Abstract
Breast cancer is the most common cancer in the UK. Advances in the methods of early diagnosis as well as newer and more effective treatments have led to improvements of disease-free and overall survival over the last decade. Almost one-third of breast cancers present with an aggressive form characterized by increased expression of human epidermal growth receptor 2 (HER2) proteins. A targeted treatment using monoclonal antibodies against HER2 expression such as trastuzumab has been shown to improve survival. Unfortunately, there is a degree of cardiotoxicity associated with these agents, as inhibition of HER2 pathways can also impair cardioprotective pathways. In the present review, we discuss the mechanisms by which trastuzumab might affect vascular homeostasis leading to endothelial dysfunction. We also provide suggestions for future research examining the effects of trastuzumab on the vasculature in breast cancer.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Breast cancer is the most common cancer in the UK and accounts for 30% of all new cancer cases in females with a greater incidence in women over the age of 60 years.Citation1 Fortunately, a variety of treatments including surgical resection, adjuvant chemotherapy, hormonal therapy, and radiation therapy have been shown to improve survival and reduce the risk of tumor reoccurrence. Approximately 15%–25% of patients present with aggressive breast cancer characterized by increased expression of human epidermal growth receptor 2 (HER2) proteins in the breast tissue.Citation1 It is postulated that HER2-positivity increases the likelihood of invasion and survival of tumor cells at the site of metastasis.Citation2 Therefore, patients with HER2-positive tumors may also have increased resistance to common anticancer treatments, such as chemotherapy and radiation therapy.Citation2
In these patients, targeted treatment using monoclonal antibodies such as trastuzumab (Herceptin) can be used to reduce tumor recurrence and improve survival.Citation3,Citation4 Trastuzumab selectively binds to the extracellular domain of HER2 receptors, where it inhibits downstream signaling pathways, resulting in a reduced proliferation of tumor cells. This is achieved by identifying tumor cells for immune destruction, and then, by initiation of antibody-dependent cellular cytotoxicity, causing apoptosis of tumor cells.Citation5 In addition, trastuzumab undergoes internalization (endocytosis) into the tumor cells and subsequently increases the expression of HER2 on the cellular surface. This enhances the immune effects of trastuzumab and reduces tumor expression.Citation5 However, trastuzumab is also associated with an increased risk of cardiotoxicity which manifests clinically as congestive heart failure (CHF).Citation6 Trastuzumab-mediated cardiotoxicity appears to be independent of drug dose, has not been shown to be associated with structural changes in cardiomyocytes, and is fully reversible following cessation of treatment.Citation7 Cardiotoxicity is more likely to occur in patients who have preexisting hypertension, a history of smoking, obesity, family history of cardiovascular disease (CVD), and previous coronary artery disease – all of which are well-established risk factors for cardiac events.Citation8,Citation9 CVD and its sequelae are strong predictors of mortality in patients with breast cancer, this association being independent of breast cancer stage.Citation10 It is therefore possible that trastuzumab-related cardiotoxicity might be mediated by adverse drug effects on the coronary vasculature. In this review, we summarize the biological mechanisms by which trastuzumab may affect the vasculature and contribute to CVD risk. We also provide suggestions about the investigation of trastuzumab-mediated cardiotoxicity in future research studies.
The importance of HER2 and neuregulin
The human epidermal growth receptors are tyrosine-kinase receptors and are expressed as four isoforms: HER1, HER2, HER3, and HER4. The second isoform (HER2) regulates the growth, repair, and regeneration of breast tissue,Citation11 but overexpression of HER2 receptors via polymorphisms in the erb-b2 receptor tyrosine kinase 2 oncogene can lead to uncontrolled cell growth.Citation12 This state is known as HER2-positivity and is linked with increased mortality.Citation13,Citation14 Under normal conditions, a protein called neuregulin is released by coronary microvascular endothelial cells and the endocardium.Citation15 Neuregulin binds to HER4 receptors which dimerize with HER2 receptors and increase several survival pathways in the myocardium.Citation15 The primary role of the survival pathways is to inhibit the production of reactive oxygen species (ROS) and maintain cellular integrity by reducing cell apoptosis. In an animal model of adult rat ventricular myocytes, treatment with either paclitaxel or trastuzumab resulted in damage to cardiac myofilaments which corresponded with increased intracellular calcium, reduced diastolic relaxation time, and increased oxidative stress.Citation16 These adverse changes following trastuzumab increase the risk of developing CHF (for review, see Sandoo et alCitation17). Indeed, the HER2/neuregulin pathway is integral to the preservation of sarcomeres in the cardiomyocytes.Citation18 However, neuregulin can also increase the expression of endothelial nitric oxide synthase (eNOS).Citation15 The eNOS gene is constitutively expressed in vascular endothelial cells and releases nitric oxide (NO) – a vasoactive molecule that reduces oxidative stress and prevents atherosclerosis.Citation19 NO bioavailability is increased via activation from protein kinase B as a result of the dimerization of HER2 and HER4 receptors.Citation15 Trastuzumab blocks this dimerization and therefore inhibits the cardioprotective actions of neuregulin.Citation20 Given that the myocardium is a highly vascularized territory, it is possible that abnormalities in the vasculature following trastuzumab could also contribute to CHF. In the following section, we discuss the implications of blocking HER2 using trastuzumab on vascular endothelial cells, and highlight how this may contribute to cardiovascular complications in breast cancer.
Mechanisms for trastuzumab-mediated endothelial dysfunction
eNOS
The endothelium is the delicate and permeable lining of the vasculature which allows specific vasoactive factors to regulate homeostatic processes and prevent atherosclerosis.Citation19 In a healthy vessel, eNOS continuously synthesizes NO, helping to maintain basal vasodilator tone and preventing platelet aggregation and formation of ROS.Citation19 In chronic disease states characterized by increased oxidative stress and low-grade chronic inflammation (such as breast cancer), endothelial cells can be damaged, leading to a reduction in eNOS expression and endothelial dysfunction.Citation21,Citation22
The expression of eNOS has been postulated to play an important role in regulating cardiovascular function and overall survival in breast cancer. In a recent study, Zeglinski et alCitation23 compared 60 wild-type mice with 60 mice having a congenital absence of the eNOS gene treated with either saline, doxorubicin, or trastuzumab, or both drugs simultaneously. Following simultaneous treatment with both agents, the mice with absence of the eNOS gene presented with adverse modeling of the left ventricle, reduced left ventricular systolic function, increased oxidative stress, and reduced survival when compared with the wild-type mice. These findings suggest that adequate expression of the eNOS gene is important in suppressing the generation of ROS which may in turn improve cardiac function and survival following trastuzumab treatment. Indeed, in human breast cancer patients receiving chemotherapy, polymorphisms in the eNOS gene result in poorer survival,Citation24 and expression of eNOS in the microvessels surrounding breast tumors associates with better prognosis, possibly due to a reduction in tumor progression.Citation25 In addition, polymorphisms in the Asp298 allele of the eNOS gene result in impaired NO synthesis which reduces event-free survival in patients with systolic heart failure.Citation26
Generation of ROS
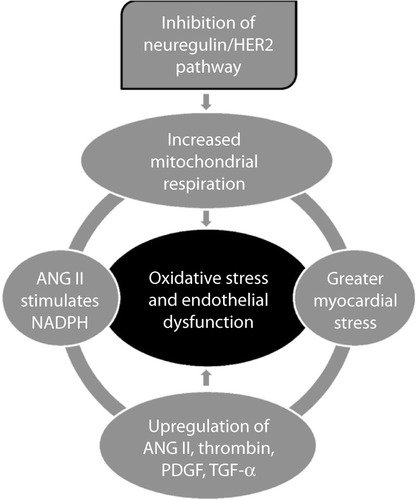
Inhibition of the neuregulin/HER2 pathway by trastuzumab allows increased production of ROS,Citation15 due to abnormalities in mitochondrial respiration in cardiomyocytes, which increases myocardial stress.Citation27 When the myocardium is increasingly stressed, angiotensin II (ANG II), thrombin, platelet-derived growth factor, and tumor growth factor-alpha are upregulated and stimulate vascular smooth muscle cells to increase formation of further ROS.Citation22 ANG II, which is a potent vasoconstrictor, also inhibits neuregulin activity in the coronary microvasculature and increases levels of nicotinamide adenine dinucleotide phosphate-oxidase (NADPH).Citation28,Citation29 NADPH has been implicated as a causal factor of endothelial dysfunction due to its ability to generate large amounts of ROS in the endothelium.Citation22 The mechanisms highlighted above represent a vicious cycle of free radical formation which will injure the endothelium causing endothelial dysfunction ().
Figure 1 The generation of reactive oxygen species following inhibition of the neuregulin/HER2 pathway.
Endothelial dysfunction and CHF
Treatment with trastuzumab does not cause morphological alterations to cardiomyocytes, and myocardial function can improve following cessation of treatment.Citation30 Although apoptosis of cardiomyocytes following trastuzumab treatment can result in CHF,Citation31 in the general population, endothelial dysfunction is one of the primary causal factors for CHF.Citation32–Citation35 On this basis, it has been hypothesized that endothelial dysfunction might be the principal mechanism for trastuzumab-mediated CHF (see ).Citation17
Figure 2 The effects of trastuzumab on the vasculature and its subsequent contribution to congestive heart failure.
Abbreviations: ANG II, angiotensin II; eNOS, endothelial nitric oxide synthase; HER, human epidermal growth receptor; NO, nitric oxide; ROS, reactive oxygen species.
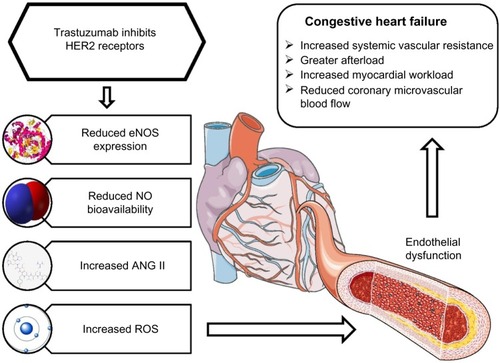
The myocardial microvasculature receives less than 5% of cardiac output yet regulates the majority of blood flow to the myocardium.Citation36 Unsurprisingly, in a 5-year prospective study of 242 CHF patients, microvascular endothelial dysfunction was independently associated with cardiac events.Citation33 Similarly, Fischer et alCitation32 revealed that large-vessel endothelial dysfunction was independently associated with higher incidence of hospitalization from CHF, cardiac transplantation, and cardiac death. These findings suggest that global alterations in the vasculature are important determinants for the development of CHF and associated comorbidities.
The central mechanisms in the development of CHF involve the reduction in L-arginine and tetrahydrobiopterin (BH4) concentrations – both essential substrates for the synthesis of NO.Citation37 In particular, absence of the NO cofactor BH4 can increase the production of ROS, perpetuating myocardial damage. The conversion of L-arginine to NO by eNOS requires the presence of BH4 which helps the transfer of electrons produced at the eNOS site to an oxidase domain, resulting in activation of oxygen. The activated oxygen is then “coupled” with L-arginine oxidation to produce NO.Citation38 However, in the absence of BH4 or L-arginine, eNOS produces superoxide molecules instead of NO.Citation38 Levels of BH4 are reduced in CHF,Citation39 and the consequent reduction in NO will impact on coronary vasorelaxation and basal blood flow, both of which are important physiological factors for the development of CHF.Citation40
The renin–angiotensin system also plays an important role in regulating the release of NO from vascular endothelial cells. In CHF patients presenting with decreased vasodilatory function of the large arteries, angiotensin-converting enzyme (ACE) inhibitors improve vasodilatation as a result of increased NO bioavailability.Citation41 Similarly, endothelial function improves following administration of ANG II receptor antagonists concomitantly with ACE inhibitors.Citation42 In breast cancer patients treated with trastuzumab, concomitant administration of ACE inhibitors, ANG II receptor antagonists, and beta-blockers improves left ventricular ejection fraction over the course of treatment.Citation43 Given that trastuzumab can reduce NO production and increase production of ANG II via inhibition of neuregulin, it is plausible that CHF resulting from trastuzumab treatment is likely to be mediated, in part, by endothelial dysfunction of the coronary vasculature.
Role of classical CVD risk in potentiating trastuzumab-mediated cardiotoxicity
The link between endothelial dysfunction and development of CHF in trastuzumab-treated patients could be mediated by several classical CVD risk factors. Associations between CVD risk factors implicated in endothelial dysfunction and trastuzumab-mediated cardiotoxicity have been reported.Citation8,Citation9 The presence of severe hypertension in particular appears to be an important factor in the development of heart failure following trastuzumab treatment.Citation8,Citation44 Hypertension usually occurs when NO bioavailability is reduced, allowing vasoconstrictors such as endothelin-1 and ANG II to increase systemic vascular resistance.Citation45 The direct consequence is an augmentation in afterload and intracavitary pressures of the left ventricle. If the increased myocardial stress is coupled with poor myocardial blood flow, cardiac workload will be increased, leading to profound damage of the myocardium and CHF.Citation46 Thus, the impact of CVD risk status is important in understanding the impact of trastuzumab treatment on the vasculature.
Proposals for future research studies
The investigation of endothelial dysfunction following trastuzumab treatment has received little attention in the literature to date, yet it is clear that abnormalities in the vasculature following inhibition of HER2 could be implicated in the development of trastuzumab-mediated cardiotoxicity. At present, several noninvasive assessments which examine vasodilatory function and arterial structure can be utilized in patients with breast cancer.Citation19 These peripheral vascular assessments correlate well with assessments in the coronary circulationCitation47,Citation48 and are strong predictors of cardiac events in a variety of clinical populations at risk of CVD,Citation49,Citation50 including postmenopausal women.Citation51 Thus, we suggest that, in addition to further in vitro and animal model work, studies should also be conducted to 1) identify whether there is evidence of endothelial dysfunction in trastuzumab-treated patients when compared to non-trastuzumab-treated patients and healthy controls; 2) establish the time course of endothelial dysfunction by conducting long-term prospective studies examining endothelial function at pretreatment baseline and at multiple follow-up points; and 3) assess the impact of classical CVD risk factors and their control on the above. Such studies will enable better understanding of the processes that cause CHF following trastuzumab treatment, inform possible pharmacological and nonpharmacological interventions, and eventually improve cardiovascular outcomes in this patient population.
Disclosure
The authors report no conflicts of interest in this work.
References
- Breast cancer statistics [webpage on the internet]LondonCancer Research UK [updated November 27, 2014 Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/breast/Accessed January 15, 2015
- YuDHungMCOverexpression of ErbB2 in cancer and ErbB2-targeting strategiesOncogene2000196115612111156524
- SlamonDJLeyland-JonesBShakSUse of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2N Engl J Med200134478379211248153
- Piccart-GebhartMJProcterMLeyland-JonesBHerceptin Adjuvant (HERA) Trial Study TeamTrastuzumab after adjuvant chemotherapy in HER2-positive breast cancerN Engl J Med20053531659167216236737
- MolinaMACodony-ServatJAlbanellJRojoFArribasJBaselgaJTrastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cellsCancer Res2001614744474911406546
- ZeglinskiMLudkeAJassalDSSingalPKTrastuzumab-induced cardiac dysfunction: a ‘dual-hit’Exp Clin Cardiol201116707422065936
- FloydJDNguyenDTLobinsRLBashirQDollDCPerryMCCardiotoxicity of cancer therapyJ Clin Oncol2005237685769616234530
- WadhwaDFallah-RadNGrenierDTrastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: a retrospective studyBreast Cancer Res Treat200911735736419082707
- GunaldiMDumanBBAfsarCURisk factors for developing cardiotoxicity of trastuzumab in breast cancer patients: an observational single-centre studyJ Oncol Pharm Pract Epub 172015
- SatarianoWARaglandDRThe effect of comorbidity on 3-year survival of women with primary breast cancerAnn Intern Med19941201041108256968
- ChoHSMasonKRamyarKXStructure of the extracellular region of HER2 alone and in complex with the Herceptin FabNature200342175676012610629
- SeshadriRFirgairaFAHorsfallDJMcCaulKSetlurVKitchenPClinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study GroupJ Clin Oncol199311193619428105035
- BerchuckAKamelAWhitakerROverexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancerCancer Res199050408740911972347
- SlamonDJGodolphinWJonesLAStudies of the HER-2/neu proto-oncogene in human breast and ovarian cancerScience19892447077122470152
- LemmensKDoggenKDe KeulenaerGWRole of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failureCirculation200711695496017709650
- PentassugliaLTimolatiFSeifrizFAbudukadierKSuterTMZuppingerCInhibition of ErbB2/neuregulin signaling augments paclitaxel-induced cardiotoxicity in adult ventricular myocytesExp Cell Res20073131588160117400210
- SandooAKitasGDCarmichaelAREndothelial dysfunction as a determinant of trastuzumab-mediated cardiotoxicity in patients with breast cancerAnticancer Res2014341147115124596352
- KuramochiYGuoXSawyerDBNeuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytesJ Mol Cell Cardiol20064122823516769082
- SandooAvan ZantenJJMetsiosGSCarrollDKitasGDThe endothelium and its role in regulating vascular toneOpen Cardiovasc Med J2010430231221339899
- PentassugliaLSawyerDBThe role of Neuregulin-1beta/ErbB signaling in the heartExp Cell Res200931562763718801360
- WilcoxJNSubramanianRRSundellCLExpression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vesselsArterioscler Thromb Vasc Biol199717247924889409218
- CaiHHarrisonDGEndothelial dysfunction in cardiovascular diseases: the role of oxidant stressCirc Res20008784084411073878
- ZeglinskiMPremeczSLernerJCongenital absence of nitric oxide synthase 3 potentiates cardiac dysfunction and reduces survival in doxorubicin- and trastuzumab-mediated cardiomyopathyCan J Cardiol20143035936724484915
- ChoiJYBarlowWEAlbainKSNitric oxide synthase variants and disease-free survival among treated and untreated breast cancer patients in a Southwest Oncology Group clinical trialClin Cancer Res2009155258526619671875
- MortensenKHolckSChristensenIJEndothelial cell nitric oxide synthase in peritumoral microvessels is a favorable prognostic indicator in premenopausal breast cancer patientsClin Cancer Res199951093109710353743
- McNamaraDMHolubkovRPostavaLEffect of the Asp298 variant of endothelial nitric oxide synthase on survival for patients with congestive heart failureCirculation20031071598160212668492
- GrazetteLPBoeckerWMatsuiTInhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathyJ Am Coll Cardiol2004442231223815582322
- NakagamiHTakemotoMLiaoJKNADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophyJ Mol Cell Cardiol20033585185912818576
- CardinaleDColomboASandriMTPrevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibitionCirculation20061142474248117101852
- Di CosimoSHeart to heart with trastuzumab: a review on cardiac toxicityTarget Oncol2011618919522125051
- GordonLIBurkeMASinghATBlockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathwaysJ Biol Chem20092842080208719017630
- FischerDRossaSLandmesserUEndothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or deathEur Heart J200526656915615801
- de BerrazuetaJRGuerra-RuizAGarcía-UnzuetaMTEndothelial dysfunction, measured by reactive hyperaemia using strain-gauge plethysmography, is an independent predictor of adverse outcome in heart failureEur J Heart Fail20101247748320354033
- HornigBMaierVDrexlerHPhysical training improves endothelial function in patients with chronic heart failureCirculation1996932102148548890
- HornigBArakawaNKohlerCDrexlerHVitamin C improves endothelial function of conduit arteries in patients with chronic heart failureCirculation1998973633689468210
- BrutsaertDLFransenPAndriesLJDe KeulenaerGWSysSUCardiac endothelium and myocardial functionCardiovasc Res1998382812909709389
- LinkeARecchiaFZhangXHintzeTHAcute and chronic endothelial dysfunction: implications for the development of heart failureHeart Fail Rev20038879712652162
- AlkaitisMSCrabtreeMJRecoupling the cardiac nitric oxide synthases: tetrahydrobiopterin synthesis and recyclingCurr Heart Fail Rep2012920021022711313
- MoensALTakimotoETocchettiCGReversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategyCirculation20081172626263618474817
- SeddonMMelikianNDworakowskiREffects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivoCirculation20091192656266219433760
- HornigBArakawaNHaussmannDDrexlerHDifferential effects of quinaprilat and enalaprilat on endothelial function of conduit arteries in patients with chronic heart failureCirculation199898284228489860785
- GiannattasioCAchilliFGrappioloARadial artery flow-mediated dilatation in heart failure patients: effects of pharmacological and nonpharmacological treatmentHypertension2001381451145511751734
- OlivaSCioffiGFrattiniSItalian Cardio-Oncological NetworkAdministration of angiotensin-converting enzyme inhibitors and β-blockers during adjuvant trastuzumab chemotherapy for nonmetastatic breast cancer: marker of risk or cardioprotection in the real world?Oncologist20121791792422673631
- HerrmannJHerrmannSMHaddadTCNew-onset heart failure in association with severe hypertension during trastuzumab therapyMayo Clin Proc2014891734173925441402
- NadarSBlannADLipGYEndothelial dysfunction: methods of assessment and application to hypertensionCurr Pharm Des2004103591360515579056
- BuusNHBøttcherMHermansenFSanderMNielsenTTMulvanyMJInfluence of nitric oxide synthase and adrenergic inhibition on adenosine-induced myocardial hyperemiaCirculation20011042305231011696470
- AndersonTJUehataAGerhardMDClose relation of endothelial function in the human coronary and peripheral circulationsJ Am Coll Cardiol199526123512417594037
- TakaseBHamabeASatomuraKClose relationship between the vasodilator response to acetylcholine in the brachial and coronary artery in suspected coronary artery diseaseInt J Cardiol2005105586616207546
- GokceNKeaneyJFJrHunterLMPredictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular diseaseJ Am Coll Cardiol2003411769177512767663
- SchächingerVBrittenMBZeiherAMPrognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart diseaseCirculation20001011899190610779454
- RossiRNuzzoAOriglianiGModenaMGPrognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal womenJ Am Coll Cardiol200851997100218325438
