Abstract
The MitraClip (MC) system is a device for percutaneous, transseptal edge-to-edge reconstruction of the mitral valve (MV) in patients with severe mitral regurgitation (MR) not eligible for surgery. Recently, a number of studies have underlined the therapeutic benefit of the MC system for patients with extreme and high risk for MV surgery suffering from either degenerative or functional MR. The MC procedure shows negligible intraprocedural mortality, low periprocedural complication rates, and a significant reduction in MR, as well as an improvement in functional capacity and most importantly quality of life. Presently, the MC system has become an additional interventional tool in the concert of surgical methods. It hereby enlarges the spectrum of MV repair for the Heart Team. Lately, many reviews focused on the MC system. The current review describes the developments in the treatment of MR with the MC system.
Introduction
With increasing age, the prevalence of mitral regurgitation (MR) is rapidly growing.Citation1 Aging of the world’s population challenges health care professionals worldwide to develop new and less invasive treatment options for the elderly.
The MitraClip (MC) system is by now an established interventional therapy for severe MR in a very selective group of patients with high or extreme risk for conventional surgery (class IIB, evidence class C, recommendation, European Guidelines)Citation2 – namely the elderly, patients with multiple comorbidities, and/or patients with severely reduced ejection fraction.
Mitral valve repair (MVR) – despite the absence of randomized trials – is the gold standard for the treatment of severe MR. If repair is not suitable, mitral valve replacement (MVRx) is the only surgical option.Citation3 In young and low surgical risk patients, results are excellent; the mortality rate for MVR is low with 1.4%, and for MVRx with 1.6%. However, in octogenerians and patients with high surgical risk, 30-day mortality has been shown to be substantially higher with 11.0% for MVR and 18.9% for MVRx. For the latter, there is a great need for a less invasive interventional therapy. The MC device (Abbott Laboratories, Abbott Park, IL, USA) is a transvenous, transseptal, edge-to-edge repair system for patients with high surgical risk for the treatment of severe functional (FMR) and degenerative MR (DMR). In 2005, the first results of the Endovascular Valve Edge-to-Edge Repair Study (EVEREST) I trial were published.Citation5 At present, over 22,000 patients (as of April 2015, according to the manufacturer Abbott Laboratories) have been treated worldwide ().
Table 1 MitraClip clinical trials and commercial use with corresponding number of patients
In the USA, the commercial use of the MC system started in 2014 with a steadily growing number and holds a current market share of 20%–25% (). Globally, the etiology of MR is 2/3 FMR and 1/3 DMR ().
Figure 1 Worldwide experience with the MitraClip procedure from September 2008 until April 2015.
Abbreviations: APAC, Asia-Pacific; CALA, Caribbean and Latin America.
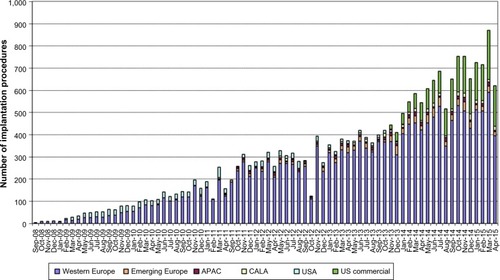
Figure 2 Etiology of mitral regurgitation according to the manufacturer Abbott Laboratories (as of April 30, 2015).
Abbreviations: DMR, degenerative mitral regurgitation; FMR, functional mitral regurgitation.
European guidelines recommend MC treatment for both FMR and DMR,Citation2 whereas the MC system is approved only for DMR in the USA. After 10 years of clinical experience with percutaneous MVR, this review focuses on the results of recent registries and clinical studies, such as EVEREST II,Citation6–Citation10 ACCESS-EU,Citation11,Citation12 GRASP,Citation13,Citation14 TRAMI,Citation15–Citation17 and one meta-analysis.Citation18
The MitraClip: eligibility criteria and procedure
To guarantee safe positioning of the clip, anatomical eligibility criteria are recommended. A coaptation length of ≥2 mm, a coaptation depth of <11 mm, and in the case of degenerative disease, a flail gap of <10 mm and a flail width of <15 mm are favorable.Citation19 The MC system consists of a steerable guide catheter that is introduced transfemoral, and through echocardiographic guiding transseptal into the left atrium (). The clip delivery system can be introduced through the guide catheter. Once the delivery system is completely introduced into the guide, the operator can move the MC in all dimensions under echocardiographic guidance and fluoroscopic confirmation. In the area of the MR, the clip is guided directly above the leaflets by 3D-echo, and the orientation of the clip arms should be perpendicular to the line of coaptation.
Figure 3 The MitraClip system.
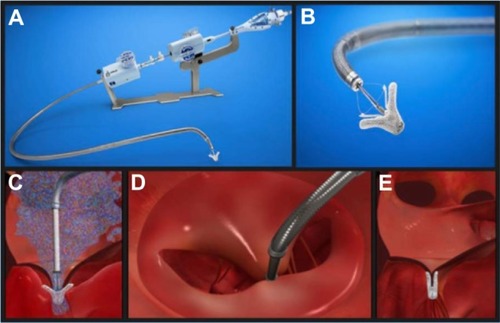
The MC is lowered through the valve into the left ventricle to load the leaflets on the clip arms. The grippers fix the leaflets to the clip arms, and then the arms are closed and the MC can be released from the delivery system ().
Echocardiography is the most important guiding modality for all steps of the procedure, especially during clip positioning, whereas fluoroscopy is only essential for wiring, transseptal puncture, and control of coaxial alignment of the clip to the line of coaptation during transvalvular maneuvering. Further use of fluoroscopy as a second tool of visualization is optional and operator-dependent. Fluoroscopy duration is approximately 25 minutes. Contrast agents are not needed.Citation12
Conventional management of MR in comparison to the MC system
Standard of care for severe MR is surgical treatment, preferably mitral valve (MV) reconstruction before MVRx.Citation20 So far, the EVEREST II trial is the only randomized controlled trial comparing MV surgery (MVS) and MC.Citation10 Of note, patients in this study were eligible for surgery in contrast to the majority of MC studies. In this study, MC shows a clear inferiority regarding acute efficacy in MR reduction as well as an inferiority in the composite end point of primary efficacy (freedom of death, MVS or reoperation, and MR grade 3+ or 4+) at 1-year follow-up in the intent-to-treat analysis (73% for MVS vs 55% for MC, P=0.007). Following MC, 20% of the patients had to be reoperated compared to 2.2% after MVS (4-year follow-up: 24.8% and 5.5%, respectively). More patients had MR grade 2+ after MC therapy. However, patients showed less symptomatic heart failure according to the New York Heart Association (NYHA) class when compared to those who underwent MVS. Interestingly, at 4-year follow-up the composite end point of freedom from death, surgery, or MR ≥ grade 3+ showed a rate of 39.8% in the MC group and 53.4% after MVS, a difference that was no longer statistically significant (P=0.07). The interventional and surgical long-term results proved to be stable at 4 years with 25% vs 5.5% (MC vs MVS) of the patients requiring surgery for MV dysfunction. Remarkably, if a good result after 6 months was found with the MC, the likelihood of recurrent MR was low, and there was no evidence of late device-related complications.Citation10
A meta-analysis of 21 studies with 6,463 patients compared the outcome of MVS (n=3,265) and MC (n=3,198) demonstrating similar high rates of procedural success (MVS 98% vs MC 96%).Citation21 However, while MVS was superior with respect to 30-day technical failure rate (0.6% vs 3.2%, P=0.002), outcome for MC was superior to MVS in the pooled key safety analysis at 30 days (mortality: 3.3%, 95% confidence interval [CI] 2.6–4.2 vs 16.2%, 95% CI 13.0–20.0; stroke: 1.1%, 95% CI 0.6–1.6 vs 4.5%, 95% CI 3.6–5.3; bleeding: 4.2%, 95% CI 3.0–7.0 vs 59.0%, 95% CI 50.0–67.0; prolonged mechanical ventilation: 1.7%, 95% CI 1.1–2.2 vs 36.3%, 95% CI 33.1–40.0). These results were shown despite a higher surgical risk profile in the patient group treated with MC (eg, mean left ventricular ejection fraction [LVEF] of 38% vs 52% [MC vs MVS]).Citation21 One-year mortality of MC patients was 13%. Since there is no long-term data available for MVS, results are not comparable. A further limitation of this meta-analysis is the fact that most of these studies on MC therapy were derived from registries or retrospectively analyzed case series; they are not randomized or controlled, especially for the patient population characteristics.
MC therapy has to be compared to the best medical therapy in heart failure patients with severe MR. This group of patients have a 1-year mortality of 20%, a 5-year mortality of 50%, and a high rate of recurrent hospitalization for heart failure.Citation22 Trials prospectively comparing best medical treatment with interventional therapy are ongoing. The currently enrolling studies COAPT (ClinicalTrials.gov registration number: NCT01626079) and RESHAPE-HF (ClinicalTrials.gov registration number: NCT01772108) will address this important question in the near future.
Mortality and safety
The largest meta-analysis comprising 2,980 patients from 16 studies (12 European and four North American) demonstrated a very low intra-procedural mortality of only 0.1%. Nevertheless, 30-day mortality increased to 4.2%, and all-cause mortality during a mean follow-up of 310 days was 15.8%.Citation18 Thirty-day mortality ranged between 0.9%Citation14 and 4.7%Citation12 in most clinical trials. Recent trials report a high 1-year mortality ranging between 12% and 18.2%.Citation12,Citation14,Citation20 Significant comorbidities expressed by a high logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) I of 23.4%±1.5% most likely accounts for the high mortality rate during the first year.Citation18 In the GRASP registry, 55% of deaths during the first year were attributed to noncardiac reasons.Citation14
Complications
In the meta-analysis, the most relevant procedure-associated complication was major bleeding (requiring transfusion) with 9.7% followed by stroke/transient ischemic attack (1.3%), chordal rupture (0.8%), pericardial tamponade (0.7%), and myocardial infarction (0.4%).Citation18 The ACCESS registry reports even lower rates of stroke (0.7%) and bleeding complications (3.8%).Citation12 In summary, complication rates associated with MC are low (), particularly when compared to those associated with transcatheter aortic valve replacement. Here, arterial puncture and calcification of vessels and the aortic annulus increase the risk of transient ischemic attack as well as stroke, ranging from 4.0% to 6.7%,Citation23 and of major vascular complications, ranging from 8.2%Citation24 to 16.2%.Citation23
Table 2 Complications of percutaneous mitral valve repair
Heart failure and MC
MR in combination with congestive heart failure has a very poor prognosis.Citation22,Citation25 Reverse left ventricular (LV) remodeling and improvements of symptoms after MVS in patients with advanced LV dysfunction have been reported in several studies.Citation26,Citation27 Thirty-day mortality ranges between 8% and 9%.Citation26,Citation28 In this regard, MC is a promising, minimally invasive percutaneous treatment technique.Citation29 In a study, 108 patients with predominantly FMR and LV dysfunction (mean LVEF 28%±11% with LVEF <40% in 88% of patients) demonstrated an impressively low 30-day mortality of only 1.8%.Citation30 Most importantly, there are accumulating data that the MC appears to be not only safe but also efficacious in patients with FMR.Citation30 In this regard, the ACCESS-EU registry showed a similar clinical improvement in patients with an LVEF ≤30% vs >30%. In addition, there is some evidence of reverse remodeling after successful treatment with the MC.Citation31
There is an ongoing discussion whether a severely depressed LV function might be susceptible to further acute reduction in an LVEF induced by surgical correction of MR. Interestingly, the occurrence of the so-called afterload mismatch was frequently found (26%) in a study comprising 73 patients with FMR and severely reduced LVEF (27%±9%).Citation32 Comparison of patients with vs without afterload mismatch after MC revealed that LV end-diastolic diameter (71±8 mm vs 67±7 mm, P=0.02) and LV end-systolic diameter (57±9 mm vs 53±7 mm, P=0.04) were larger, pulmonary pressure was higher (49±10 mmHg vs 40±10 mmHg, P=0.04), and right ventricular dysfunction was more prevalent (68% vs 31%, P=0.049). The authors suggested that afterload mismatch was the consequence of an abrupt increase in LV end-systolic wall stress after MR correction on a preexisting status of absent or reduced contractile reserve. Fortunately, the observed hemodynamic deterioration in patients was a transient phenomenon and did not translate into an adverse outcome at 12 months (1-year survival: 81.2% vs 75.2%, P=0.44). This study observed no difference in the need for inotropes between patients with and without afterload mismatch in the early postoperative time period.Citation32
The previously mentioned study comprising only patients with FMR and reduced LVEF reported that 57.7% of patients required inotropic support on the intensive care unit and 13% of patients were transiently bridged with intra-aortic balloon counter pulsation (IABP) underlining the incidence of a transient window of aggravated heart failure immediately after intervention.Citation30
Procedural success, long-term outcome, and predictors of procedural success
Acute procedural success is defined as a reduction in MR to ≤grade 2+. A recent meta-analysis showed acute procedural success in 91.4% of the patients. Persistent MR reduction was found in 85.3% of the patients at 30-days follow-up and in 86.9% at a mean follow-up of 310 days (ranging from 80 days to 4 years).Citation18 In only 3% of the patients, the therapy failed, and no clip could be implanted. A single-center study in 108 patients with predominantly FMR and LV dysfunction (mean LVEF 28%±11% with 88% having an LVEF <40%) showed a procedural success rate of 99%.Citation30
The MC procedure is associated with a low mortality rate. However, it is of great importance to better define predictors of adverse clinical outcome. Only few studies have addressed this topic. In a study of selected FMR patients with severe LV dysfunction, univariate analysis demonstrated an adverse outcome for pre-interventional logistic EuroSCORE I ≥20% (hazards ratio [HR] 4.4, 95% CI 1.8–9.5, P=0.01) and pre-interventional proBNP >1,600 pg/mL (HR 21.2, 95% CI 2.5–38, P=0.01), a need for post-interventional IABP treatment (HR 3.8, 95% CI 1.2–13.5, P=0.02), and peri-interventional occurrence of acute kidney injury (HR 4.1, 95% CI 2–16, P=0.01).Citation30 These findings are in line with results of a single-center study (65% FMR, 35% DMR) analyzing predictors of midterm clinical and survival outcome (all-cause mortality or hospitalization): NYHA IV at baseline (HR 2.4, 95% CI 1.4–4.3, P=0.002) and glomerular filtration rate <60 mL/min/1.73 m2 (HR 2.05, 95% CI 1.1–4.0, P=0.03), Sociey of Thoracic Surgeons score >12% (HR 2.20, 95% CI 1.3–3.8, P=0.004), and failure of procedural success (HR 2.66, 95% CI 1.4–5.0, P=0.002).Citation33
In a study of 300 MC patients (68% FMR, 32% DMR), regurgitant orifice area ≥70.8 mm2 and trans-mitral pressure gradient ≥4 mmHg in combination with an MV orifice area ≤3.0 cm2 (assessed by echocardiography) were defined as predictors of increased risk for procedural failure.Citation34
Quality of life
Functional capacity according to the NYHA class showed a relief of symptoms (86% in the NYHA class I and II) 1 year after treatment.Citation30 The meta-analysis showed an improved functional capacity according to the NYHA (class I and II) in 76.6% of the patients.Citation18 Moreover, the analysis demonstrated an improvement in LVEF, 6-minute walk distance, and quality of life.Citation18 The gain in 6-minute walk distance (260.6±13.6 m at baseline vs 359.8±24.9 m at follow-up) was larger than the gain being described after cardiac resynchronization therapy.Citation23 Similar data were reported for FMR patients with severe LV dysfunction (328.7±80.1 m; mean improvement 108 m). Most interestingly, there were clear signs of LV reverse remodeling with an increase in LVEF (27%±9.8% to 34.7%±10.4%, P=0.02 at 1-year follow-up) and with a decrease in LV end-diastolic volume as well as in LV end-systolic volume.Citation30
Conclusion
For patients with severe MR and high surgical risk, the treatment with the MC has meanwhile evolved as the therapy of choice in Europe (FMR and DMR) and North America (DMR). There is a class IIb (level of evidence C) recommendation in the European guidelines for interventional mitral device therapy in severe symptomatic FMR as well as DMR in all patients with high surgical risk. Additionally, life expectancy has to exceed 1 year, and the Heart Team (cardiologist, cardiac surgeon) should mutually agree that patients are ineligible for surgery.Citation2 Additionally, MR pathology has to meet special criteria for intervention by defined echocardiographic parameters.Citation6,Citation35 North American 2014 guidelines for the management of valvular heart diseaseCitation20 strictly follow the results of the EVEREST I and II trials and recommend interventional mitral device therapy only for DMR.Citation6,Citation7,Citation10 In conclusion, the MC system has evolved as the most important transcatheter MVR therapy till date. Intraprocedural mortality is low, adverse events are seldom, and short- as well as long-term results are satisfactory.
The number of case reports and smaller case series, where the MC device was used as a bailout strategy apart from current guideline recommendations, is rapidly growing. These reports show that with increasing device experience, the anatomical criteria for the applicability of MC implantation broaden. Current guideline-based indications might therefore underestimate the potential of MC therapy.
In the case of severe coaptation failure, two MCs were implanted via a double-guide approach with two simultaneously introduced clip delivery systems. The first clip was initially used to improve coaptation between the posterior and anterior leaflet for the second clip to be positioned for principal MR treatment. Once a successful grasp was performed with the second clip, the first clip was reopened and optimized (“mitral titration technique”).Citation36
The MC was used after MVR to reduce residual severe MR.Citation37,Citation38
The MC was implanted in a series of patients with left ventricular outflow tract (LVOT) obstruction with subsequent MR due to systolic anterior movement of the anterior leaflet in hypertropic obstructive cardiomyopathy patients. Not only MR but also subvalvular LVOT gradient was successfully reduced.Citation39,Citation40
The MC was used as primary rescue therapy for patients with severe MR in cardiogenic shock and/or critically ill.Citation41–Citation43
The MC was implanted in a trileaflet MV.Citation44
These cases demonstrate the potential of the device and might inspire reconsideration of the use of MC in future trials. Due to the remarkably low peri-interventional risk, the MC might be considered as a bailout therapy for treatment of severe MR even in critically ill patients.
However, new devices for the therapy of MR are entering the market, ultimately broadening the spectrum of patients being treated by catheter-based techniques in the near future. In line with surgical reconstruction, a combined use of different interventional devices, for example, MC and annuloplasty device, may be the future of interventional MV therapy.
Disclosure
F Deuschl received speakers honoraria and traveling support from Abbott Laboratories. U Schaefer received research funding, speakers honoraria, and traveling support from Abbott Laboratories, and is a faculty member at Crossroads Abbott Laboratories in Brussels. E Lubos received research funding, speakers honoraria, and traveling support from Abbott Laboratories. The authors report no other conflicts of interest in this work.
References
- NkomoVTGardinJMSkeltonTNGottdienerJSScottCGEnriquez-SaranoMBurden of valvular heart diseases: a population-based studyLancet200636895401005101110.1016/S0140-6736(06)69208-816980116
- VahanianAAlfieriOAndreottiFGuidelines on the management of valvular heart disease (version 2012)Eur Heart J201233192451249610.1093/eurheartj/ehs10922922415
- BonowROCarabelloBAChatterjeeK2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic SurgeonsCirculation200811815e523e66110.1161/CIRCULATIONAHA.108.19074818820172
- ChikweJGoldstoneABPassageJA propensity score-adjusted retrospective comparison of early and mid-term results of mitral valve repair versus replacement in octogenariansEur Heart J201132561862610.1093/eurheartj/ehq33120846993
- FeldmanTWassermanHSHerrmannHCPercutaneous mitral valve repair using the edge-to-edge technique: six-month results of the EVEREST phase I clinical trialJ Am Coll Cardiol200546112134214010.1016/j.jacc.2005.07.06516325053
- FeldmanTFosterEGlowerDDPercutaneous repair or surgery for mitral regurgitationN Engl J Med2011364151395140610.1097/01.SA.0000410146.91957.d621463154
- WhitlowPLFeldmanTPedersenWRAcute and 12-month results with catheter-based mitral valve leaflet repair: The EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk StudyJ Am Coll Cardiol201259213013910.1016/j.jacc.2011.08.06722222076
- LimDSReynoldsMRFeldmanTImproved functional status and quality of life in prohibitive surgical risk patients with degenerative mitral regurgitation after transcatheter mitral valve repairJ Am Coll Cardiol201464218219210.1016/j.jacc.2013.10.02124184254
- GlowerDDKarSTrentoAPercutaneous mitral valve repair for mitral regurgitation in high-risk patients: results of the EVEREST II studyJ Am Coll Cardiol201464217218110.1016/j.jacc.2013.12.06225011722
- MauriLFosterEGlowerDD4-Year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitationJ Am Coll Cardiol201362431732810.1016/j.jacc.2013.04.03023665364
- ReichenspurnerHSchillingerWBaldusSClinical outcomes through 12 months in patients with degenerative mitral regurgitation treated with the mitraclip® device in the ACCESS-EUrope phase I trialEur J Cardiothoracic Surg201344428028810.1093/ejcts/ezt321
- MaisanoFFranzenOBaldusSPercutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the Mitraclip therapy in EuropeJ Am Coll Cardiol201362121052106110.1016/j.jacc.2013.02.09423747789
- AttizzaniGFOhnoYCapodannoDGender-related clinical and echocardiographic outcomes at 30-day and 12-month follow up after MitraClip implantation in the GRASP registryCatheter Cardiovasc Interv201585588989710.1002/ccd.2571525367550
- GrassoCCapodannoDScanduraSOne- and twelve-month safety and efficacy outcomes of patients undergoing edge-to-edge percutaneous mitral valve repair (from the GRASP registry)Am J Cardiol2013111101482148710.1016/j.amjcard.2013.01.30023433761
- WiebeJFrankeJLubosEPercutaneous mitral valve repair with the mitraclip system according to the predicted risk by the logistic EuroSCORE: preliminary results from the German Transcatheter Mitral Valve Interventions (TRAMI) registryCatheter Cardiovasc Interv201459880059159810.1002/ccd.2549324664460
- SchillingerWHünlichMBaldusSAcute outcomes after MitraClip® therapy in highly aged patients: results from the German TRAnscatheter Mitral valve Interventions (TRAMI) registryEuroIntervention201391849010.4244/EIJV9I1A1323579108
- BaldusSSchillingerWFranzenOMitra Clip therapy in daily clinical practice: initial results from the German transcatheter mitral valve interventions (TRAMI) registryEur J Heart Fail20121491050105510.1093/eurjhf/hfs07922685268
- VakilKRoukozHSarrafMSafety and efficacy of the MitraClip® system for severe mitral regurgitation: a systematic reviewCatheter Cardiovasc Interv201484112913610.1002/ccd.2534724323764
- FeldmanTKarSRinaldiMPercutaneous mitral repair with the MitraClip system. Safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohortJ Am Coll Cardiol200954868669410.1016/j.jacc.2009.03.07719679246
- NishimuraRAOttoCMBonowRO2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice GuidelinesCirculation201412923e65010.1161/CIR.0000000000000029
- PhilipFAthappanGTuzcuEMSvenssonLGKapadiaSRMitraClip for severe symptomatic mitral regurgitation in patients at high surgical risk: a comprehensive systematic reviewCatheter Cardiovasc Interv201484458159010.1002/ccd.2556424905665
- GoelSSBajajNAggarwalBPrevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of mitraclip for this unmet needJ Am Coll Cardiol201463218518610.1016/j.jacc.2013.08.72324036029
- LindeCGoldMRAbrahamWTLong-term impact of cardiac resynchronization therapy in mild heart failure: 5-year results from the resynchronization reverses remodeling in systolic left ventricular dysfunction (REVERSE) studyEur Heart J201334332592259910.1093/eurheartj/eht16023641006
- PopmaJJAdamsDHReardonMJTranscatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgeryJ Am Coll Cardiol201463191972198110.1016/j.jacc.2014.02.55624657695
- RossiADiniFLFaggianoPIndependent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathyHeart201197201675168010.1136/hrt.2011.22578921807656
- De BonisMTaramassoMGrimaldiAThe GeoForm annuloplasty ring for the surgical treatment of functional mitral regurgitation in advanced dilated cardiomyopathyEur J Cardiothoracic Surg201140248849510.1016/j.ejcts.2010.11.048
- De BonisMLapennaEVerziniARecurrence of mitral regurgitation parallels the absence of left ventricular reverse remodeling after mitral repair in advanced dilated cardiomyopathyAnn Thorac Surg200885393293910.1016/j.athoracsur.2007.11.02118291174
- BraunJBaxJJVersteeghMIMPreoperative left ventricular dimensions predict reverse remodeling following restrictive mitral annuloplasty in ischemic mitral regurgitationEur J Cardiothoracic Surg200527584785310.1016/j.ejcts.2004.12.031
- De BonisMLapennaELa CannaGMitral valve repair for functional mitral regurgitation in end-stage dilated cardiomyopathy: role of the “edge-to-edge” techniqueCirculation20051129 Suppl1402140810.1161/CIRCULATIONAHA.104.525188
- TaramassoMMaisanoFLatibAClinical outcomes of MitraClip for the treatment of functional mitral regurgitationEuroIntervention201410674675224469474
- GrayburnPAFosterESangliCRelationship between the magnitude of reduction in mitral regurgitation severity and left ventricular and left atrial reverse remodeling after mitraclip therapyCirculation2013128151667167410.1161/CIRCULATIONAHA.112.00103924014834
- MelisurgoGAjelloSPappalardoFAfterload mismatch after mitraclip insertion for functional mitral regurgitationAm J Cardiol2014113111844185010.1016/j.amjcard.2014.03.01524837263
- PulsMTichelbäckerTBleckmannAFailure of acute procedural success predicts adverse outcome after percutaneous edge-to-edge mitral valve repair with MitraClipEuroIntervention20149121407141710.4244/EIJV9I12A23824972141
- LubosESchlüterMVettorazziEMitraClip therapy in surgical high-risk patients: identification of echocardiographic variables affecting acute procedural outcomeJACC Cardiovasc Interv20147439440210.1016/j.jcin.2013.12.19824630887
- ArmstrongEJRogersJHSwanCHEchocardiographic predictors of single versus dual MitraClip device implantation and long-term reduction of mitral regurgitation after percutaneous repairCatheter Cardiovasc Interv201382467367910.1002/ccd.2464522936628
- SchaeferUFrerkerCKreidelFSimultaneous double clipping delivery guide strategy for treatment of severe coaptation failure in functional mitral regurgitationHeart Lung Circ20152419810210.1016/j.hlc.2014.09.00825308769
- GrassoCAttizzaniGFOhnoYCatheter-based edge-to-edge mitral valve repair after percutaneous mitral valve annuloplasty failureJACC Cardiovasc Interv20147e8586 Epub 2014 Jun 1824954567
- GrassoCOhnoYAttizzaniGFPercutaneous mitral 547 valve repair with the MitraClip system for severe mitral 548 regurgitation in patients with surgical mitral valve repair 549 failureJ Am Coll Cardiol20146383683824161329
- SchäferUKreidelFFrerkerCMitraClip implantation as a new treatment strategy against systolic anterior motion-induced outflow tract obstruction in hypertrophic obstructive cardiomyopathyHeart Lung Circ2014235e131e13510.1016/j.hlc.2014.01.00724698439
- SchäferUFrerkerCThielsenTTargeting systolic anterior motion and left ventricular outflow tract obstruction in hypertrophic obstructed cardiomyopathy with a MitraClipEuroIntervention201410.4244/EIJY14M08_13.
- PlegerSTChorianopoulosEKrumsdorfUKatusHAPercutaneous edge-to-edge repair of mitral regurgitation as a bail-out strategy in critically ill patientsJ Invasive Cardiol2013252697223388223
- ZuernCSSchreieckJWeigHJGawazMMayAEPercutaneous mitral valve repair using the MitraClip in acute cardiogenic shockClin Res Cardiol2011100871972110.1007/s00392-011-0324-121607532
- CouturePCloutier-GillL-ADucharmeABonanRAsgarAWMitraClip intervention as rescue therapy in cardiogenic shock: one-year follow-upCan J Cardiol20143091108.e151108.e1610.1016/j.cjca.2014.03.04525151290
- FreixaXHayamiDBasmadjianANAsgarAWMitraClip repair of a “trileaflet” regurgitant mitral valveEuroIntervention201511335510.4244/EIJY14M07_0525022225
