Abstract
Patients infected with the human immunodeficiency virus (HIV) have an increased cardiovascular risk. Although initially this increased risk was attributed to metabolic alterations associated with antiretroviral treatment, in recent years, the attention has been focused on the HIV disease itself. Inflammation, immune system activation, and endothelial dysfunction facilitated by HIV infection have been identified as key factors in the development and progression of atherosclerosis. In this review, we describe the epidemiology and pathogenesis of cardiovascular disease in patients with HIV infection and summarize the latest knowledge on the relationship between traditional and novel inflammatory, immune activation, and endothelial dysfunction biomarkers on the cardiovascular risk associated with HIV infection.
Importance of cardiovascular disease in patients infected with the human immunodeficiency virus
In regions with universal access to care and antiretroviral therapy (ART), the prognosis of patients infected with the human immunodeficiency virus (HIV) has improved substantially.Citation1–Citation4 In the last 2 decades, the incidence and mortality of acquired immunodeficiency syndrome (AIDS)-defining illnesses (associated with severe immunosuppression in advanced stages of HIV infection) have been dramatically reduced, whereas the role of non-AIDS comorbidities has risen.Citation5,Citation6 A marked decrease in overall mortality due to AIDS-defining causes has been observed, while the proportion of deaths from other causes has increased, including those caused by cardiovascular disease (CVD).Citation6–Citation9 Similarly, hospitalizations due to AIDS-defining illnesses have decreased in European and North American populations, while admissions for non-AIDS diseases and CVD have increased.Citation10,Citation11
Patients with HIV infection have higher atherosclerotic CVD rates than the general population.Citation12 HIV patients experience more clinical cardiovascular events (coronary heart diseaseCitation13–Citation15 and peripheral artery disease),Citation16 and subclinical cardiovascular damage (elevation of intima–media thickness [IMT],Citation17 coronary calcification,Citation18 abnormal ankle–brachial index,Citation19 silent myocardial ischemia,Citation20 or endothelial dysfunction).Citation21 In addition, the incidence of ischemic stroke in the HIV-infected population is considerably high, particularly in young adults, although no studies have determined whether stroke risk is greater in this population.Citation22,Citation23
Besides atherosclerotic coronary heart disease, other cardiac abnormalities have been associated with HIV infection.Citation24 Thus, in the pre-ART era, a high incidence of dilated cardiomyopathy with left ventricular dysfunction related to viral myocarditis was reported, mainly during the AIDS stage.Citation24 More recent studies, in settings with unrestricted access to ART, reported a low prevalence of dilated cardiomyopathy, but a high burden of subclinical myocardial disease (cardiac steatosis, myocardial fibrosis, and alterations in cardiac function) in HIV patients as compared to uninfected controls.Citation25 Moreover, HIV patients may be at an increased risk of sudden arrhythmic death. The prevalence of prolonged corrected QT, a major risk for polymorphous ventricular tachycardia (Torsades de pointes) and sudden arrhythmic death, is also increased in HIV patients.Citation26 In this regard, sudden cardiac death rate (due to atherosclerotic and/or arrhythmic causes) is 4.5-fold higher in HIV-infected patients than among those observed in the general population.Citation27
On the other hand, the mean age of the HIV population is increasing due to the effectiveness of ART and increased life expectancy, favoring the development of age-associated comorbidities, many of which are related to CVD, such as type 2 diabetes mellitus (T2DM), hypertension (HT), and chronic kidney disease (CKD). In the Swiss HIV Cohort Study,Citation28 which gathered patient data from 1990–2010, the proportion of patients older than 50 years increased from less than 5% to just over 30%. It is expected that if this trend continues, in the next decade, 50% of patients in this cohort will be above 50 years.
In addition to chronological aging, it is believed that HIV patients experience accelerated biological aging, thus contributing to the early development of age-related comorbidities.Citation29–Citation31 Patients with HIV infection have a higher prevalence of noninfectious comorbidities commonly seen in the elderly (T2DM, CVD, osteoporosis, and CKD).Citation32–Citation35 An interesting case-control study in an Italian population found a prevalence of comorbidity in HIV patients equivalent to that observed in control individuals 10–15 years older.Citation32 As seen in these studies, we are witnessing a change in the patterns of morbidity and mortality among the HIV-infected population, leading researchers to shift the focus to non-AIDS comorbidity, where CVD plays a central role.
Etiopathogenesis of cardiovascular disease in patients with HIV infection
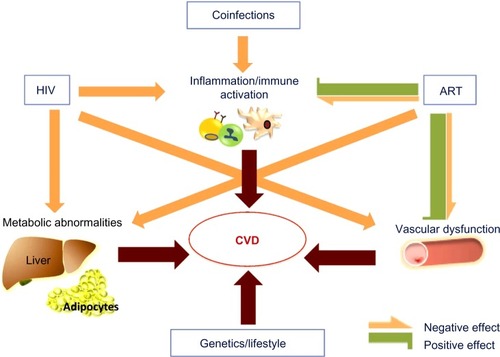
The higher cardiovascular risk observed in patients with HIV infection is due to a combination of several determinants, including factors related to antiretroviral treatment, the inherent impact of the infection, a high prevalence of traditional cardiovascular risk factors among HIV-infected individuals, and the presence of other factors that occur more frequently in these patients: coinfection with hepatitis C virus (HCV), coinfection and the replication of herpes family viruses, or the development of CKD ().
Figure 1 Determining factors of CVD in HIV-infected individuals.
Abbreviations: HIV, human immunodeficiency virus; ART, antiretroviral therapy; CVD, cardiovascular disease; HCV, hepatitis C virus; CMV, cytomegalovirus.
Antiretroviral drugs
Initially, the elevated cardiovascular risk observed in HIV patients was attributed to metabolic alterations associated with ART, due particularly to the effect of viral protease inhibitors (PI), whose introduction into clinical practice coincided with the first reported cases of ischemic heart disease in HIV patients.Citation36,Citation37 This relationship between CVD and ART was subsequently confirmed in epidemiological studies.Citation12 The most representative study, the Data Collection on Adverse Events of Anti-HIV Drugs Study (DAD Study),Citation38 showed a significant increase in acute myocardial infarction (AMI) incidence upon exposure to ART, with an increased AMI risk of 26% after 6 years of treatment. The increased cardiovascular risk associated with ART has been attributed to deleterious metabolic effects of these drugs, which favor the development of hypercholesterolemia and hypertriglyceridemia, insulin resistance, and T2DM.Citation39 These alterations may occur either independently or as part of other disorders, such as metabolic syndrome or lipodystrophy. To a greater or lesser extent, the appearance of these entities has been reported in patients treated with PI drugs, nucleoside/nucleotide analog reverse transcriptase inhibitors (NRTIs) and non-nucleoside/nucleotide reverse transcriptase inhibitors. These alterations depend on the type of drug administered, and drugs belonging to the same family may have different effects. While these adverse effects have decreased with the new generation of antiretroviral drugs,Citation40 the initiation of ART is usually associated with increased plasma lipids (triglycerides and total cholesterol with elevations in low-density lipoprotein [LDL] cholesterol [LDL-C] and, to a lesser extent, high-density lipoprotein cholesterol [HDL-C]) which, in part, could also be related to the weight gain observed after starting ART.Citation41
In addition to these metabolic effects, the use of some antiretroviral drugs may induce other proatherogenic effects. Studies conducted in vitro and in healthy volunteers have revealed that some antiretrovirals – mainly PIs, but also NRTIs – could impair endothelial function, and increase oxidative stress and inflammation, inducing the early onset of senescence markers in endothelial and mononuclear cells.Citation42–Citation45 However, the data of many clinical studies suggest that ART dampens inflammation, immune activation and endothelial dysfunction in HIV-infected patients, probably through the suppression of HIV replication, as we further discuss.
HIV infection
In recent years, the ART-dependent atherosclerosis hypothesis has been challenged. Evidence from experimental and observational studies, especially the findings of the Strategies for Management of Anti-Retroviral Therapy Study (SMART Study),Citation46 have redirected attention from ART to the consequences of HIV infection itself. In this study, two different therapeutic strategies were compared. In the first group, ART was interrupted when a recovery of the immune system was achieved and CD4+ lymphocytes were above 350 cells/mL, whereas in the second group, ART was maintained continuously, regardless of CD4+ levels. Patients in whom the treatment was discontinued showed increased complications associated with immunosuppression and an increased risk of all-cause mortality. Moreover, patients assigned to discontinuous therapy exhibited an unexpected increase in cardiovascular outcomes in spite of less exposure to antiretroviral drugs.Citation46 These results suggested that uncontrolled HIV infection might have a greater influence on CVD than ART.
HIV infection may increase cardiovascular risk by several mechanisms: 1) persistent inflammation and immune activation; 2) endothelial damage; 3) increased thrombotic activity; 4) higher oxidative stress; and 5) indirect metabolic disorders. HIV infection leads to the activation of several inflammatory pathways, causing the release of cytokines and endothelial adhesion molecule expression that facilitate adhesion and the transmigration of leukocytes.Citation47 There is a close relationship between endothelial dysfunction and inflammation/immune activation. Various cytokines induce endothelial activation and alter its functionality.Citation48 Additionally, HIV produces direct endothelial cell damage, increasing endothelial permeability, favoring apoptosis and increasing the expression of adhesion molecules (E-selectin, VCAM-1, and ICAM-1).Citation49–Citation54
HIV infection is also accompanied by immune activation, as determined by the elevation of several activation markers on monocytes/macrophages (sCD163, sCD14, and CD14+/CD16+ monocyte expansion) and the increased proportion of activated CD8 T-lymphocytes human leukocyte antigen (HLA)-DR+CD38+.Citation55–Citation60 Monocytes/macrophages play a central role in the genesis and development of atherosclerosis. Thus, in atherosclerotic plaques, macrophages phagocyte modified lipoproteins, promote proinflammatory and chemotactic cytokine secretion, and mediate cholesterol efflux from the arterial wall.Citation61 HIV infection increases the proportion of CD14+/CD16+ monocytes, as it exhibits an activated phenotype with increased secretion of proinflammatory cytokines.Citation58,Citation59 Furthermore, HIV blocks the adenosine triphosphate-binding cassette transporter A1 (ABCA-1) pathway, suppressing reverse cholesterol transport from arterial wall macrophages to HDL particles, and favoring the accumulation of foam macrophages within atherosclerotic plaques.Citation62
Inflammation and immune activation are also associated with increased thrombotic activity, with the elevation of biomarkers such as D-dimer (a fibrin degradation product that may be elevated in response to inflammatory stimuli and bacterial translocation), von Willebrand factor, and fibrinogen.Citation63
HIV also induces oxidative stress, impairing the mechanisms of DNA repair and promoting the accumulation of oxidative lesions.Citation64 Furthermore, HIV promotes atherogenesis through its metabolic effects, mainly decreasing HDL-C and apoA1, decreasing LDL particle clearance, as well as increasing triglycerides and very LDL-C. This pattern is associated with a high prevalence of proatherogenic small and dense LDL particles.Citation65–Citation67 It has been proposed that circulating HDL particles in different proinflammatory conditions are functionally less active and are therefore less atheroprotective, reducing their ability to perform cholesterol efflux.Citation68 However, this scenario has not been explored specifically in HIV infection.
Inflammation, immune activation, and prothrombotic state driven by HIV infection may participate not only in primary atherosclerosis, but also in occlusive complications after arterial revascularization procedures. It has been reported that HIV patients may have higher rates of stent restenosis and stent thrombosis after percutaneous coronary intervention.Citation69–Citation71 However, the results are not homogeneous and some studies report no differences in revascularization rates or major cardiac events after percutaneous coronary intervention in HIV-positive patients compared to age- and sex-matched HIV-negative subjects.Citation72,Citation73
Traditional cardiovascular risk factors
HIV patients show a high prevalence of certain traditional cardiovascular risk factors, such as smoking, dyslipidemia, and T2DM.Citation12,Citation74–Citation76 The importance of these cardiovascular risk factors has been demonstrated in several cohort studies, highlighting a strong association between HIV and CVD, one that is even stronger than the link between ART and CVD. However, it is important to note that both ART and HIV infection may also induce dyslipidemia and diabetes.
Lifestyle
Smoking is very common in HIV patients, more so than in the general population; between 35% and 72% of HIV-infected individuals smoke depending on the population analyzed.Citation77–Citation81 Dietary habits also appear to be worse in HIV patients. In a case-control study that included 356 HIV-infected patients and 162 healthy volunteers, it was found that infected patients had significantly higher trans-, saturated-, and total fat and cholesterol consumption.Citation82 One potential explanation for this difference is that without appropriate dietary control, many HIV patients have a greater caloric intake in order to compensate for lipoatrophy. Interestingly, in HIV patients that started ART, the incidence of dyslipidemia was significantly lower in those subjects who initiated a hypocaloric and low saturated fat diet as compared with patients who did not undergo a dietary intervention.Citation41
Metabolic cardiovascular risk factors
Patients with HIV infection share the same predisposing factors for the development of dyslipidemia, diabetes, or metabolic syndrome as the general population.Citation74 However, in these individuals, other factors coexist such as the HIV infection itself, ART, and/or HCV coinfection, which could explain the higher prevalence of abnormal carbohydrate and lipid metabolism observed in this population. In the DAD study,Citation76 the prevalence of dyslipidemia at baseline was 45.9%. It is expected that this prevalence would decrease with the use of lipid-lowering and antiretroviral drugs with a better metabolic profile. However, recent data from the Spanish cohort CoRISCitation81 showed that the prevalence remained elevated, as 27% of patients exhibited hypercholesterolemia, 36% low HDL cholesterol, and 19% hypertriglyceridemia. The frequency of abnormal carbohydrate metabolism has decreased in recent years, revealing a presence of diabetes of 17% in older cohorts,Citation76 although a recent analysis found only 2.9% of the cohort to be diabetic.Citation81 Patients with HIV infection also have a high prevalence of metabolic syndrome, around 25%.Citation81,Citation83,Citation84
Hypertension
The prevalence of HT in patients with HIV infection does not appear to be greater than in non-HIV individuals.Citation73 A recent meta-analysis showed lower levels of systolic and diastolic blood pressure in HIV-infected patients compared to HIV-negative individuals.Citation85 Interestingly, it has been hypothesized that in spite of normal brachial blood pressure, HIV patients may have higher arterial stiffness than healthy controls,Citation86,Citation87 emerging as an early marker of vascular damage related to HT; however, the data remain conflicting.Citation88
Additional factors
In addition to the HIV infection itself, other factors may be involved in the increased vascular risk in patients with HIV infection, such as the presence of chronic coinfections (HCV or herpes family viruses) and CKD.Citation40 HCV infection promotes inflammation, platelet activation, endothelial dysfunction, and increased production of reactive oxygen species.Citation89 In the general population, HCV infection has been associated with a higher prevalence of subclinical atherosclerosisCitation90–Citation92 and overt CVD.Citation93–Citation95 Similarly, in HIV patients, HCV coinfection has been associated with a higher frequency of atherosclerotic plaques (carotid or femoral),Citation96 stroke, and AMI when compared to non-HCV coinfected patients.Citation97,Citation98
Latent infection with a virus of the herpes family has atherogenic effectsCitation99 and is associated with subclinical atherosclerosis.Citation100,Citation101 It has been hypothesized that herpes viruses may have more serious cardiovascular effects in HIV-infected patients, contributing to immune activation and inflammation. Cytomegalovirus (CMV) infection has been associated with atherosclerosis in HIV-infected patients. CMV-specific T-cell responses and CMV immunoglobulin G levels have been associated with increased carotid atherosclerosis.Citation102,Citation103 Moreover, other herpes viruses have been implicated. A case-control study that evaluated the association between subclinical coronary atherosclerosis and markers of CMV, herpes simplex virus 1 and 2, and human herpes virus type 8 in HIV patients, showed that herpes simplex virus 2 was independently associated with the presence of subclinical coronary atherosclerosis, supporting the proatherogenic role of the herpes virus in this population.Citation104
Finally, CKD is more frequent in HIV patients, and this factor is widely associated with CVD.Citation105 This higher prevalence of renal disease can be explained by several factors, including a direct effect of HIV, the potential nephrotoxicity of some antiretroviral agents, the presence of traditional cardiovascular risk factors such as diabetes, and the development of atherosclerosis, which is bidirectionally associated with CKD.Citation106 It is important to note that although some antiretroviral drugs may cause renal toxicity, many studies have shown that ART slows the decline of the estimated glomerular filtration rate, probably related to the effect of HIV viral suppression on glomerular function.Citation107–Citation109
Inflammation, immune activation, and endothelial dysfunction in patients with HIV infection
Multiple biomarkers of inflammation, immune activation, and endothelial dysfunction associated with CVD in the general population have been studied in HIV patients (). Numerous inflammatory mediators and adhesion molecules are elevated in the HIV population, including high-sensitive C-reactive protein (hsCRP), interleukin (IL)-6, soluble receptors of tumor necrosis factor (sTNFR), soluble vascular cell adhesion molecule-1 (sVCAM-1), soluble intercellular adhesion molecule-1 (sICAM-1), or asymmetric dimethylarginine (ADMA).Citation110–Citation114 Some of these biomarkers have been associated with the presence of subclinical vascular disease and/or cardiovascular events in HIV-infected patients. Thus, hsCRP predicts the risk of CVD and all-cause mortality in the HIV population.Citation115,Citation116 High levels of IL-6 are also associated with an increased risk of death from any cause and cardiovascular events.Citation116–Citation118 Moreover, sTNFR has been associated with non-AIDS-defining morbid events during ART, including CVD.Citation119 In HIV patients, the levels of sVCAM-1 have been associated with carotid IMT and carotid plaques,Citation120,Citation121 whereas ADMA levels have been related with coronary calcium score, all of which are surrogate markers of atherosclerosis.Citation122
Table 1 Biomarkers of inflammation, immune activation, and endothelial dysfunction associated with CVD in the general population and performance in HIV-infected patients
Immune activation markers are also elevated in the HIV population. Soluble markers such as sCD163 and sCD14, and the proportion of activated subsets of monocytes (CD14+/CD16+) and CD8 T-cells (HLA-DR+CD38+), are increased in HIV patients.Citation56–Citation60,Citation123,Citation124 Our group has recently reported that HIV patients also have lower levels of soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK), a multifunctional cytokine involved in various atherogenic processes through its interaction with Fn14 and CD163.Citation124 sCD163 levels have been associated with the presence of subclinical atherosclerosis in patients with HIV infection. In 102 young HIV-infected men who were asymptomatic and had a low or undetectable viral load, sCD163 levels were independently associated with the percentage of noncalcified coronary plaques in both the overall analysis and in the 69 patients with undetectable viral RNA.Citation125 These results were replicated in a study with 60 HIV-infected women that showed higher levels of sCD163 in those with noncalcified coronary plaques.Citation126 Furthermore, in a similar scenario, sCD163 levels correlated positively with inflammation, as measured by aortic positron emission tomography.Citation127 In both cases, neither the presence of coronary plaques nor vascular inflammation was associated with other biomarkers of CVD, such as hsCRP or D-dimer. sCD14 levels and CD14+/CD16+ monocytes have also been associated with coronary artery calcium score.Citation128,Citation129 These results suggest that monocyte–macrophage activation could play a central role in both coronary and aortic atherosclerosis in HIV patients on effective ART, independently of classical inflammatory biomarkers. In apparent contrast to these results, a study of 60 HIV-infected patients receiving ART with controlled viral replication found no association between carotid IMT and plaques with sCD14, sCD163, or proinflammatory monocyte subsets. However, the IMT was correlated with concentrations of fibrinogen and sTNFR-I, and subjects with carotid plaques had higher levels of sVCAM-1 and a higher percentage of CD38+HLA-DR+ CD8 T-cells.Citation120 These results are not necessarily contradictory and may reflect the specific role of different immune responses in the genesis of different atherosclerotic lesions. Supporting this hypothesis, it has been recently reported that in HIV-treated patients, sCD14 was independently associated with coronary artery calcium, while T-cell activation and systemic inflammation biomarkers, but not sCD14, correlated with carotid IMT.Citation128 To clarify this issue, more studies are needed to assess the relationship of these biomarkers with the incidence of different atherosclerotic lesions in different vascular territories.
Role of ART in the control of inflammation, endothelial dysfunction, and immune activation
Contrary to the initial perception, a number of data support the notion that ART has a net protective effect on CVD in HIV patients.Citation46 It is hypothesized that this effect is mediated by ART’s ability to reduce inflammation, immune activation, and endothelial dysfunction by suppressing HIV replication ().
It has been reported that ART reduces inflammation and endothelial dysfunction biomarkers.Citation130–Citation135 A decrease in sVCAM-1, sICAM-1 and hsCRP was observed in 115 HIV-infected patients after 2 months and 14 months of antiretroviral treatment.Citation117 In a further study, sTNFR-I, sTNFR-II, sVCAM-1, sICAM-1, and IL-6 plasma levels were significantly decreased after 24 weeks and 96 weeks of ART.Citation131 Additionally, ADMA levels also seem to be reduced after successful ART with suppression of viral replication.Citation134,Citation135
A number of studies have also shown that immune activation decreases after ART. Data from two case-control studies suggest that sCD163 plasma levels decrease after ART.Citation123,Citation124 Moreover, ART-treated patients have a lower proportion of proinflammatory CD14+/CD16+ monocytes than patients who never received ART or those who discontinued this therapy.Citation123,Citation136 However, a reduction of sCD14 levels with ART has not been observed with similar concentrations before and after receiving treatment.Citation137,Citation138
It is important to note that even in individuals with spontaneously suppressed viremia, the so-called “controllers”, ART reduced immune activation markers, specifically T-cell activation, and provoked a higher reduction of viremia.Citation139 These patients showed higher subclinical CVD burden (coronary plaques or increased carotid IMT) than healthy individuals, in spite of maintaining suppressed viremia.Citation140,Citation141 Pereyra et alCitation141 showed that controller HIV patients had a similar coronary disease burden as HIV patients on ART who exhibited suppressed viremia. In the study, controller HIV patients showed higher levels of sCD163, but no significant differences were observed in hsCRP plasma levels.Citation141 These data support the key role of monocytes/macrophages in HIV-associated coronary atherosclerosis and the importance of ART in the reduction of activation of these cells, even in low viral replication situations.
However, it is important to note that ART did not completely reverse inflammation, immune activation, and endothelial dysfunction in HIV patients. In a study involving 781 patients from the SMART study and 8,500 HIV-seronegative control subjects from two large population-based studies (Multi-Ethnic Study of Atherosclerosis [MESA] study and Coronary Artery Development in Young Adults [CARDIA] study), the HIV patients had higher concentrations of hsCRP, IL-6, D-dimer, and C cystatin as compared with uninfected subjects, even when viral replication was suppressed with ART. Citation110 Similar results were observed in a small case-control study, where ART reduced the concentrations of sVCAM-1, hsCRP, and sTNFR-II, although they remained elevated when compared with healthy subjects in spite of viral suppression.Citation124 Also, sCD163 plasma levels decreased with ART but remained elevated as compared to healthy controls.Citation123,Citation124
Altogether, these results support the claim that ART reduces immune activation, inflammation, and endothelial activation, explaining the favorable net effect on cardiovascular risk, in spite of the deleterious metabolic effects. Therefore, ART is a key factor in preventing CVD in patients with HIV. However, in spite of ART, these patients maintain a persistent state of immune activation, inflammation, and endothelial activation that could justify the increased frequency of CVD observed in HIV patients, even with stable suppression of viral replication.
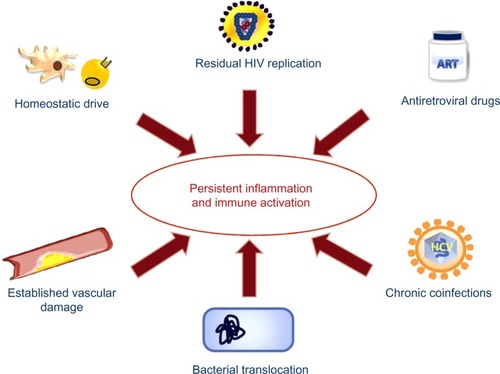
Factors potentially involved in persistent inflammation and immune activation in HIV patients
Several factors could explain the persistent state of immune activation, inflammation, and endothelial activation in spite of the suppression of viral replication after ART ().
Figure 2 Factors implicated in persistent inflammation and immune activation in HIV patients.
Abbreviations: HIV, human immunodeficiency syndrome; ART, antiretroviral therapy; HCV, hepatitis C virus.
Homeostatic drive and time to ART initiation
Sustained viral replication may promote an immune/inflammatory response that cannot be reversed after a certain point. Therefore, starting ART before reaching this immune/inflammatory set point may prevent the state of persistent inflammation and immune activation. Supporting this hypothesis, the results of several studies have demonstrated that starting ART with low CD4 counts and/or a lowest CD4 nadir was associated with worse immunologic outcomes, even if patients achieved effective viral suppression.Citation142–Citation144 Treating HIV infection in the acute phase significantly reduces the proportion of activated CD38+HLA-DR+ CD8 T-cells when compared to nontreated patients.Citation145,Citation146 In the Options Project,Citation147 patients who started ART in the first 6 months after infection had a lower proportion of activated CD8 T-cells than those who initiated treatment 2 years or more after the infection. In another interesting study, Burdo et alCitation123 showed that patients with chronic HIV infection experienced a decrease of sCD163 levels after 3 months of ART; however, the sCD163 plasma concentration remained elevated as compared with controls. By contrast, in patients with early HIV infection (<1 year postinfection), the sCD163 levels at 3 months of treatment were similar to those of controls.Citation123 All together, these studies suggest that early ART could result in the decreased activation of CD8 T-cells and monocytes–macrophages, and they support the hypothesis that the activation of these cells could be reversed to normal levels with early ART.
Residual viral replication
Despite receiving effective ART, HIV patients may have residual viral replication below the detection limits of the techniques commonly used, and/or they may have episodes of transient viral replication. It has been hypothesized that adding an additional antiretroviral drug could control this residual viral replication and reduce inflammation and immune activation, although the data are controversial. The results on the effect of ART intensification with maraviroc or raltegravir are contradictory, and the effect on the reduction of the inflammatory response, in any case, is modest.Citation148–Citation152
ART-dependent effects
Some antiretroviral drugs can induce endothelial dysfunction and oxidative stress and promote an inflammatory response. Drugs such as ritonavir, indinavir, lopinavir, zidovudine, and abacavir have been associated with these deleterious effects.Citation42–Citation45 It has been suggested that other anti-retroviral drugs, such as raltegravir, may have an additional beneficial effect on these processes, independently of the suppression of viral replication. Some studies have shown that substituting a PI or non-nucleoside/nucleotide reverse transcriptase inhibitors with raltegravir reduces the levels of circulating inflammatory markers such as IL-6, hsCRP, or D-dimer.Citation153–Citation155
Coinfections
Chronic coinfections with HCV or herpes viruses, very common in the HIV population, might activate inflammatory and immunological mechanisms, although HIV viral replication is suppressed.
Bacterial translocation
HIV causes damage in the intestinal mucosa, favoring the translocation of bacterial products that stimulate immune activation.Citation156–Citation158 During acute infection phases, HIV severely damages the lymphoid tissue associated with the intestinal mucosa, with a massive depletion of T-cell lymphocytes. In patients with controlled chronic HIV infection, this lymphoid tissue cannot fully recover, and a predisposition to bacterial translocation persists in spite of ART.Citation159,Citation160
Established atherosclerosis
Atherosclerosis is, by itself, a process that is accompanied by persistent inflammation and immune activation.Citation161 The development of atherosclerotic lesions prior to the start of the suppressive ART could be partially responsible for the persistent elevation of inflammation and immune activation biomarkers (intraplaque foam cells and endothelial dysfunction). In this regard, the use of rosuvastatin in patients receiving ART has revealed reduced circulating levels of sCD14 and decreased CD14+/CD16+ monocytes, but it did not affect systemic inflammatory biomarkers.Citation162,Citation163
Conclusion
Patients with HIV infection are at increased cardiovascular risk due to the HIV infection itself, and due to other factors such as ART-associated metabolic abnormalities, lifestyle, or the coexistence of chronic infections. ART was initially proposed as the causal factor for CVD in these patients; however, there is growing evidence supporting a net beneficial effect on CVD risk, presumably through the control of viral replication and the subsequent reduction of inflammation and immune activation. Nevertheless, in spite of optimal treatment, HIV patients maintain a state of persistent inflammation and immune activation that facilitates the development of CVD. In this regard, strategies to control these processes, in addition to ART and the treatment of traditional cardiovascular risk factors, may be useful in CVD prevention for HIV patients.
Acknowledgments
We thank Isabel Martin (Hospital Quirón San Camilo, Madrid) for her assistance in the design and preparation of the figures. We thank Oliver Shawn (Instituto de Investigaciones Sanitarias Fundación Jiménez Díaz [IIS-FJD]) for his editorial assistance. This work has been supported by grants from FIS (Programa Miguel Servet: CP10/00479, PI13/00802 and PI14/00883) and Spanish Society of Nephrology to Juan Antonio Moreno.
Disclosure
The authors report no conflicts of interest in this work.
References
- PalellaFJDelaneyKMMoormanACDeclining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study InvestigatorsN Engl J Med1998338138538609516219
- MocroftALedergerberBKatlamaCEuroSIDA study groupDecline in the AIDS and death rates in the EuroSIDA study: an observational studyLancet20033629377222912853195
- LimaVDHoggRSHarriganPRContinued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapyAIDS200721668569217413689
- LuzPMBruyandMRibeiroSIPEC/FIOCRUZ Cohort and the Aquitaine ANRS C03 Study GroupAIDS and non-AIDS severe morbidity associated with hospitalizations among HIV-infected patients in two regions with universal access to care and antiretroviral therapy, France and Brazil, 2000–2008: hospital-based cohort studiesBMC Infect Dis20141427824885790
- PalellaFJJrBakerRKMoormanACHIV Outpatient Study InvestigatorsMortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient studyJ Acquir Immune Defic Syndr2006431273416878047
- CrumNFRiffenburghRHWegnerSTriservice AIDS Clinical ConsortiumComparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) erasJ Acquir Immune Defic Syndr200641219420016394852
- MorlatPRoussillonCHenardSANRS EN20 Mortalité 2010 Study GroupCauses of death among HIV-infected patients in France in 2010 (national survey): trends since 2000AIDS20142881181119124901259
- FrenchALGawelSHHershowRTrends in mortality and causes of death among women with HIV in the United States: a 10-year studyJ Acquir Immune Defic Syndr200951439940619487953
- MocroftAReissPGasiorowskiJEuroSIDA Study GroupSerious fatal and nonfatal non-AIDS-defining illnesses in EuropeJ Acquir Immune Defic Syndr201055226227020700060
- BerrySAFleishmanJAMooreRDGeboKAHIV ResearchNetworkTrends in reasons for hospitalization in a multisite United States cohort of persons living with HIV, 2001–2008J Acquir Immune Defic Syndr201259436837522240460
- EngsigFNHansenABGerstoftJKronborgGLarsenCSObelNInpatient admissions and outpatient visits in persons with and without HIV infection in Denmark, 1995–2007AIDS201024345746119786845
- CurrierJSLundgrenJDCarrAWorking Group 2Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapyCirculation20081182e29e3518566319
- TriantVALeeHHadiganCGrinspoonSKIncreased acute myo-cardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus diseaseJ Clin Endocrinol Metab20079272506251217456578
- IslamFMWuJJanssonJWilsonDPRelative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysisHIV Med201213845346822413967
- CurrierJSTaylorABoydFCoronary heart disease in HIV-infected individualsJ Acquir Immune Defic Syndr200333450651212869840
- PalaciosRAlonsoIHidalgoAPeripheral arterial disease in HIV patients older than 50 years of ageAIDS Res Hum Retroviruses20082481043104618620492
- LorenzMWStephanCHarmjanzABoth long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosisAtherosclerosis2008196272072617275008
- KingsleyLACuervo-RojasJMuñozASubclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: Multicenter AIDS Cohort StudyAIDS200822131589159918670218
- PeriardDCavassiniMTafféPSwiss HIV Cohort StudyHigh prevalence of peripheral arterial disease in HIV-infected personsClin Infect Dis200846576176718230043
- CarrAGrundBNeuhausJSMART Study InvestigatorsAsymptomatic myocardial ischaemia in HIV-infected adultsAIDS200822225726718097228
- SolagesAVitaJAThorntonDJEndothelial function in HIV-infected personsClin Infect Dis20064291325133216586393
- SenSRabinsteinAAElkindMSPowersWJRecent developments regarding human immunodeficiency virus infection and strokeCerebrovasc Dis201233320921822261608
- OvbiageleBNathAIncreasing incidence of ischemic stroke in patients with HIV infectionNeurology201176544445021248273
- De CastroSd’AmatiGGalloPFrequency of development of acute global left ventricular dysfunction in human immunodeficiency virus infectionJ Am Coll Cardiol1994244101810247930192
- HollowayCJNtusiNSuttieJComprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patientsCirculation2013128881482223817574
- VallecilloGMojalSRoquerARisk of QTc prolongation in a cohort of opioid-dependent HIV-infected patients on methadone maintenance therapyClin Infect Dis20135781189119423899678
- MoyersBSSecemskyEAVittinghoffEEffect of left ventricular dysfunction and viral load on risk of sudden cardiac death in patients with human immunodeficiency virusAm J Cardiol201411371260126524521717
- HasseBLedergerberBFurrerHSwiss HIV Cohort StudyMorbidity and aging in HIV-infected persons: the Swiss HIV cohort studyClin Infect Dis201153111130113921998280
- DeeksSGPhillipsANHIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidityBMJ2009338a317219171560
- DeeksSGHIV infection, inflammation, immunosenescence, and agingAnnu Rev Med20116214115521090961
- EffrosRBFletcherCVGeboKAging and infectious diseases: workshop on HIV infection and aging: what is known and future research directionsClin Infect Dis200847454255318627268
- GuaraldiGOrlandoGZonaSPremature age-related comorbidities among HIV-infected persons compared with the general populationClin Infect Dis201153111120112621998278
- KirkJBGoetzMBHuman immunodeficiency virus in an aging population, a complication of successJ Am Geriatr Soc200957112129213819793157
- DeeksSGPhillipsANHIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidityBMJ2009338a317219171560
- GouletJLFultzSLRimlandDAging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity?Clin Infect Dis200745121593160118190322
- HenryKMelroeHHuebschJSevere premature coronary artery disease with protease inhibitorsLancet1998351911213289643798
- GalletBPulikMGenetPChedinPHiltgenMVascular complications associated with use of HIV protease inhibitorsLancet19983519120195819599654285
- Friis-MøllerNSabinCAWeberRData Collection on Adverse Events of Anti-HIV Drugs (DAD) Study GroupCombination antiretroviral therapy and the risk of myocardial infarctionN Engl J Med2003349211993200314627784
- HadiganCMeigsJBCorcoranCMetabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophyClin Infect Dis200132113013911118392
- HemkensLGBucherHCHIV infection and cardiovascular diseaseEur Heart J201435211373138124408888
- LazzarettiRKKuhmmerRSprinzEPolanczykCARibeiroJPDietary intervention prevents dyslipidemia associated with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected individuals: a randomized trialJ Am Coll Cardiol2012591197998822402068
- ConklinBFuWLinPLundsenAYaoQChenCHIV protease inhibitor ritonavir induces endothelial dysfunction in porcine arteriesJ Surg Res2003114249
- ShankarSSDubéMPGorskiJCKlaunigJESteinbergHOIndinavir impairs endothelial function in healthy HIV-negative menAm Heart J2005150593316290967
- LagathuCEustaceBProtMSome HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophagesAntivir Ther200712448950017668557
- LefèvreCAuclairMBoccaraFPremature senescence of vascular cells is induced by HIV protease inhibitors: implication of prelamin A and reversion by statinArterioscler Thromb Vasc Biol201030122611262020884875
- El-SadrWMLundgrenJNeatonJDStrategies for Management of Antiretroviral Therapy (SMART) Study GroupCD4+ count-guided interruption of antiretroviral treatmentN Engl J Med2006355222283229617135583
- FisherSDMillerTLLipshultzSEImpact of HIV and highly active anti-retroviral therapy on leukocyte adhesion molecules, arterial inflammation, dyslipidemia, and atherosclerosisAtherosclerosis2006185111116297390
- MuHChaiHLinPHYaoQChenCCurrent update on HIV-associated vascular disease and endothelial dysfunctionWorld J Surg200731463264317372667
- EugeninEAMorgelloSKlotmanMEHuman immunodeficiency virus (HIV) infects human arterial smooth muscle cells in vivo and in vitro: implications for the pathogenesis of HIV-mediated vascular diseaseAm J Pathol200817241100111118310503
- OshimaTFloresSCVaitaitisGHIV-1 Tat increases endothelial solute permeability through tyrosine kinase and mitogen-activated protein kinase-dependent pathwaysAIDS200014547548210780709
- JiaHLohrMJezequelSCysteine-rich and basic domain HIV-1 Tat peptides inhibit angiogenesis and induce endothelial cell apoptosisBiochem Biophys Res Commun2001283246947911327725
- HuangMBKhanMGarcia-BarrioMPowellMBondVCApoptotic effects in primary human umbilical vein endothelial cell cultures caused by exposure to virion-associated and cell membrane-associated HIV-1 gp120J Acquir Immune Defic Syndr200127321322111464139
- DhawanSPuriRKKumarADuplanHMassonJMAggarwalBBHuman immunodeficiency virus-1-tat protein induces the cell surface expression of endothelial leukocyte adhesion molecule-1, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 in human endothelial cellsBlood1997904153515449269771
- RenZYaoQChenCHIV-1 envelope glycoprotein 120 increases intercellular adhesion molecule-1 expression by human endothelial cellsLab Invest200282324525511896203
- SandlerNGSeretiICan early therapy reduce inflammation?Curr Opin HIV AIDS201491727924247669
- LiovatASRey-CuilléMALécurouxCAcute plasma biomarkers of T cell activation set-point levels and of disease progression in HIV-1 infectionPLoS One2012710e4614323056251
- BurdoTHSoulasCOrzechowskiKIncreased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasmaPLoS Pathog201064e100084220419144
- HearpsACMaisaAChengWJHIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapyAIDS201226784385322313961
- MartinGEGouillouMHearpsACAge-associated changes in monocyte and innate immune activation markers occur more rapidly in HIV infected womenPLoS One201381e5527923365694
- ArmahKAMcGinnisKBakerJHIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activationClin Infect Dis201255112613622534147
- CroweSMWesthorpeCLMukhamedovaNJaworowskiASviridovDBukrinskyMThe macrophage: the intersection between HIV infection and atherosclerosisJ Leukoc Biol201087458959819952353
- MujawarZRoseHMorrowMPHuman immunodeficiency virus impairs reverse cholesterol transport from macrophagesPLoS Biol2006411e36517076584
- FunderburgNTMarkers of coagulation and inflammation often remain elevated in ART-treated HIV-infected patientsCurr Opin HIV AIDS201491808624275673
- AukrustPLunaLUelandTImpaired base excision repair and accumulation of oxidative base lesions in CD4+ T cells of HIV-infected patientsBlood2005105124730473515705786
- GrunfeldCPangMDoerrlerWLipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndromeJ Clin Endocrinol Metab1992745104510521373735
- RoseHHoyJWoolleyIHIV infection and high density lipoprotein metabolismAtherosclerosis20081991798618054941
- OhJHegeleRAHIV-associated dyslipidaemia: pathogenesis and treatmentLancet Infect Dis200771278779618045561
- NorataGDPirilloACatapanoALHDLs, immunity, and atherosclerosisCurr Opin Lipidol201122541041621881500
- D’AscenzoFCerratoEAppletonDPercutaneous coronary intervention and surgical revascularization in HIV Database (PHD) Study InvestigatorsPrognostic indicators for recurrent thrombotic events in HIV-infected patients with acute coronary syndromes: use of registry data from 12 sites in Europe, South Africa and the United StatesThromb Res2014134355856425064035
- MatetzkySDomingoMKarSAcute myocardial infarction in human immunodeficiency virus-infected patientsArch Intern Med2003163445746012588205
- SegevACantorWJStraussBHOutcome of percutaneous coronary intervention in HIV-infected patientsCatheter Cardiovasc Interv200668687988117086530
- BoccaraFTeigerECohenAPercutaneous coronary intervention in HIV infected patients: immediate results and long term prognosisHeart200692454354416537777
- BadrSMinhaSKitabataHSafety and long-term outcomes after percutaneous coronary intervention in patients with human immunodeficiency virusCatheter Cardiovasc Interv Epub2282014
- SavèsMChêneGDucimetièrePFrench WHO MONICA Project and the APROCO (ANRS EP11) Study GroupRisk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general populationClin Infect Dis200337229229812856222
- BrownTTColeSRLiXAntiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort studyArch Intern Med2005165101179118415911733
- Friis-MøllerNWeberRReissPDAD study groupCardiovascular disease risk factors in HIV patients – association with antiretroviral therapy. Results from the DAD studyAIDS20031781179119312819520
- SmithCJLevyISabinCAKayaEJohnsonMALipmanMCCardiovascular disease risk factors and antiretroviral therapy in an HIV-positive UK populationHIV Med200452889215012647
- SantosJPalaciosRGonzálezMRuizJMárquezMAtherogenic lipid profile and cardiovascular risk factors in HIV-infected patients (Nétar Study)Int J STD AIDS2005161067768016212715
- GlassTRUngsedhapandCWolbersMSwiss HIV Cohort StudyPrevalence of risk factors for cardiovascular disease in HIV-infected patients over time: the Swiss HIV Cohort StudyHIV Med20067640441016903986
- KaplanRCKingsleyLASharrettARTen-year predicted coronary heart disease risk in HIV-infected men and womenClin Infect Dis20074581074108117879928
- MasiáMPérez-CachafeiroSLeyesMGrupo de estudio de riesgo cardiovascular de CoRISCardiovascular risk in human immunodeficiency virus-infected patients in Spain. CoRIS cohort, 2011Enferm Infecc Microbiol Clin2012309517527 Spanish22551653
- JoyTKeoghHMHadiganCDietary fat intake and relationship to serum lipid levels in HIV-infected patients with metabolic abnormalities in the HAART eraAIDS200721121591160017630554
- MondyKOvertonETGrubbJMetabolic syndrome in HIV-infected patients from an urban, midwestern US outpatient populationClin Infect Dis200744572673417278068
- PalaciosRSantosJGonzálezMRuizJMárquezMIncidence and prevalence of the metabolic syndrome in a cohort of naive HIV-infected patients: prospective analysis at 48 weeks of highly active antiretroviral therapyInt J STD AIDS200718318418717362552
- DillonDGGurdasaniDRihaJAfrican Partnership for Chronic Disease Research (APCDR)Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysisInt J Epidemiol20134261754177124415610
- SchillaciGDe SocioGVPucciGAortic stiffness in untreated adult patients with human immunodeficiency virus infectionHypertension200852230831318559718
- LekakisJIkonomidisIPaliosJAssociation of highly active antiretroviral therapy with increased arterial stiffness in patients infected with human immunodeficiency virusAm J Hypertens200922882883419556973
- EcheverríaPBonjochAMoltóJPulse wave velocity as index of arterial stiffness in HIV-infected patients compared with a healthy populationJ Acquir Immune Defic Syndr2014651505623982659
- AdinolfLERestivoLZampinoRChronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosisAtherosclerosis2012221249650222385985
- IshizakaNIshizakaYTakahashiEAssociation between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickeningLancet2002359930113313511809259
- AlyanOKacmazFOzdemirOHepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score systemCirc J200872121960196518957787
- VassalleCMasiniSBianchiFZucchelliGCEvidence for association between hepatitis C virus seropositivity and coronary artery diseaseHeart200490556556615084562
- LeeMHYangHIWangCHHepatitis C virus infection and increased risk of cerebrovascular diseaseStroke201041122894290020966408
- YounossiZMStepanovaMNaderFYounossiZElsheikhEAssociations of chronic hepatitis C with metabolic and cardiac outcomesAliment Pharmacol Ther201337664765223384408
- HsuYHMuoCHLiuCYHepatitis C virus infection increases the risk of Developing peripheral arterial disease: a 9-year population-based cohort studyJ Hepatol Epub9252014
- SosnerPWangermezMChagneau-DerrodeCLe MoalGSilvainCAtherosclerosis risk in HIV-infected patients: the influence of hepatitis C virus co-infectionAtherosclerosis2012222127427722417840
- BedimoRWestfallAOMugaveroMDrechslerHKhannaNSaagMHepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patientsHIV Med201011746246820163481
- GillisJSmiejaMCesconAOHTN Cohort Study GroupRisk of cardiovascular disease associated with HCV and HBV coinfection among antiretroviral-treated HIV-infected individualsAntivir Ther201419330931724429380
- SutherlandMRRaynorCMLeenknegtHWrightJFPryzdialELCoagulation initiated on herpesvirusesProc Natl Acad Sci U S A1997942513510135149391056
- SunYPeiWWuYJingZZhangJWangGHerpes simplex virus type 2 infection is a risk factor for hypertensionHypertens Res200427854154415492472
- Espinola-KleinCRupprechtHJBlankenbergSAtheroGene InvestigatorsImpact of infectious burden on extent and long-term prognosis of atherosclerosisCirculation20021051152111772870
- HsuePYHuntPWSinclairEIncreased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responsesAIDS200620182275228317117013
- ParrinelloCMSinclairELandayALCytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected womenJ Infect Dis2012205121788179622492856
- HechterRCBudoffMHodisHNHerpes simplex virus type 2 (HSV-2) as a coronary atherosclerosis risk factor in HIV-infected men: multicenter AIDS cohort studyAtherosclerosis2012223243343622472456
- IslamFMWuJJanssonJWilsonDPRelative risk of renal disease among people living with HIV: a systematic review and meta-analysisBMC Public Health20121223422439731
- MaggiPBartolozziDBonfantiPRenal complications in HIV disease: between present and futureAIDS Rev2012141375322297503
- KalayjianRCFranceschiniNGuptaSKSuppression of HIV-1 replication by antiretroviral therapy improves renal function in persons with low CD4 cell counts and chronic kidney diseaseAIDS200822448148718301060
- ChoiAIShlipakMGHuntPWMartinJNDeeksSGHIV-infected persons continue to lose kidney function despite successful antiretro-viral therapyAIDS200923162143214919684507
- KalayjianRCLauBMechekanoRNRisk factors for chronic kidney disease in a large cohort of HIV-1 infected individuals initiating antiretroviral therapy in routine careAIDS201226151907191522824630
- NeuhausJJacobsDRBakerJVMarkers of inflammation, coagulation, and renal function are elevated in adults with HIV infectionJ Infect Dis2010201121788179520446848
- CalzaLPocaterraDPavoniMPlasma levels of VCAM-1, ICAM-1, E-Selectin, and P-Selectin in 99 HIV-positive patients versus 51 HIV-negative healthy controlsJ Acquir Immune Defic Syndr200950443043219322038
- MelendezMMMcNurlanMAMynarcikDCKhanSGelatoMCEndothelial adhesion molecules are associated with inflammation in subjects with HIV diseaseClin Infect Dis200846577578018225982
- HilemanCOCarmanTLLongeneckerCTRate and predictors of carotid artery intima media thickness progression in antiretroviral-naive HIV-infected and uninfected adults: a 48-week matched prospective cohort studyAntivir Ther201318792192923756436
- KurzKTeerlinkTSarclettiMWeissGZangerleRFuchsDPlasma concentrations of the cardiovascular risk factor asymmetric dimethylarginine (ADMA) are increased in patients with HIV-1 infection and correlate with immune activation markersPharmacol Res200960650851419651212
- TriantVAMeigsJBGrinspoonSKAssociation of C-reactive protein and HIV infection with acute myocardial infarctionJ Acquir Immune Defic Syndr200951326827319387353
- KullerLHTracyRBellosoWINSIGHT SMART Study GroupInflammatory and coagulation biomarkers and mortality in patients with HIV infectionPLoS Med2008510e20318942885
- DuprezDANeuhausJKullerLHINSIGHT SMART Study GroupInflammation, coagulation and cardiovascular disease in HIV-infected individualsPLoS One201279e4445422970224
- NordellADMcKennaMBorgesÁHDuprezDNeuhausJNeatonJDINSIGHT SMART, ESPRIT Study GroupsSILCAAT Scientific Committee.Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulationJ Am Heart Assoc201433e00084424870935
- TenorioARZhengYBoschRJSoluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatmentJ Infect Dis201421081248125924795473
- LongeneckerCTFunderburgNTJiangYMarkers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individualsHIV Med201314638539023332012
- RossACRizkNO’RiordanMARelationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapyClin Infect Dis20094971119112719712036
- JangJJBerkheimerSBMerchantMKrishnaswamiAAsymmetric dimethylarginine and coronary artery calcium scores are increased in patients infected with human immunodeficiency virusAtherosclerosis2011217251451721549379
- BurdoTHLentzMRAutissierPSoluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapyJ Infect Dis2011204115416321628670
- BeltránLMMuñoz HernándezRde Pablo BernalRSReduced sTWEAK and increased sCD163 levels in HIV-infected patients: modulation by antiretroviral treatment, HIV replication and HCV co-infectionPLoS One201493e9054124594990
- BurdoTHLoJAbbaraSSoluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patientsJ Infect Dis201120481227123621917896
- FitchKVSrinivasaSAbbaraSNoncalcified coronary atherosclerotic plaque and immune activation in HIV-infected womenJ Infect Dis2013208111737174624041790
- SubramanianSTawakolABurdoTHArterial inflammation in patients with HIVJAMA2012308437938622820791
- LongeneckerCTJiangYOrringerCESoluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infectionAIDS201428796997724691204
- BakerJVHullsiekKHSinghACDC SUN Study InvestigatorsImmunologic predictors of coronary artery calcium progression in a contemporary HIV cohortAIDS201428683184024370480
- KristoffersenUSKofoedKKronborgGGigerAKKjaerALebechAMReduction in circulating markers of endothelial dysfunction in HIV-infected patients during antiretroviral therapyHIV Med2009102798719200170
- McComseyGAKitchDDaarESInflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavirAIDS201226111371138522546988
- CalmyAGayet-AgeronAMontecuccoFSTACCATO Study GroupHIV increases markers of cardiovascular risk: results from a randomized, treatment interruption trialAIDS200923892993919425222
- WolfKTsakirisDAWeberRErbPBattegayMSwiss HIV Cohort StudyAntiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1J Infect Dis2002185445646211865397
- KurzKTeerlinkTSarclettiMWeissGZangerleRFuchsDAsymmetric dimethylarginine concentrations decrease in patients with HIV infection under antiretroviral therapyAntivir Ther20121761021102722892398
- BakerJVNeuhausJDuprezDINSIGHT SMART Study GroupHIV replication, inflammation, and the effect of starting antiretroviral therapy on plasma asymmetric dimethylarginine, a novel marker of endothelial dysfunctionJ Acquir Immune Defic Syndr201260212813422421746
- JaworowskiAElleryPMaslinCLNormal CD16 expression and phagocytosis of Mycobacterium avium complex by monocytes from a current cohort of HIV-1-infected patientsJ Infect Dis2006193569369716453265
- KamatAMisraVCassolEA plasma biomarker signature of immune activation in HIV patients on antiretroviral therapyPLoS One201272e3088122363505
- MalherbeGSteelHCCassolSCirculating biomarkers of immune activation distinguish viral suppression from nonsuppression in HAART-treated patients with advanced HIV-1 subtype C infectionMediators Inflamm2014201419841324808634
- HatanoHYuklSAFerreALProspective antiretroviral treatment of asymptomatic, HIV-1 infected controllersPLoS Pathog2013910e100369124130489
- HsuePYHuntPWSchnellARole of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosisAIDS20092391059106719390417
- PereyraFLoJTriantVAIncreased coronary atherosclerosis and immune activation in HIV-1 elite controllersAIDS201226182409241223032411
- HoJEScherzerRHechtFMThe association of CD4+ T-cell counts and cardiovascular risk in treated HIV diseaseAIDS20122691115112022382147
- van LelyveldSFGrasLKesselringAATHENA national observational cohort studyLong-term complications in patients with poor immunological recovery despite virological successful HAART in Dutch ATHENA cohortAIDS201226446547422112603
- GrantPMKitchDMcComseyGALow baseline CD4+ count is associated with greater bone mineral density loss after antiretroviral therapy initiationClin Infect Dis201357101483148823943825
- ChevalierMFPetitjeanGDunyach-RémyCThe Th17/Treg ratio, IL-1RA and sCD14 levels in primary HIV infection predict the T-cell activation set point in the absence of systemic microbial translocationPLoS Pathog201396e100345323818854
- VinikoorMJCopeAGayCLAntiretroviral therapy initiated during acute HIV infection fails to prevent persistent T-cell activationJ Acquir Immune Defic Syndr201362550550823314410
- JainVHartogensisWBacchettiPAntiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir sizeJ Infect Dis201320881202121123852127
- WilkinTJLalamaCMMcKinnonJA pilot trial of adding maraviroc to suppressive antiretroviral therapy for suboptimal CD4+ T-cell recovery despite sustained virologic suppression: ACTG A5256J Infect Dis2012206453454222740718
- HuntPWShulmanNSHayesTLThe immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trialBlood2013121234635464623589670
- MassanellaMNegredoEPuigJRaltegravir intensification shows differing effects on CD8 and CD4 T cells in HIV-infected HAART-suppressed individuals with poor CD4 T-cell recoveryAIDS201226182285229323018435
- HatanoHScherzerRWuYA randomized controlled trial assessing the effects of raltegravir intensification on endothelial function in treated HIV infectionJ Acquir Immune Defic Syndr201261331732522918156
- HatanoHStrainMCScherzerRIncrease in 2-long terminal repeat circles and decrease in D-dimer after raltegravir intensification in patients with treated HIV infection: a randomized, placebo-controlled trialJ Infect Dis201320891436144223975885
- SilvaEFCharreauIGourmelBANRS 138 EASIER Study GroupDecreases in inflammatory and coagulation biomarkers levels in HIV-infected patients switching from enfuvirtide to raltegravir: ANRS 138 substudyJ Infect Dis2013208689289723801606
- MartínezED’AlbuquerquePMLlibreJMSPIRAL Trial GroupChanges in cardiovascular biomarkers in HIV-infected patients switching from ritonavir-boosted protease inhibitors to raltegravirAIDS201226182315232623018438
- MasiáMMartínezEPadillaSGatellJMGutiérrezFEndothelial function in HIV-infected patients switching from a boosted protease inhibitor-based regimen to raltegravir: a substudy of the SPIRAL studyJ Antimicrob Chemother201368240941323075691
- KlattNRFunderburgNTBrenchleyJMMicrobial translocation, immune activation, and HIV diseaseTrends Microbiol201321161323062765
- BrenchleyJMPriceDASchackerTWMicrobial translocation is a cause of systemic immune activation in chronic HIV infectionNat Med200612121365137117115046
- WilsonEMSeretiIImmune restoration after antiretroviral therapy: the pitfalls of hasty or incomplete repairsImmunol Rev2013254134335423772630
- MehandruSPolesMATenner-RaczKPrimary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tractJ Exp Med2004200676177015365095
- MavignerMCazabatMDuboisMAltered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individualsJ Clin Invest20121221626922156200
- LegeinBTemmermanLBiessenEALutgensEInflammation and immune system interactions in atherosclerosisCell Mol Life Sci2013702038476923430000
- FunderburgNTJiangYDebanneSMRosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapyClin Infect Dis201458458859524253250
- EckardARJiangYDebanneSMFunderburgNTMcComseyGAEffect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapyJ Infect Dis201420981156116424415784
- RidkerPMCushmanMStampferMJTracyRPHennekensCHInflammation, aspirin, and the risk of cardiovascular disease in apparently healthy menN Engl J Med1997336149739799077376
- RidkerPMHennekensCHBuringJERifaiNC-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in womenN Engl J Med20003421283684310733371
- RidkerPMBuringJECookNRRifaiNC-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American womenCirculation2003107339139712551861
- BallantyneCMHoogeveenRCBangHLipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) studyArch Intern Med2005165212479248416314544
- FordESGreenwaldJHRichtermanAGTraditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infectionAIDS201024101509151720505494
- CesariMPenninxBWNewmanABInflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study)Am J Cardiol200392552252812943870
- FolsomARChamblessLEBallantyneCMAn assessment of incremental coronary risk prediction using C-reactive protein and other novel risk markers: the atherosclerosis risk in communities studyArch Intern Med2006166131368137316832001
- TzoulakiIMurrayGDLeeAJRumleyALoweGDFowkesFGRelative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh Artery StudyCirculation2007115162119212717404162
- DaneshJKaptogeSMannAGLong-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic reviewPLoS Med200854e7818399716
- TakeiYDi TullioMRHommaSSoluble tumor necrosis factor receptor 1 level is associated with left ventricular hypertrophy: the northern Manhattan studyAm J Hypertens200922776376919390513
- ValgimigliMCeconiCMalaguttiPTumor necrosis factor-alpha receptor 1 is a major predictor of mortality and new-onset heart failure in patients with acute myocardial infarction: the Cytokine-Activation and Long-Term Prognosis in Myocardial Infarction (C-ALPHA) studyCirculation2005111786387015699251
- UelandTKjekshusJFrølandSSPlasma levels of soluble tumor necrosis factor receptor type I during the acute phase following complicated myocardial infarction predicts survival in high-risk patientsJ Am Coll Cardiol200546112018202116325035
- NilssonLSzymanowskiASwahnEJonassonLSoluble TNF receptors are associated with infarct size and ventricular dysfunction in ST-elevation myocardial infarctionPLoS One201382e5547723405158
- MorenoJAMuñoz-GarcíaBMartín-VenturaJLThe CD163-expressing macrophages recognize and internalize TWEAK: potential consequences in atherosclerosisAtherosclerosis2009207110311019473660
- AristoteliLPMøllerHJBaileyBMoestrupSKKritharidesLThe monocytic lineage specific soluble CD163 is a plasma marker of coronary atherosclerosisAtherosclerosis2006184234234715979079
- MorenoJADejouvencelTLabreucheJPeripheral artery disease is associated with a high CD163/TWEAK plasma ratioArterioscler Thromb Vasc Biol20103061253126220299688
- KnudsenAMøllerHJKatzensteinTLSoluble CD163 does not predict first-time myocardial infarction in patients infected with human immunodeficiency virus: a nested case-control studyBMC Infect Dis20131323023692821
- ReinerAPLangeEMJennyNSSoluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adultsArterioscler Thromb Vasc Biol201333115816423162014
- KelesidisTKendallMAYangOOHodisHNCurrierJSBiomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infectionJ Infect Dis2012206101558156723066162
- RogacevKSSeilerSZawadaAMCD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney diseaseEur Heart J2011321849220943670
- RogacevKSCremersBZawadaAMCD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiographyJ Am Coll Cardiol201260161512152022999728
- HeineGHUlrichCSeibertECD14(++)CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patientsKidney Int200873562262918160960
- JaipersadASShantsilaALipGYShantsilaEExpression of monocyte subsets and angiogenic markers in relation to carotid plaque neovascularization in patients with pre-existing coronary artery disease and carotid stenosisAnn Med20141119
- BergKELjungcrantzIAnderssonLElevated CD14++CD16-monocytes predict cardiovascular eventsCirc Cardiovasc Genet20125112213122238190
- BarbourJDJalbertECChowDCReduced CD14 expression on classical monocytes and vascular endothelial adhesion markers independently associate with carotid artery intima media thickness in chronically HIV-1 infected adults on virologically suppressive anti-retroviral therapyAtherosclerosis20142321525824401216
- HwangSJBallantyneCMSharrettARCirculating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) studyCirculation19979612421942259416885
- BlankenbergSRupprechtHJBickelCCirculating cell adhesion molecules and death in patients with coronary artery diseaseCirculation2001104121336134211560847
- HilemanCOLongeneckerCTCarmanTLMcComseyGAC-reactive protein predicts 96-week carotid intima media thickness progression in HIV-infected adults naive to antiretroviral therapyJ Acquir Immune Defic Syndr201465334034424220288
- SchnabelRBlankenbergSLubosEAsymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene StudyCirc Res2005975e53e5916100045
- LeongTZylbersteinDGrahamISwedish-Irish-Norwegian CollaborationAsymmetric dimethylarginine independently predicts fatal and nonfatal myocardial infarction and stroke in women: 24-year follow-up of the population study of women in GothenburgArterioscler Thromb Vasc Biol200828596196718292394
- ParikhRVScherzerRNittaEMIncreased levels of asymmetric dimethylarginine are associated with pulmonary arterial hypertension in HIV infectionAIDS201428451151924469026
- Blanco-ColioLMMartín-VenturaJLMuñóz-GarcíaBIdentification of soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK) as a possible biomarker of subclinical atherosclerosisArterioscler Thromb Vasc Biol200727491692217272745
- Jelić-IvanovićZBujisićNSpasićSBogavac-StanojevićNSpasojević-KalimanovskaVKotur-StevuljevićJCirculating sTWEAK improves the prediction of coronary artery diseaseClin Biochem20094213–141381138619505454
- YilmazMISonmezAOrtizASoluble TWEAK and PTX3 in nondialysis CKD patients: impact on endothelial dysfunction and cardiovascular outcomesClin J Am Soc Nephrol20116478579221330486
- ChorianopoulosERosenbergMZugckCWolfJKatusHAFreyNDecreased soluble TWEAK levels predict an adverse prognosis in patients with chronic stable heart failureEur J Heart Fail200911111050105619875405
- UrbonavicieneGMartin-VenturaJLLindholtJSImpact of soluble TWEAK and CD163/TWEAK ratio on long-term cardiovascular mortality in patients with peripheral arterial diseaseAtherosclerosis2011219289289921962403
