Abstract
Aneurysms at non-branching sites in the supraclinoid internal carotid artery (ICA) can be classified as “blood blister-like aneurysms” (BBAs), which have blood blister-like configurations and fragile walls. While surgical treatment for the BBA in the acute stage is recommended, the optimal surgical procedure remains controversial. In the study reported here, we describe the case of a 37-year-old woman with a ruptured BBA in the ophthalmic segment of the right ICA who underwent wrap-clipping with external carotid artery–internal carotid artery bypass by intraoperative estimation of the measurement of cortical cerebral blood flow (CoBF) using a thermal diffusion flow probe. Trapping of the ICA in the acute stage of subarachnoid hemorrhage may result in ischemic complications secondary to hemodynamic hypoperfusion or occlusion of the perforating artery, and/or delayed vasospasm, even with concomitant bypass surgery. We believe that it is important to perform scheduled external carotid artery–internal carotid artery bypass before trapping of the ICA in patients with a ruptured BBA in the acute stage of subarachnoid hemorrhage and to perform wrap-clipping rather than trapping. This would provide much more CoBF if a reduction of CoBF occurs after trapping occlusion of the ICA including a ruptured BBA according to intraoperative CoBF monitoring. As far as we are aware, the case reported here is the first report on high-flow bypass and wrap-clipping for a ruptured BBA of the ICA using intraoperative monitoring of cerebral hemodynamics.
Introduction
While surgical treatment for a ruptured blood blister-like aneurysm (BBA) of the supraclinoid internal carotid artery (ICA) in the acute stage is recommended, BBAs tend to rupture intraoperatively, resulting in the formation of a large ICA defect.Citation1,Citation2 Various surgical procedures, such as parallel clipping with or without the cotton-reinforced technique,Citation1,Citation3 suturing and covering the aneurysm with an encircling clip,Citation4 vascular closure staple clips,Citation5 wrap-clipping,Citation6,Citation7 and trapping with external carotid artery–internal carotid artery (EC-IC) bypass,Citation2,Citation8–Citation10 have been used for the management of BBA of the ICA. While the optimal surgical strategy remains controversial, trapping with EC-IC bypass is a useful surgical strategy.Citation2,Citation8–Citation10 If reconstruction of the cerebral circulation with EC-IC bypass is performed, tolerance for ischemia should be carefully determined. Even if a patent EC-IC bypass is reconstructed based on an accurate evaluation of cortical cerebral blood flow (CoBF) demand by preoperative monitoring, such as the balloon occlusion test, ischemic complications can occur secondary to hemodynamic hypoperfusion or occlusion of the perforating artery. In addition, delayed cerebral vasospasm may develop between 4 and 14 days after subarachnoid hemorrhage (SAH). Therefore, it is essential to consider both repair of the ruptured BBA as well as the potential for cerebral ischemia; in that regard, quantification of cerebral blood flow with intraoperative monitoring tools is necessary. Carter and AtkinsonCitation11 first described quantitative CoBF measurement by the thermal diffusion method using a cortical thermal sensor. Ogawa et alCitation12 described a significant relationship between intraoperative CoBF and electroencephalographic changes as well as a correlation between postoperative neurological deficits and the degree of residual CoBF during temporary arterial occlusion.
In the present report, we describe a case of a patient with a ruptured BBA in the ophthalmic segment of the ICA who underwent wrap-clipping with EC-IC bypass by intraoperative estimation using measurement of CoBF.
Case report
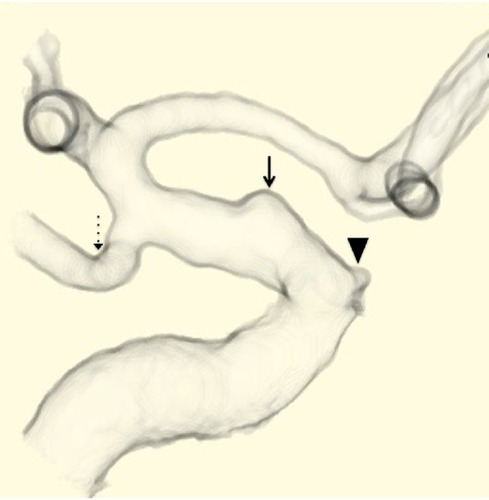
A 37-year-old woman had been an uninterrupted state of good health before she experienced sudden onset of severe headache, vomiting, and deep coma. She was transferred to our hospital, and a computed-tomography (CT) scan revealed diffuse SAH in the basal cistern. Computed-tomography angiography (CTA) demonstrated a BBA in the ophthalmic segment of the right ICA ().
Figure 1 Preoperative computed-tomography angiography demonstrates a ruptured blood blister-like aneurysm (solid-line arrow) in the ophthalmic segment of the right internal carotid artery. The arrow head indicates ophthalmic artery, and the dotted-line arrow indicates the posterior communicating artery.
One day after the onset of SAH, she recovered from Grade V SAH to Grade IV SAH, according to the Hunt and Hess classification,Citation13 and underwent craniotomy for treatment of the ruptured BBA. The ICA balloon occlusion test was not performed. Measurement of motor evoked potentials could not be performed due to mechanical errors. Under general anesthesia, the cervical common carotid artery, external carotid artery, and ICA were exposed. After a frontotemporal craniotomy with preservation of the superficial temporal artery, the Sylvian fissure was opened widely. She underwent high-flow saphenous vein graft bypass between the right cervical external carotid artery and M2 portion before approaching the ruptured BBA. Any subarachnoid hematoma surrounding the intracranial ICA was carefully removed without dislodging the relatively dense clot on the anterior wall of the artery. During the procedure, the cervical ICA was occluded intermittently to avoid intraoperative bleeding from the lesion. The anterior clinoid process was removed using an ultrasonic surgical aspirator (Sonopet UST-2001, Stryker, Kalamazoo, MI, USA).
An abnormal arterial lesion on the anterior wall of the ICA (as viewed through an operating microscope) was wider than the abnormal hemispheric bulge visualized on preoperative CTA. For CoBF measurements, a calibrated stick-type thermal probe (BTG-III, Biomedical Science, Shinjuku, Japan) was placed on the inferior frontal gyrus of the frontal lobe and superior frontal gyrus of the temporal lobe near the Sylvian fissure within the standard frontotemporal craniotomy,Citation12,Citation14 and the test clip-trapping between just distal to the origin of the ophthalmic artery and just proximal to the origin of the posterior communicating artery, including the ruptured BBA, was performed with simultaneous and continuous measurement of the CoBF.
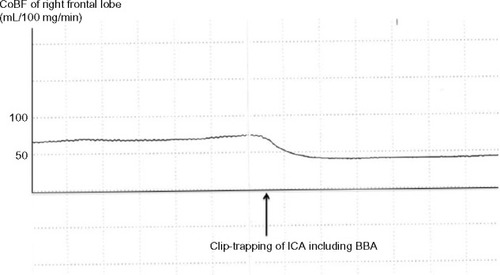
First, the CoBF during clip-trapping of the ICA under functioning high-flow bypass was measured, and the ratio of the value to the CoBF immediately before test clip-trapping of the ICA was calculated in the frontal and temporal lobes. Prior to the beginning of the present study, the CoBF was measured immediately after dural incision for 5 minutes in ten patients undergoing neck clipping for an un-ruptured cerebral aneurysm. The ratio of the lowest value to the highest value in the period ranged from 0.92 to 0.99 (mean ± standard deviation [SD]; 0.96±0.03).Citation14 Thus, in the present study, any value less than 1.0–3.0 SD (0.9) was defined as a significant reduction of the CoBF ratio. In the present case, the CoBF decreased immediately after test clip-trapping of the ICA in the frontal lobe and the temporal lobe and returned to pre-clip-trapping levels immediately after de-clamping of the ICA. CoBF ratios were 0.56 and 0.70 in the right frontal lobe () and temporal lobe, respectively. Consequently, the patient underwent wrap-clipping with EC-IC bypass to provide greater blood flow.
Figure 2 The cortical cerebral blood flow (CoBF) in the frontal lobe decreases immediately after clip-trapping between the area just distal of the origin of the ophthalmic artery and the area just proximal to the origin of the posterior communicating artery, including the ruptured blood blister-like aneurysm (BBA), in the internal carotid artery (ICA). CoBF after clip-trapping decreases by 56% in the right frontal lobe and returns to pre-clamping levels immediately after de-clamping of the ICA.
The diameter of the abnormal arterial lesion along the long axis of the ICA and the distance between the origin of the ophthalmic artery and the origin of the posterior communicating artery were measured, and a strip of 0.1 mm thick polytetrafluoroethylene (PTFE) membrane (Gore-Tex®; WL Gore and Associates, Flagstaff, AZ, USA), which is often used as a dural substitute, was trimmed with scissors to match the diameter and distance. After temporarily occluding the cervical ICA, the intracranial ICA that included the lesion was carefully wrapped with the strip of PTFE, and one clip was applied parallel to the vessel, so that the clip blade gripped the arterial wall beyond the lesion. These procedures were successfully accomplished without intraoperative bleeding.
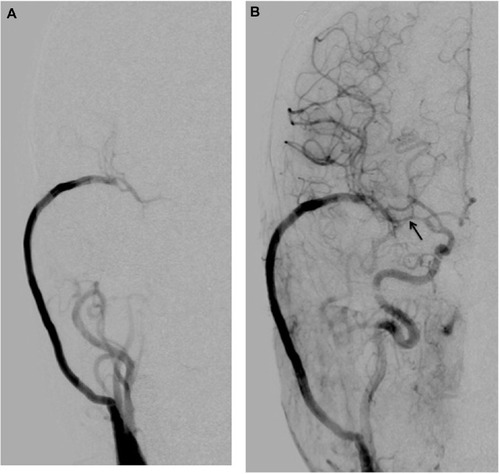
Right carotid angiography performed 7 days after surgery demonstrated resolution of the BBA and no stenosis in the affected intracranial ICA; however, moderate narrowing of the proximal segment was present in the middle cerebral artery (MCA). The ipsilateral MCA area supplied the patent high-flow bypass during the arterial early phase of angiography (). During the arterial late phase, anterograde filling via the ICA was shown ().
Figure 3 Postoperative right carotid angiogram performed 7 days after surgery demonstrates that (A) the ipsilateral middle cerebral artery (MCA) area is supplied by the patent high-flow bypass during the arterial early phase of angiography; and (B) resolution of the blood blister-like aneurysm and no stenosis in the affected intracranial internal carotid artery (ICA), however, moderate narrowing (arrow) of the proximal segment in the MCA. During the arterial late phase, anterograde filling via the ICA is shown (B).
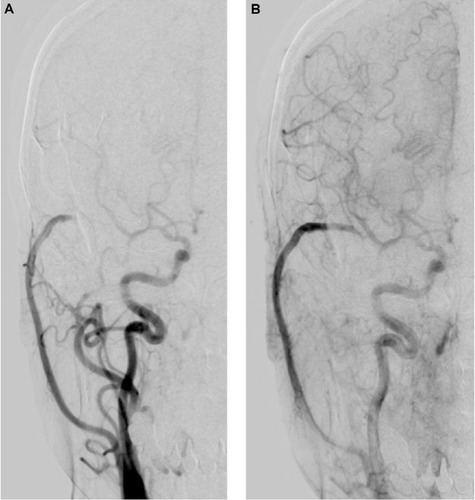
The patient had an uneventful postoperative course, and new ischemic brain lesions were not demonstrated in postoperative magnetic resonance images. Follow-up right carotid angiography performed 1 year after the surgery revealed resolution of the BBA and no stenosis. Further, the ipsilateral MCA area was supplied by anterograde filling via the ICA during the arterial early phase of angiography (). During the arterial late phase, retrograde filling via the patent high-flow bypass was shown ().
Figure 4 (A) Follow-up right carotid angiogram performed 1 year after the surgery reveals that the ipsilateral middle cerebral artery area is supplied by anterograde filling via the internal carotid artery during the arterial early phase of angiography. (B) During the arterial late phase, retrograde filling via the patent high-flow bypass is shown.
Discussion
In the case described here, the patient with a ruptured BBA of the ICA underwent wrap-clipping with high-flow bypass. Our previous report suggested that wrap-clipping of BBA using PTFE resulted in favorable patient outcomes.Citation7 Wrap-clipping may be advantageous in terms of preserving anterograde flow when it is safely and completely applicable and may be suitable for BBAs that are located far from the anterior clinoid process that project anteriorly or anteromedially and are located at the communicating segment of the ICA. On the other hand, forced trapping after intraoperative bleeding when approaching BBA is associated with high morbidity and mortality.Citation15 In our current surgical strategy for a ruptured BBA, at the ophthalmic or communicating segment of the ICA in the vicinity of the anterior clinoid process, it is recommended to perform bypass surgery before approaching the BBA.
Results of scheduled trapping after EC-IC bypass strategy have been relatively good;Citation2 however, cerebral ischemia due to hemodynamic hypoperfusion may develop. Furthermore, ischemic complications may occur immediately after surgery due to occlusion of the perforating artery.Citation8 Some perforating arteries might be directly sacrificed by parent artery occlusion, such as clip-trapping, and postoperative perforator infarction has been observed in cases of paraclinoid aneurysms but not in cases of intracavernous aneurysms.Citation8 Perforating arteries might be occluded by thrombotic or embolic mechanisms due to acute and excessive thrombosis in the lumen of the occluded parent artery.Citation8 In addition, delayed angiographic vasospasm has been observed in more than 90% of patients after SAH, regardless of whether or not it was symptomatic.Citation16
In the present case, the ipsilateral MCA area supplied the patent high-flow bypass during the arterial early phase, according to angiography that was performed 7 days after surgery. This angiographic finding might be the result of salvage flow from the high-flow bypass due to moderate narrowing of the proximal segment in the MCA. Initially, the patient had severe neurological deficits, including deep coma and poor pupillary response. Patients with poor-grade SAH have been reported to have delayed ischemic neurological deficits due to cerebral vasospasm.Citation17 Therefore, we intentionally used high-flow bypass to prevent delayed ischemic neurological deficits due to cerebral vasospasm.
One possible concern is that trapping occlusion of the ICA in the acute stage of SAH may provoke ischemic complications due to hemodynamic hypoperfusion or occlusion of the perforating artery, delayed vasospasm, and/or early global cerebral hypoperfusion, even with concomitant bypass surgery. We believe that it is important to perform scheduled EC-IC bypass before trapping of the ICA in patients with a ruptured BBA in the acute stage of SAH and to utilize wrap-clipping rather than trapping. What is novel in the management of our case is that this surgical strategy will provide much more CoBF if reduction of CoBF develops after trapping occlusion of the ICA, including a ruptured BBA, when conducting intraoperative CoBF monitoring, and may prevent ischemic complications. Several groups of investigators have used endovascular treatment as primary treatment;Citation18,Citation19 however, clinical and angiographic results have been unsatisfactory. Recently, the application of flow diverters, such as the pipeline embolization device, for the treatment of complex aneurysms has been reported,Citation20 and long-term clinical and angiographic surveillance is recommended.
Conclusion
As far as we are aware, this is the first report on high-flow bypass and wrap-clipping for a ruptured BBA of the ICA using intraoperative monitoring of cerebral hemodynamics. This surgical strategy using intraoperative monitoring may become recognized as one surgical treatment option for a ruptured BBA in the ICA.
Acknowledgments
This work was supported by a grant from Japan Society for the Promotion of Science Kakenhi (grant no 23592103).
Disclosure
The authors report no conflict of interest in this work.
References
- NakagawaFKobayashiSTakemaeTSugitaKAneurysms protruding from the dorsal wall of the internal carotid arteryJ Neurosurg19866533033083734880
- ShimizuHMatsumotoYTominagaTNon-saccular aneurysms of the supraclinoid internal carotid artery trunk causing subarachnoid hemorrhage: acute surgical treatments and review of literaturesNeurosurg Rev201033220521620521339
- KalaniMYZabramskiJMKimLJLong-term follow-up of blister aneurysms of the internal carotid arteryNeurosurgery201373610261033 discussion 103324056320
- YanakaKMeguroKNoseTRepair of a tear at the base of a blister-like aneurysm with suturing and an encircling clip: technical noteNeurosurgery200250121822111844255
- YanagisawaTMizoiKSugawaraTDirect repair of a blisterlike aneurysm on the internal carotid artery with vascular closure staple clips. Technical noteJ Neurosurg2004100114614914743928
- AbeMTabuchiKYokoyamaHUchinoABlood blisterlike aneurysms of the internal carotid arteryJ Neurosurg19988934194249724116
- KuboYOgasawaraKTomitsukaNOtawaraYWatanabeMOgawaAWrap-clipping with polytetrafluoroethylene for ruptured blisterlike aneurysms of the internal carotid artery. Technical noteJ Neurosurg2006105578578717121147
- MurakamiKShimizuHMatsumotoYTominagaTAcute ischemic complications after therapeutic parent artery occlusion with revascularization for complex internal carotid artery aneurysmsSurg Neurol2009714434441 discussion 44118617245
- KamijoKMatsuiTAcute extracranial-intracranial bypass using a radial artery graft along with trapping of a ruptured blood blister–like aneurysm of the internal carotid artery. Clinical articleJ Neurosurg2010113478178519929189
- KawashimaAOkadaYKawamataTOndaHKuboOHoriTSuccessful treatment of a blood blister-like aneurysm of the internal carotid artery by trapping with a high-flow bypassJ Clin Neurosci200815779780018406147
- CarterLPAtkinsonJRCortical blood flow in controlled hypotension as measured by thermal diffusionJ Neurol Neurosurg Psychiatry19733669069134772724
- OgawaASatoHSakuraiYYoshimotoTLimitation of temporary vascular occlusion during aneurysm surgery. Study by intraoperative monitoring of cortical blood flowSurg Neurol19913664534571759185
- HuntWEHessRMSurgical risk as related to time of intervention in the repair of intracranial aneurysmsJ Neurosurg196828114205635959
- KuboYOgasawaraKTomitsukaNOtawaraYKakinoSOgawaARevascularization and parent artery occlusion for giant internal carotid artery aneurysms in the intracavernous portion using intraoperative monitoring of cerebral hemodynamicsNeurosurgery20065814350 discussion 43–5016385328
- MelingTRSortebergABakkeSJSlettebøHHernesniemiJSortebergWBlood blister-like aneurysms of the internal carotid artery trunk causing subarachnoid hemorrhage: treatment and outcomeJ Neurosurg2008108466267118377243
- InagawaTSite of ruptured intracranial saccular aneurysms in patients in Izumo City, JapanCerebrovasc Dis2010301728420484905
- SchmidtJMWartenbergKEFernandezAFrequency and clinical impact of asymptomatic cerebral infarction due to vasospasm after subarachnoid hemorrhageJ Neurosurg200810961052105919035719
- ÇinarCOranİBozkayaHOzgirayEEndovascular treatment of ruptured blister-like aneurysms with special reference to the flow-diverting strategyNeuroradiology201355444144723322455
- WalshKMMoskowitzSIHuiFKSpiottaAMMultiple overlapping stents as monotherapy in the treatment of ‘blister’ pseudoaneurysms arising from the supraclinoid internal carotid artery: a single institution series and review of the literatureJ Neurointerv Surg20146318419423543733
- PistocchiSBlancRBartoliniBPiotinMFlow diverters at and beyond the level of the circle of willis for the treatment of intracranial aneurysmsStroke20124341032103822282890
