over 18 cohorts of ∼45,000 women with normal and hypertensive pregnancies. In this study they discovered an increase of 2.39 mmHg in systolic pressure, 1.35 mmHg in diastolic pressure, and an 0.32 kg/m2 increase in body mass index in the offspring (4–30 years old) of preeclamptic mothers versus the offspring of normal pregnant women.Citation148
Conclusion
The clinical symptoms of PE can be resolved after delivery of the placenta, however women and their offspring affected by PE have double the risk for subsequent cardiovascular complications such as heart disease, stroke, and venous thromboembolism over the 5–15 years after delivery and these women have greater risks of dying from cerebrovascular disease after pregnancy than women who had a healthy pregnancy.Citation149–Citation151 A more recent study examined the incidence of long-term atherosclerotic morbidity in preeclamptic women and the risk is greater for patients with severe and recurrent episodes of PE.Citation152 Moreover, endothelial dysfunction is a common feature of pregnancies with PE, atherosclerosis, and cardiovascular disease and thus, endothelial dysfunction could serve as an underlying mechanism in the development of cardiovascular disease in preeclamptic women or their offspring. Furthermore, PE women exhibit chronic inflammation indicative of immunological memory with the activation of CD4+ T helper cells and the secretion of specialized IgG in the form of the AT1-AA. The prevalence of AT1-AA, immunological memory with NK or T-cells and the sequela of endothelial dysfunction could all mediate the development of CVD or cardiovascular events later in life among PE women. In addition, because of this prolonged chronic inflammation, PE women may be at risk for autoimmune diseases later in life. In recent years, a number of small studies or cases have been published that suggest use of the monoclonal antibody, rituximab, for the treatment of lymphoma or autoimmune disease is safe during pregnancy.Citation153–Citation159 However, these same groups performed follow-up studies that identified the drug within the nasal cavity of newborns born to pregnant macaques treated during pregnancy. Therefore, this avenue of treatment may prove to be deleterious for the immune development during the first few months of life of the newborn. With the development of immune suppression therapies proving successful for the treatment of cancer and lymphomas, if we can prove they are safe for both mother and infant ante and postpartum, should we consider the use of such therapies for PE women?
Acknowledgments
This research was supported by National Institutes of Health grants HL105324, HL124715, HL51971, HL78147, and HD067541.
Disclosure
The authors have no conflicts of interest to disclose.
References
- RobertsJMGammillHSPreeclampsia: recent insightsHypertension20054661243124916230510
- GilbertJSRyanMJLaMarcaBBSedeekMMurphySRGrangerJPPathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunctionAm J Physiol Heart Circ Physiol20082942H541H55018055511
- GrangerJPAlexanderBTBennettWAKhalilRAPathophysiology of pregnancy-induced hypertensionAm J Hypertens2001146 Pt 2178S185S11411754
- GrangerJPAlexanderBTLlinasMTBennettWAKhalilRAPathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunctionMicrocirculation20029314716012080413
- NorisMPericoNRemuzziGMechanisms of disease: Pre-eclampsiaNat Clin Pract Nephrol2005129811416932375
- RedmanCWSargentILLatest advances in understanding preeclampsiaScience200530857281592159415947178
- SankaralingamSArenasIALaluMMDavidgeSTPreeclampsia: current understanding of the molecular basis of vascular dysfunctionExpert Rev Mol Med20068312016438753
- SibaiBDekkerGKupfermincMPre-eclampsiaLancet2005365946178579915733721
- BrennanLJMortonJSDavidgeSTVascular dysfunction in preeclampsiaMicrocirculation201421141423890192
- GoulopoulouSDavidgeSTMolecular mechanisms of maternal vascular dysfunction in preeclampsiaTrends Mol Med2015212889725541377
- SandrimVCPaleiACMetzgerIFGomesVACavalliRCTanus-SantosJENitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endogline in preeclampsiaHypertension200852240240718574068
- MurphySRLaMarcaBBParrishMCockrellKGrangerJPControl of soluble fms-like tyrosine-1 (sFlt-1) production response to placental ischemia/hypoxia: role of tumor necrosis factor-alphaAm J Physiol Regul Integr Comp Physiol20133042R130R13523193111
- WangARanaSKarumanchiSAPreeclampsia: the role of angiogenic factors in its pathogenesisPhysiology (Bethesda)20092414715819509125
- SerranoNCCasasJPDiazLAEndothelial NO synthase genotype and risk of preeclampsia: a multicenter case-control studyHypertension200444570270715364897
- KinneyMVLawnJEHowsonCPBelizanJ15 Million preterm births annually: what has changed this year?Reprod Health201292823148557
- ChapmanABAbrahamWTZamudioSTemporal relationships between hormonal and hemodynamic changes in early human pregnancyKidney Int1998546205620639853271
- GarovicVDHaymanSRHypertension in pregnancy: an emerging risk factor for cardiovascular diseaseNat Clin Pract Nephrol200731161362217957198
- AhmedRDunfordJMehranRRobsonSKunadianVPre-eclampsia and future cardiovascular risk among women: a reviewJ Am Coll Cardiol201463181815182224613324
- Muller-DeileJSchifferMPreeclampsia from a renal point of view: Insides into disease models, biomarkers and therapyWorld J Nephrol20143416918125374810
- GibsonHMPlasma volume and glomerular filtration rate in pregnancy and their relation to differences in fetal growthJ Obstet Gynaecol Br Commonw19738012106710744761381
- VikseBEIrgensLMLeivestadTSkjaervenRIversenBMPreeclampsia and the risk of end-stage renal diseaseN Engl J Med2008359880080918716297
- GarovicVDWagnerSJTurnerSTUrinary podocyte excretion as a marker for preeclampsiaAm J Obstet Gynecol20071964320.e1e717403404
- CraiciIMWagnerSJBaileyKRPodocyturia predates proteinuria and clinical features of preeclampsia: longitudinal prospective studyHypertension20136161289129623529165
- AitaKEtohMHamadaHAcute and transient podocyte loss and proteinuria in preeclampsiaNephron Clin Pract20091122c65c7019390204
- MatsubaraKMatsubaraYHyodoSKatayamaTItoMRole of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsiaJ Obstet Gynaecol Res201036223924720492372
- RobertsJMVon Versen-HoeynckFMaternal fetal/placental interactions and abnormal pregnancy outcomesHypertension2007491151617116760
- ShaamashAHElsnosyEDMakhloufAMZakhariMMIbrahimOAHM EL-dMaternal and fetal serum nitric oxide (NO) concentrations in normal pregnancy, pre-eclampsia and eclampsiaInt J Gynaecol Obstet200068320721410699190
- MurphySRLaMarcaBCockrellKAranyMGrangerJPL-arginine supplementation abolishes the blood pressure and endothelin response to chronic increases in plasma sFlt-1 in pregnant ratsAm J Physiol Regul Integr Comp Physiol20123022R259R26322071155
- SandrimVCMontenegroMFPaleiACIncreased circulating cell-free hemoglobin levels reduce nitric oxide bioavailability in preeclampsiaFree Radic Biol Med201049349350020510352
- PimentelAMPereiraNRCostaCAL-arginine-nitric oxide pathway and oxidative stress in plasma and platelets of patients with pre-eclampsiaHypertens Res201336978378823575380
- MolnarMSutoTTothTHertelendyFProlonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, pro-teinuria, thrombocytopenia, and intrauterine growth retardationAm J Obstet Gynecol19941705 Pt 1145814667909994
- VosatkaRJHassounPMHarvey-WilkesKBDietary L-arginine prevents fetal growth restriction in ratsAm J Obstet Gynecol199817822422469500481
- NeriIMazzaVGalassiMCVolpeAFacchinettiFEffects of L-arginine on utero-placental circulation in growth-retarded fetusesActa Obstet Gynecol Scand19967532082128607330
- MoncadaSHiggsAThe L-arginine-nitric oxide pathwayN Engl J Med199332927200220127504210
- MoncadaSHiggsEAThe discovery of nitric oxide and its role in vascular biologyBr J Pharmacol2006147Suppl 1S193S20116402104
- NelsonSHSteinslandOSWangYYallampalliCDongYLSanchezJMIncreased nitric oxide synthase activity and expression in the human uterine artery during pregnancyCirc Res200087540641110969039
- SandrimVCPaleiACCavalliRCeNOS haplotypes associated with gestational hypertension or preeclampsiaPharmacogenomics J200891014671473
- SandrimVCPaleiACLuizonMRIzidoro-ToledoTCCavalliRCTanus-SantosJEeNOS haplotypes affect the responsiveness to antihypertensive therapy in preeclampsia but not in gestational hypertensionPharmacogenomics J2010101404519704415
- NorisMTodeschiniMCassisPL-arginine depletion in preeclampsia orients nitric oxide synthase toward oxidant speciesHypertension200443361462214744923
- EleuterioNMPaleiACRangel MachadoJSTanus-SantosJECavalliRCSandrimVCRelationship between adiponectin and nitrite in healthy and preeclampsia pregnanciesClin Chim Acta201342311211523643962
- SandrimVCPaleiACMetzgerIFCavalliRCDuarteGTanus-SantosJEInterethnic differences in ADMA concentrations and negative association with nitric oxide formation in preeclampsiaClin Chim Acta201041119–201457146020570587
- MaynardSEMinJYMerchanJExcess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsiaJ Clin Invest2003111564965812618519
- SankaralingamSXuHDavidgeSTArginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsiaCardiovasc Res201085119420319684035
- AlpoimPNGodoiLCFreitasLGGomesKBDusseLMAssessment of L-arginine asymmetric 1 dimethyl (ADMA) in early-onset and late-onset (severe) preeclampsiaNitric Oxide201333818223876347
- Mutlu-TurkogluUAykac-TokerGIbrahimogluLAdemogluEUysalMPlasma nitric oxide metabolites and lipid peroxide levels in preeclamptic pregnant women before and after deliveryGynecol Obstet Invest199948424725010592426
- AlexanderBTKassabSEMillerMTReduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxideHypertension20013741191119511304523
- CrewsJKHerringtonJNGrangerJPKhalilRADecreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant ratHypertension2000351 Pt 236737210642326
- WalshSKEnglishFAJohnsEJKennyLCPlasma-mediated vascular dysfunction in the reduced uterine perfusion pressure model of preeclampsia: a microvascular characterizationHypertension200954234535119564546
- AmaralLMCorneliusDCHarmonAMoseleyJMartinJNJrLaMarcaB17-hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat modelHypertension201565122523125368030
- LamarcaBEndothelial dysfunction. An important mediator in the pathophysiology of hypertension during pre-eclampsiaMinerva Ginecol201264430932022728575
- CartyDMAndersonLADuncanCNPeripheral arterial tone: assessment of microcirculatory function in pregnancyJ Hypertens201230111712322052066
- SpasojevicMSmithSAMorrisJMGalleryEDPeripheral arterial pulse wave analysis in women with pre-eclampsia and gestational hypertensionBJOG2005112111475147816225565
- ClarkDESmithSKHeYA vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulationBiol Reprod1998596154015489828203
- MurphySRLaMarcaBBCockrellKGrangerJPRole of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant ratsHypertension201055239439820026766
- PruthiDKhankinEVBlantonRMExposure to experimental preeclampsia in mice enhances the vascular response to future injuryHypertension201565486387025712723
- NooriMDonaldAEAngelakopoulouAHingoraniADWilliamsDJProspective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertensionCirculation2010122547848720644016
- WolfMHubelCALamCPreeclampsia and future cardiovascular disease: potential role of altered angiogenesis and insulin resistanceJ Clin Endocrinol Metab200489126239624315579783
- VaccaPMorettaLMorettaAMingariMCOrigin, phenotype and function of human natural killer cells in pregnancyTrends Immunol2011321151752321889405
- CidMCKleinmanHKGrantDSSchnaperHWFauciASHoffmanGSEstradiol enhances leukocyte binding to tumor necrosis factor (TNF)-stimulated endothelial cells via an increase in TNF-induced adhesion molecules E-selectin, intercellular adhesion molecule type 1, and vascular cell adhesion molecule type 1J Clin Invest199493117257506711
- GrangerJPAlexanderBTLlinasMTBennettWAKhalilRAPathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunctionHypertension2001383 Pt 271872211566964
- KeiserSDVeillonEWParrishMREffects of 17-hydroxypro-gesterone on tumor necrosis factor-alpha-induced hypertension during pregnancyAm J Hypertens200922101120112519745821
- LaMarcaBBCockrellKSullivanEBennettWGrangerJPRole of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant ratsHypertension2005461828615928030
- LaMarcaBParrishMRayLFHypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1Hypertension200954490590919704104
- NovotnySRWallaceKHeathJActivating autoantibodies to the angiotensin II type I receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic ratsAm J Physiol Regul Integr Comp Physiol201230210R1197R120122461177
- TaylorRNde GrootCJChoYKLimKHCirculating factors as markers and mediators of endothelial cell dysfunction in preeclampsiaSemin Reprod Endocrinol199816117319654604
- WallaceKRichardsSDhillonPCD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancyHypertension201157594995521464392
- WallaceKNovotnySHeathJHypertension in response to CD4(+) T cells from reduced uterine perfusion pregnant rats is associated with activation of the endothelin-1 systemAm J Physiol Regul Integr Comp Physiol20123032R144R14922647295
- WuCFHuangFDSuiRFSunJXPreeclampsia serum upregulates CD40/CD40L expression and induces apoptosis in human umbilical cord endothelial cellsReprod Biol Endocrinol2012102822510585
- ZhouCCIraniRADaiYAutoantibody-mediated IL-6-dependent endothelin-1 elevation underlies pathogenesis in a mouse model of preeclampsiaJ Immunol2011186106024603421482739
- BenyoDFMilesTMConradKPHypoxia stimulates cytokine production by villous explants from the human placentaJ Clin Endocrinol Metab1997825158215889141553
- ConradKPBenyoDFPlacental cytokines and the pathogenesis of preeclampsiaAm J Reprod Immunol19973732402499127646
- RobertsJMPearsonGCutlerJLindheimerMPregnancy NWGoRoHDSummary of the NHLBI Working Group on Research on Hypertension During PregnancyHypertension200341343744512623940
- RobertsJMLainKYRecent Insights into the pathogenesis of pre-eclampsiaPlacenta200223535937212061851
- LamarcaBThe role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsiaMinerva Ginecol201062210512020502423
- LaMarcaBBBennettWAAlexanderBTCockrellKGrangerJPHypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alphaHypertension20054641022102516144982
- ZenclussenACFestSJoachimRKlappBFArckPCIntroducing a mouse model for pre-eclampsia: adoptive transfer of activated Th1 cells leads to pre-eclampsia-like symptoms exclusively in pregnant miceEur J Immunol200434237738714768042
- SaitoSUmekageHSakamotoYIncreased T-helper-1-type immunity and decreased T-helper-2-type immunity in patients with preeclampsiaAm J Reprod Immunol199941529730610378024
- SaitoSSakaiMSasakiYTanebeKTsudaHMichimataTQuantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsiaClin Exp Immunol1999117355055510469061
- ToldiGRigoJJrStenczerBVasarhelyiBMolvarecAIncreased prevalence of IL-17-producing peripheral blood lymphocytes in pre-eclampsiaAm J Reprod Immunol201166322322921306467
- SaitoSNakashimaAShimaTItoMTh1/Th2/Th17 and regulatory T-cell paradigm in pregnancyAm J Reprod Immunol201063660161020455873
- SaitoSTh17 cells and regulatory T cells: new light on pathophysiology of preeclampsiaImmunol Cell Biol201088661561720498674
- JianjunZYaliHZhiqunWMingmingZXiaZImbalance of T-cell transcription factors contributes to the Th1 type immunity predominant in pre-eclampsiaAm J Reprod Immunol2010631384519912158
- Darmochwal-KolarzDKludka-SternikMTabarkiewiczJThe predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsiaJ Reprod Immunol2012932758122370101
- El-KabarityRHNaguibAHSerum levels of IL-18, IL-12 and TH-1/TH-2 ratio in patients with pre-eclampsiaEgypt J Immunol20111811823082474
- DongMHeJWangZXieXWangHPlacental imbalance of Th1-and Th2-type cytokines in preeclampsiaActa Obstet Gynecol Scand200584878879316026406
- SaitoSSakaiMTh1/Th2 balance in preeclampsiaJ Reprod Immunol200359216117312896820
- BorzychowskiAMCroyBAChanWLRedmanCWSargentILChanges in systemic type 1 and type 2 immunity in normal pregnancy and pre-eclampsia may be mediated by natural killer cellsEur J Immunol200535103054306316134082
- WallaceKCorneliusDCScottJCD4+ T cells are important mediators of oxidative stress that cause hypertension in response to placental ischemiaHypertension20146451151115825259742
- Laresgoiti-ServitjeEA leading role for the immune system in the pathophysiology of preeclampsiaJ Leukoc Biol201394224725723633414
- DhillionPWallaceKHerseFIL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancyAm J Physiol Regul Integr Comp Physiol20123034R353R35822718806
- CorneliusDCWallaceKKippronoLDhillionPMoseleyJLamarcaBEndothelin-1 is not a Mechanism of IL-17 Induced Hypertension during PregnancyMed J Obstet Gynecol201311pii:1006
- CorneliusDCHoggJPScottJAdministration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancyHypertension20136261068107324060899
- MaddurMSMiossecPKaveriSVBayryJTh17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategiesAm J Pathol2012181181822640807
- VitoratosNEconomouEIavazzoCPanoulisKCreatsasGMaternal serum levels of TNF-alpha and IL-6 long after delivery in preeclamptic and normotensive pregnant womenMediators Inflamm2010201090864921253506
- KvehaugenASDechendRRamstadHBTroisiRFugelsethDStaffACEndothelial function and circulating biomarkers are disturbed in women and children after preeclampsiaHypertension2011581636921606387
- ToldiGSaitoSShimaTThe frequency of peripheral blood CD4+ CD25high FoxP3+ and CD4+ CD25- FoxP3+ regulatory T cells in normal pregnancy and pre-eclampsiaAm J Reprod Immunol201268217518022510013
- TilburgsTRoelenDLvan der MastBJDifferential distribution of CD4(+)CD25(bright) and CD8(+)CD28(−) T-cells in decidua and maternal blood during human pregnancyPlacenta200627Suppl AS47S5316442616
- Santner-NananBPeekMJKhanamRSystemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsiaJ Immunol2009183117023703019915051
- PrinsJRBoelensHMHeimwegJPreeclampsia is associated with lower percentages of regulatory T cells in maternal bloodHypertens Pregnancy200928330031119412837
- GeigerTLTauroSNature and nurture in Foxp3(+) regulatory T cell development, stability, and functionHum Iimmunol2012733232239
- PetersonRARegulatory T-cells: diverse phenotypes integral to immune homeostasis and suppressionToxicol Pathol201240218620422222887
- ShimaTSasakiYItohMRegulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic miceJ Reprod Immunol201085212112920439117
- BettiniMVignaliDARegulatory T cells and inhibitory cytokines in autoimmunityCurr Opin Immunol200921661261819854631
- ArckPCHecherKFetomaternal immune cross-talk and its consequences for maternal and offspring’s healthNat Med201319554855623652115
- AluvihareVRKallikourdisMBetzAGRegulatory T cells mediate maternal tolerance to the fetusNat Immunol20045326627114758358
- JorgensenKTPedersenBVJacobsenSBiggarRJFrischMNational cohort study of reproductive risk factors for rheumatoid arthritis in Denmark: a role for hyperemesis, gestational hypertension and pre-eclampsia?Ann Rheum Dis201069235836319289384
- JorgensenKTHarpsoeMCJacobsenSJessTFrischMIncreased risk of rheumatoid arthritis in women with pregnancy complications and poor self-rated health: a study within the Danish National Birth CohortRheumatology (Oxford)20145381513151924692576
- PerittDRobertsonSGriGShoweLAste-AmezagaMTrinchieriGCutting Edge: Differentiation of Human NK Cells into NK1 and NK2 SubsetsThe Journal of Immunology199816111582158249834059
- KerdilesYUgoliniSVivierET cell regulation of natural killer cellsJ Exp Med201321061065106823733834
- KatsumotoTKimuraMYamashitaMSTAT6-dependent differentiation and production of IL-5 and IL-13 in murine NK2 cellsJ Immunol200417384967497515470039
- Al OmarSFlanaganBFAlmehmadiMChristmasSEThe effects of IL-17 upon human natural killer cellsCytokine201362112313023490420
- FukuiAYokotaMFunamizuAChanges of NK cells in preeclampsiaAm J Reprod Immunol201267427828622364232
- HedrickCCLymphocytes in atherosclerosisArterioscler Thromb Vasc Biol201535225325725609772
- SelathuraiADeswaerteVKanellakisPNatural killer (NK) cells augment atherosclerosis by cytotoxic-dependent mechanismsCardiovasc Res2014102112813724469537
- KossmannSSchwenkMHausdingMAngiotensin II-induced vascular dysfunction depends on interferon-gamma-driven immune cell recruitment and mutual activation of monocytes and NK-cellsArterioscler Thromb Vasc Biol20133361313131923520167
- Martinez-RodriguezJEMunne-ColladoJRasalRExpansion of the NKG2C+ natural killer-cell subset is associated with high-risk carotid atherosclerotic plaques in seropositive patients for human cytomegalovirusArterioscler Thromb Vasc Biol201333112653265923968979
- GuptaAKHaslerPHolzgreveWHahnSNeutrophil NETs: a novel contributor to preeclampsia-associated placental hypoxia?Semin Immunopathol200729216316717621701
- WallukatGHomuthVFischerTPatients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptorJ Clin Invest1999103794595210194466
- HerseFLaMarcaBAngiotensin II type 1 receptor autoantibody (AT1-AA)-mediated pregnancy hypertensionAm J Reprod Immunol201369441341823279165
- YangSZhongQQiuZAngiotensin II receptor type 1 autoantibodies promote endothelial microparticles formation through activating p38 MAPK pathwayJ Hypertens201432476277024609215
- ThwayTMShlykovSGDayMCAntibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activationCirculation2004110121612161915381659
- DechendRHomuthVWallukatGAT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factorCirculation2000101202382238710821814
- DragunDMullerDNBrasenJHAngiotensin II type 1-receptor activating antibodies in renal-allograft rejectionN Engl J Med2005352655856915703421
- FuMLHerlitzHSchulzeWAutoantibodies against the angiotensin receptor (AT1) in patients with hypertensionJ Hypertens200018794595310930193
- LiaoYHWeiYMWangMWangZHYuanHTChengLXAutoantibodies against AT1-receptor and alpha1-adrenergic receptor in patients with hypertensionHypertens Res200225464164612358154
- FischerTWallukatGSchneiderMPSchlembachDMunzWHomuthVHELLP syndrome in the 18th week of gestation in association with elevated angiotensin AT(1)-receptor autoantibodiesEur J Obstet Gynecol Reprod Biol200197225525711451561
- ZhangLCuiLMiaoGBZhaoWSWangSYLiuXLStudy of autoantibodies against the G-protein-coupled beta 2- and alpha 1-adren-ergic and AT1 receptors in patients with primary hypertensionZhongguo Yi Xue Ke Xue Yuan Xue Bao2002244367369 Chinese12905654
- DechendRViedtCMullerDNAT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidaseCirculation2003107121632163912668498
- XiaYWenHBobstSDayMCKellemsREMaternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cellsJ Soc Gynecol Investig20031028293
- BobstSMDayMCGilstrapLC3rdXiaYKellemsREMaternal autoantibodies from preeclamptic patients activate angiotensin receptors on human mesangial cells and induce interleukin-6 and plasminogen activator inhibitor-1 secretionAm J Hypertens200518333033615797649
- ChaiWZhangWJinZFengYKuangYZhiJAngiotensin II type I receptor agonistic autoantibody-induced apoptosis in neonatal rat cardiomyocytes is dependent on the generation of tumor necrosis factor-alphaActa Biochim Biophys Sin (Shanghai)2012441298499023089979
- JinZWangJZhangWZhangGJiaoXZhiJChanges in cardiac structure and function in rats immunized by angiotensin type 1 receptor peptidesActa Biochim Biophys Sin (Shanghai)2011431297097622037945
- WenzelKRajakumarAHaaseHAngiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant ratsHypertension2011581778421576625
- BrewerJLiuRLuYEndothelin-1, oxidative stress, and endogenous angiotensin II: mechanisms of angiotensin II type I receptor autoantibody-enhanced renal and blood pressure response during pregnancyHypertension201362588689224041954
- LaMarcaBWallukatGLlinasMHerseFDechendRGrangerJPAutoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant ratsHypertension20085261168117218852381
- LiJLaMarcaBReckelhoffJFA model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) modelAm J Physiol Heart Circ Physiol20123031H1H822523250
- DechendRGratzePWallukatGAgonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsiaHypertension200545474274615699466
- LamarcaBBrewerJWallaceKIL-6-induced pathophysiology during pre-eclampsia: potential therapeutic role for magnesium sulfate?Int J Interferon Cytokine Mediator Res2011201135964
- ParrishMRMurphySRRutlandSThe effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancyAm J Hypertens201023891191620431529
- ParrishMRWallaceKTam TamKBHypertension in response to AT1-AA: role of reactive oxygen species in pregnancy-induced hypertensionAm J Hypertens201124783584021472019
- XiaYKellemsREAngiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyondCirc Res20131131788723788505
- QuittererULotherHAbdallaSAT1 receptor heterodimers and angiotensin II responsiveness in preeclampsiaSemin Nephrol200424211511915017523
- AbdAllaSLotherHel MassieryAQuittererUIncreased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsivenessNat Med2001791003100911533702
- HubelCAWallukatGWolfMAgonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsiaHypertension200749361261717210828
- WangHPZhangWHWangXFExposure to AT1 receptor autoantibodies during pregnancy increases susceptibility of the maternal heart to postpartum ischemia-reperfusion injury in ratsInt J Mol Sci2014157114951150924979132
- BarkerDJFetal origins of coronary heart diseaseBr Heart J19936931951968461215
- YeungEHRobledoCBoghossianNZhangCMendolaPDevelopmental Origins of Cardiovascular DiseaseCurr Epidemiol Rep20141191625364653
- DavisEFLazdamMLewandowskiAJCardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic reviewPediatrics20121296e1552e156122614768
- PoweCELevineRJKarumanchiSAPreeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular diseaseCirculation2011123242856286921690502
- FraserANelsonSMMacdonald-WallisCAssociations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and ChildrenCirculation2012125111367138022344039
- MoscaLBenjaminEJBerraKEffectiveness-based guidelines for the prevention of cardiovascular disease in women – 2011 update: a guideline from the American Heart AssociationJ Am Coll Cardiol201157121404142321388771
- KessousRShoham-VardiIParienteGSergienkoRSheinerELong-term maternal atherosclerotic morbidity in women with pre-eclampsiaHeart2015101644244625564558
- LeeEJAhnKHHongSCLeeEHParkYKimBSRituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy for diffuse large B-cell lymphoma in pregnancy may be associated with preterm birthObstet Gynecol Sci201457652652925469343
- MandalPKDolaiTKBagchiBGhoshMKBoseSBhattacharyyaMB cell suppression in newborn following treatment of pregnant diffuse large B-cell lymphoma patient with rituximab containing regimenIndian J Pediatr201481101092109424562617
- SangleSRLutaloPMDaviesRJKhamashtaMAD’CruzDPB-cell depletion therapy and pregnancy outcome in severe, refractory systemic autoimmune diseasesJ Autoimmun6201343555923608146
- Ojeda-UribeMAfifNDahanEExposure to abatacept or rituximab in the first trimester of pregnancy in three women with autoimmune diseasesClin Rheumatol201332569570023292481
- RingelsteinMHarmelJDistelmaierFNeuromyelitis optica and pregnancy during therapeutic B cell depletion: infant exposure to anti-AQP4 antibody and prevention of rebound relapses with low-dose rituximab postpartumMult Scler201319111544154723886825
- SarnoMAMancariRAzimHAJrColomboNPeccatoriFAAre monoclonal antibodies a safe treatment for cancer during pregnancy?Immunotherapy20135773374123829624
- BurnetteBLJentoftMAPorrataLFBoyceTGWitzigTESingle-agent rituximab for primary CNS lymphoma during pregnancy as a bridge to definitive managementJ Clin Oncol2014327e14e1724395859
