Abstract
The management of patients scheduled for surgery with a coronary stent, and receiving 1 or more antiplatelet drugs, has many controversies. The premature discontinuation of antiplatelet drugs substantially increases the risk of stent thrombosis (ST), myocardial infarction, and cardiac death, and surgery under an altered platelet function could also lead to an increased risk of bleeding in the perioperative period. Because of the conflict in the recommendations, this article reviews the current antiplatelet protocols after positioning a coronary stent, the evidence of increased risk of ST associated with the withdrawal of antiplatelet drugs and increased bleeding risk associated with its maintenance, the different perioperative antiplatelet protocols when patients are scheduled for surgery or need an urgent operation, and the therapeutic options if excessive bleeding occurs.
Introduction
Percutaneous coronary interventions (PCIs) and coronary stent placement are now frequently and increasingly used. Among coronary stents, drug-eluting stents (DESs) seem to have some advantages over bare-metal stents (BMSs), but they need more time for complete endothelialization.Citation1–Citation3 As antiplatelet therapy is a key part of the management of all these patients, during the perioperative period, its management is one of the most important safety issues and, nowadays, has many controversies.Citation4
First, the withdrawal of antiplatelet agents (APAs) seems to be associated with an increased risk of perioperative stent thrombosis (ST), myocardial infarction (MI), and death.Citation5,Citation6 Second, surgery under an altered platelet function could lead to an increased risk of bleeding, outweighing the benefit of continuous antiaggregation in some procedures, mainly those performed in a closed space.Citation7,Citation8
Because of the conflict in the recommendations, this article reviews the protocols of administration of APAs after the performance of a PCI with a DES placement and their management in patients scheduled for elective surgery or need an emergency intervention.
Antiplatelet agents
The most commonly used antiplatelet drugs are cyclooxygenase (COX) inhibitors, such as aspirin; adenosine diphosphate (ADP) receptor P2Y12 antagonist, such as clopidogrel, ticlopidine, prasugrel; and integrin αIIbβ3 (glycoprotein IIb/IIIa [GPIIb/IIIa]) receptor antagonists.Citation9,Citation10
Aspirin
The prostanoid TXA2 is the main inductor of platelet aggregation and vasoconstriction. That is, the vascular prostanoid PGI2 is the main platelet aggregation inhibitor and induces an intense vasodilatation effect. The antiplatelet action of aspirin includes the selective blocking of the platelet TXA2 synthesis by the irreversible acetylation of the enzyme COX-1, whereas COX-2-mediated PGI2 production is largely insensitive to low-dose aspirin inhibition.Citation11 The main pharmacokinetic characteristics of aspirin are known for a long time. Aspirin, the regular uncoated tablet, is rapidly absorbed in the stomach and upper intestine, reaching peak plasma levels at 30–40 minutes after ingestion (oral bioavailability 40%–50%). Enteric-coated aspirin can take up to 3–4 hours to reach peak plasma levels after its administrationCitation12 although the half-life of aspirin is close to 15–20 minutes because of its rapid clearance from the circulation. The mean life span of human platelets is approximately 8–10 days due to both platelet and megakaryocytes COX-1 inhibition by aspirin. However, as about 10%–12% of circulating platelets are replaced every 24 hours, complete recovery of platelet aggregation may occur in 50% of cases by day 3 and in 80% of cases by day 4.Citation13 The efficacy and safety of low-dose aspirin are well documented. Aspirin reduces the risks of stroke and composite outcome of stroke or death in patients with prior stroke or transient ischemic attack.Citation14 Low doses of aspirin decrease the risk of acute MI or death in patients with both unstable angina and chronic stable angina, reduce stroke or death in patients with transient cerebral ischemia,Citation15–Citation17 prevent thrombotic complications in patients with polycythemia vera,Citation18 and reduce early occlusion after aortocoronary bypass surgeryCitation19 and carotid endarterectomy.Citation20
The balance between preventing vascular occlusion and causing excess bleeding with aspirin depends critically on the absolute thrombotic vs hemorrhagic risk of the patient.Citation9 The meta-analyses by Antithrombotic Trialists’ Collaboration about the effects of antiplatelet therapy among patients at high risk of occlusive vascular diseaseCitation17 have shown that the benefits of aspirin far exceed the bleeding risks. However, it is known that some patients under aspirin therapy at usual doses (75–150 mg aspirin daily) still fail to have an optimal inhibition of thromboxane A2 pathway. These patients with such “aspirin resistance” could remain at high thrombotic risk with important clinical implications. There is a lack of uniformly accepted definition,Citation21 and the exact prevalence is unknown but has been suggested to be between 5.5% and 60%.Citation22 This phenomenon is important because in patients with some comorbidities, such as diabetes mellitus, hypertension, heart failure, inflammatory disorders, which may enhance aspirin resistance, the combination therapy, rather than aspirin-alone therapy, will be necessary to reach optimal antiplatelet inhibition.Citation22
ADP receptor antagonists
The ADP P2Y12 receptor plays a central role in the formation and stabilization of thrombus and is also essential in the complete activation of GPIIb/IIIa. Clopidogrel, ticlopidine, and prasugrel act as APAs by irreversibly binding to the ADP P2Y12 receptor on platelets, subsequently preventing ADP-associated platelet aggregation.Citation23
Clopidogrel
Clopidogrel is a parent drug. After oral administration, clopidogrel needs to be metabolized in the liver through a cytochrome P450 (CYP3A4) to active metabolite SR 26334.Citation24 Its plasma elimination half-life is approximately 8 hours. Clopidogrel produces detectable inhibition of platelet aggregation 2 hours after the administration of 400–600 mg oral doses, and as in the case of aspirin, platelet function returns to normal about 7 days after the last dose of clopidogrel. The benefits of clopidogrel for patients with an increased risk of recurrent ischemic events,Citation17,Citation25 stroke,Citation26 and acute coronary syndromes (ACS)Citation27 have been established by large, well-conducted randomized clinical trials. As aspirin and clopidogrel have different and complementary mechanisms of action, the combination therapy (dual antiplatelet therapy [DAT]) is more effective in preventing ST and in those presenting with ACS, compared with antiplatelet monotherapy.Citation28,Citation29 The American College of Cardiology/American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC) have published guidelines for the use of APAs in patients who undergo PCI.Citation30,Citation31 A current antiplatelet protocol in patients with DESs is discussed in a separate section of this article. DAT has been associated with significantly increased risk for major bleeding in studies of more than 1 month duration compared with antiplatelet monotherapy.Citation28 Infrequent complications include intracranial hemorrhage (0.4%) and severe neutropenia (0.5%).Citation26
Prasugrel
Prasugrel is the most recently marketed oral thienopyridine derivative. The main advantages of prasugrel are that it has a faster onset of action (30 minutes) than clopidogrel, and it is a more potent P2Y12 receptor antagonist, leading to higher effects of inhibition of platelet aggregation, lesser variability of response, and a lower incidence of drug resistance than clopidogrel. Citation32 Prasugrel is indicated for the prevention of atherothrombotic events in patients with ACS undergoing PCI.Citation33 In the analysis of the recent TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet inhibitioN with prasugrel – Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38) designed to compare clopidogrel with prasugrel in patients undergoing PCI, the benefit from the intensive platelet inhibitory effects associated with prasugrel was more pronounced in patients with high risk (patients with ST segment elevation MI (STEMI) and those with diabetes). Citation34,Citation35 However, this reduction in thrombotic events was associated with a significant increase in the risk of bleeding, which overcame the benefit of prasugrel in certain groups.
Integrin αIIbβ3 (GPIIb/IIa) receptor antagonists: abciximab, tirofiban, and eptifibatide
After recognizing that the interaction between fibrinogen and GPIIb/IIIa is the major final step in platelet aggregation, this GP became the target of novel antiplatelet drugs, ie, the GPIIb/IIIa antagonists, such as abciximab, tirofiban, and eptifibatide. These drugs can be used alone or in combination with clopidogrel.Citation36
Abciximab
Abciximab is a chimeric Fab fragment of the murine antihuman GPIIb/IIIa. It blocks fibrinogen ligand binding to GPIIb/IIIa. After an intravenous (IV) bolus administration, its half-life is <30 minutes. Abciximab (0.25 mg/kg IV bolus dose and 0.125 μg/kg/min infusion) produces a rapid inhibition of ADP-induced platelet aggregation with peak effects 2 hours after bolus administration. After stopping infusion, a gradual recovery of platelet function is observed and then returns to >50% of baseline in most patients within the first 24 hours, and nearly in all patients within the first 48 hours.Citation37 Abciximab is recommended as adjunct therapy in PCI. A meta-analysis of the 3 major clinical trialsCitation38–Citation40 on the use of abciximab in patients undergoing PCI showed that abciximab treatment reduced the incidence of ischemic events and all-cause mortality.Citation41 Bleeding and thrombocytopenia were the 2 most important adverse reactions associated with abciximab. Approximately 1%–2% of patients treated with abciximab have platelet counts <50,000/L, but in almost all cases, the thrombocytopenia was effectively controlled by stopping the drug administration.Citation42
Tirofiban
Tirofiban is a specific nonpeptide antagonist of GPIIb/IIIa receptors derived from tyrosine. Bolus doses of 15 μg/kg followed by tirofiban infusions 0.15 μg/kg/min produced both an inhibition of platelet aggregation induced by ADP nearly 100% and an increase of the bleeding time >30 minutes. Recovery of platelet function was achieved >50% within 4 hours after discontinuing drug infusion.Citation43 Tirofiban is effective in patients with unstable angina and non-Q-wave MICitation44 and protects patients undergoing coronary angioplasty for ACS.Citation45
Eptifibatide
Eptifibatide is a synthetic heptapeptide with high selectivity by GPIIb/IIIa receptor inhibition. Bolus dose of 180 μg/kg of eptifibatide followed by 1 μg/kg/min infusion decreases platelet-induced ATP aggregation >95% and increases bleeding time to 22 minutes when compared with control values of 7–8 minutes. After stopping eptifibatide infusion, platelet function recovers >50% of baseline aggregation within the first 4 hours.Citation46 Eptifibatide reduces the acute adverse outcome of patients undergoing PCI,Citation47 and it is also beneficial in patients with unstable angina.Citation48 However, a significant interindividual variation in the inhibitory responses to eptifibatide has been reported.Citation47
Monitoring antiplatelet effect
Tests of platelet function attempt to measure the point in the activation process reached by the platelets of an individual patient. Possible reasons for measuring platelet function in patients include screening, diagnosis, monitoring antiplatelet therapy, monitoring prohemostatic therapy, predicting thrombosis, predicting bleeding, and assessing stored platelets.Citation49 In the perioperative period, it would be helpful to identify patients who are at risk of bleeding because of platelet dysfunction related to the administration of any APA. However, none of these tests has been validated for routine clinical practice and for monitoring antiplatelet efficiency in perioperative period of coronary interventions; the reason could be a lack of standardization, poor correlation between various tests, and expensiveness of some of them.Citation50
A wide variety of tests are available for the measurement of platelet function, and each has its advantages and disadvantages. The tests available for assessing platelet function can be summarized as follows ():Citation49,Citation51
Table 1 Main platelet function test for monitoring aspirin/clopidogrel therapy
Turbidimetric platelet aggregometry could be considered the reference assay to diagnose platelet disorders. However, it needs a platelet-rich, high-volume plasma sample; the results are not completely standardized; the laboratory procedure is complex; and the test is time consuming. Vascular Health and Risk Management 2010:6
Impedance aggregometry is more physiological than the optical one, but it still requires a reasonably high sample volume, and this assay is also time consuming and expensive.
The platelet function analyzer (PFA-100) explores the platelet adhesive capacity, measuring the closure time taken for a platelet plug to occlude an aperture in a membrane impregnated with collagen and epinephrine or ADP.Citation52 Although the test has advantages such as simplicity, rapid readout, low sample volume, no requirement for sample preparation, and that aspirin and clopidogrel have been shown to prolong this closure time, there is no evident correlation between these results and an increased perioperative bleeding.
The Plateletworks analyzer measures the percentage of aggregation of whole blood before and after exposure to ADP. Its results are contradictory when compared with optical aggregometry: there is good correlation for clopidogrelCitation53 but limited usage for aspirin.Citation54 Then, it needs further studies.
Thromboelastography is a whole blood coagulation monitor that can demonstrate the alteration of platelet aggregation. However, it is unable to detect the specific defects that occur with the administration of aspirin or clopidogrel.
The Ultegra rapid platelet function assay system (known as VerifyNow®) is a whole blood, point-of-care assay that incorporates a single-use cartridge with some biochemical reagents that can detect platelet dysfunction.Citation55 It is a simple, true point-of-care device that does not need rapid pipetting and requires only a small sample volume. It has been proposed to monitor efficacy of aspirin and clopidogrel and to detect the aspirin-resistant population.
Phosphorylation of vasodilator-stimulated phosphoprotein for the measurement of P2Y12 antagonism is commercially available as a flow cytometry kit. It is dependent on the target of clopidogrel (P2Y12), and it needs low sample volume and whole blood assays, but the sample preparation is not easy, and it requires a flow cytometer and an experienced technician.
Unfortunately, none of these tests has good correlation with perioperative bleeding, so they could not predict the bleeding tendency in a patient under the effects of aspirin or clopidogrel. Further clinical investigations are necessary in this field.
Drug-eluting stents
BMSs have been developed to reduce the rate of restenosis after angioplasty. However, the restenosis rate remains high, existing in >20% of cases, leading to ischemic coronary syndromes, or requiring revascularization in >10% of cases. Although the BMS virtually eliminates the elastic recoil and premature negative remodeling, the proliferation of the endothelium is even more important and uncontrolled than with angioplasty alone. This concept was the basis for the development of DESs. By preventing neointimal hyperplasia, the DES has reduced the need for revascularization under 10%.Citation1,Citation56,Citation57 In brief, a DES is a metal stent, stainless steel or cobalt–chromium alloy, coated with a polymer containing a drug with antiproliferative properties. There are drugs from the limus family (sirolimus or rapamycin, everolimus, pimecrolimus, zotarolimus, biolimus, tacrolimus), paclitaxel (Taxol®)Citation58().
Table 2 Types of drug-eluting stent by composition
During angioplasty, the atherosclerotic material and subendothelial tissue are exposed to the blood stream, initiating the process of thrombus formation and obstruction of the coronary lumen. The metallic surface of the stent is an additional thrombogenic stimulus that persists until it is covered with endothelium. In the case of BMS, it is expected to be coated within 4–8 weeks, whereas in DES, the drug not only inhibits cell proliferation but also causes endothelial dysfunction and a local inflammatory reaction, impairing the normal healing process of an arterial injury; therefore, the thrombotic risk may persist even for years.Citation59,Citation60
Current antiplatelet protocols in patients with DESs
APA is recommended in the treatment of patients who had undergone PCI. After the combination therapy using aspirin and clopidogrel was introduced, the incidence of ST was reduced to <1% of the patients,Citation61 and it became unusual after the first month in BMS. However, ST may occur months to years after the implantation of DES in association with premature cessation of APA. The 2007 update of the ACC/AHA/Society for Cardiovascular Angiography and Interventions (SCAI) 2005 guideline for the use of APA in patients who undergo PCI is summarized in .Citation62,Citation63
Figure 1 The 2007 update on the recommendations of the ACC/AHA/SCAI 2005–2007 last-updated guidelines on use of antiplatelet drugs in patients who undergo PCI.
Abbreviations: PCI, percutaneous coronary intervention; LD, loading dose; BMS, bare-metal stent; DES, drug-eluting stent; UA, unstable angina; NSTEMI, non-ST segment elevation myocardial infarction; STEMI, ST segment elevation myocardial infarction.
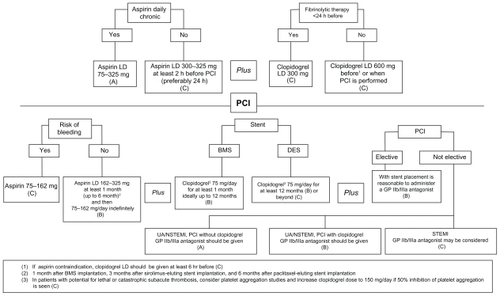
APA before or during PCI
Patients who were already receiving daily long-term aspirin therapy should be given 75–325 mg of aspirin before PCI (level of evidence: A).
Patients who were not receiving daily long-term aspirin therapy should be given 300–325 mg of aspirin at least 2 hours (preferably 24 hours) before PCI (level of evidence: C).
A clopidogrel loading dose (LD), usually of 600 mg, should be administered before or during PCI (level of evidence: C).
In patients undergoing PCI after 12–24 hours of fibrinolytic therapy, a clopidogrel oral LD of 300 mg may be considered (level of evidence: C).
If clopidogrel is given at the time of PCI, supplementation with GPIIb/IIIa receptor antagonists may be beneficial (level of evidence: B).
In patients with absolute contraindication to aspirin, a single administration of clopidogrel both 300 and 600 mg LD at least 6 hours before PCI, supplemented with or without GPIIb/IIIa antagonists, at the time of PCI is recommended (level of evidence: C).
There is an agreement about the benefit of higher LD of clopidogrel in patients undergoing PCI. Several authors have analyzed the effects of different LDs (300 or 600 mg) on the inhibition of platelet aggregation, myonecrosis (troponin I release), and clinical outcomes in patients undergoing PCI. Greater dose of clopidogrel has shown an increase of antiplatelet effect and a significant decrease of major adverse cardiac events, in comparison with 300 mg LD, which supports a clinical benefit of higher dose of clopidogrel in patients subjected to PCI.Citation64,Citation65
APA after PCI
In patients subjected to PCI and with no allergies or increased risk of bleeding, aspirin 162–325 mg daily should be given for at least 1 month after BMS implantation, 3 months after sirolimus-eluting stent implantation, and 6 months after paclitaxel-eluting stent implantation. Afterward, a daily dose of 75–162 mg of aspirin should be maintained indefinitely (level of evidence: B).
The use of low-dose aspirin is supported by data from post hoc analysis of the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study.Citation66 No differences in the efficacy among the 3 aspirin dose groups (<100 mg, 101–199 mg, and ≥200 mg) in combination with clopidogrel have been found. However, an increase of major bleeding incidence has been reported with the highest dose of aspirin either alone or with the combination of clopidogrel.Citation67 Therefore, these data suggest an optimal daily dosage of aspirin between 75 and 100 mg to minimize bleeding risk (level of evidence: C).
In post-PCI patients receiving a BMS, clopidogrel should be given for a minimum of 1 month, whereas it should be given only for a minimum of weeks for patients with an increased risk of bleeding (level of evidence: B).
In all post-PCI patients with DES and without high risk of bleeding, clopidogrel 75 mg daily should be given for at least 12 months (level of evidence: B).
These recommendations are based on the antiplatelet regimen used in clinical trials conducted to achieve the approval of US Food and Drug Administration (low-risk lesions in lowrisk patients). The anticipated times have been taken for the predicted metal stent struts adequate endothelialization time, which reduces the ST risk efficiently. The effect of continuous use of clopidogrel in patients receiving BMS or DES at 6 and 12 months was analyzed by an observational study including 3,165 patients. Clopidogrel did not modify the incidence of death or MI at 24 months in patients with BMS. However, the continuous use of clopidogrel was associated with lower rates of death or MI in patients with DES.Citation68 The American Heart Association Science Advisory (AHAA) stresses the importance of 12 months DAT and provides reports about the potential hazards associated with premature discontinuation of these drugs, and thus, they recommend that the prolongation of clopidogrel therapy beyond 1 year may be considered in patients undergoing DES placement (level of evidence: C).
In a recent meta-analysis, a triple therapy (aspirin plus thienopyridine plus cilostazol) has been evaluated to know the effect of adding cilostazol to a dual antiplatelet regimen to prevent stent restenosis.Citation69 The 6-month restenosis rates are significantly lower with triple therapy vs DAT, which seems a promising way to reduce cardiac events in patients with coronary artery stent.
Bleeding and thrombotic risk stratification in the perioperative period
The perioperative management of APA must be based on the optimal assessment of benefit – risk relationship. This includes the stratification of the perioperative hemorrhagic risk associated with the continuation of APAs throughout surgery and the stratification of the thrombotic risk associated with the discontinuation of APA ().Citation7,Citation8,Citation70–Citation72
Table 3 Proposed stratification of the hemorrhagic risk related with the continuation of antiplatelet agents through the perioperative period and the thrombotic risk associated with their discontinuation
Bleeding risk
The perioperative risk for hemorrhage depends on the type of APA, the monotherapy or combination therapy, and the surgical procedures. As a general rule, the risk of bleeding probably increases when the patient receives a dual therapy or when antiplatelet therapy is associated with an anticoagulant drug (heparin, low-molecular-weight heparin, fondaparinux, warfarin). When aspirin is given as monotherapy, the surgical bleeding and its related complications have been calculated to increase by a factor 1.5,Citation73 but it is controversial if this result could be extended to all surgical procedures.Citation72 Indeed, the evidence on the risk of bleeding in patients under the effect of APA is also controversial, and the results of several recent studies are not fully conclusive.Citation74
Finally, the increased bleeding risk with aspirin does not mean necessarily an increase in mortality or morbidity, an increase in transfusion requirements,Citation75 or a worse result in the surgical procedure, with the exception of surgery in a closed space, and possibly transurethral prostatectomy. Moreover, the risk of surgical bleeding is also increased in these patients due to DAT. Burger et alCitation73 have demonstrated, through a meta-analysis of 41 studies involving 49,590 patients undergoing different types of noncardiac surgery, that the bleeding risk is multiplied by a factor 1.5 when aspirin treatment is continued. Yet, this is not accompanied by the increased risk of major bleeding complications, with the exception of cases in which minor bleeding may have catastrophic results, such as intracranial surgery. Also prostate surgery, transurethral resection, and radical surgery are generally considered high risk for bleeding, but in the last few years, the improved resection techniques and laparoscopic approach have helped to reduce the risk of hemorrhage in these cases.Citation76
There are no randomized controlled studies that evaluate the risk of bleeding when continuing treatment with clopidogrel in noncardiac surgery. In some case series, the continuation of clopidogrel in the perioperative period is not associated with excessive bleeding.Citation77 With regard to coronary bypass surgery, the CURE trial showed no major bleeding among patients who continued clopidogrel treatment up to 5 days before surgery vs patients who discontinued clopidogrel 5 days or more before surgery, without any difference in mortality.Citation78
In conclusion, in noncardiac surgery, bleeding could be considered as a “minor” complication, and severe events are more frequently of cardiac origin.
Thrombotic risk
ST is a severe complication that is associated with a high incidence of acute MI (22%–90%) and death (30%–45%).Citation79–Citation81 The current procedure of DAT with aspirin and thienopyridine (usually clopidogrel) has reduced the incidence of ST to around 2% in the long term.Citation82,Citation83 According to the time elapsed since the stent implantation, ST is considered “early” in the first 30 days, “late” from 30 days to 1 year, and “very late” after 1 year.Citation84 The first 30 days after stent placement is the period of higher risk of thrombosis, with an incidence of 60%–75% of cases with ST.Citation82 Although early ST is generally considered to be related to the procedure, late and very late events are less frequent, and discontinuation of antiplatelet therapy seems to be an important risk factor.Citation84
Nevertheless, over the past few years, some observational studies found that late and very late DES thromboses occurred more frequently than reported in previous randomized controlled trials, with an increased risk of mortality.Citation85 It appeared to be related to the premature discontinuation of antiplatelet therapy and with the higher use of DESs in off-label indication (around 60% of cases in real-world practice). Citation86 Thus, the safety of DES was questioned.
Recent studies with longer follow-up period allow us to summarize the current situation. In a recent Swedish observational study of 17,198 patients followed up for 1–4 years after stent placement, no significant differences in the risk of death or MI were found between both types of stent, in the case of either on-label or off-label use.Citation87 Lastly, Kirtane et alCitation88 have published an extensive meta-analysis of DES vs BMS in on-label and off-label uses. They conclude that the use of DES compared with BMS is associated with a significant reduction (46%–55%) in target vessel revascularization. These results seem to be consistent in demonstrating that DES use is safe for a broad range of lesions and patients at various risk levels.
However, there are different time patterns for the ST depending on the type of stent. Although early thrombosis is more frequent in BMS, the incidence of late and very late thromboses is higher in DESs.Citation82,Citation89 Several studies have identified multiple factors that contribute to ST ().Citation90 Some scoring systems have been recently developed to estimate the risk for ST; one of them include 8 significant predictors, with a stratification of patients in low, medium, and high risk groups, and has been validated using 1-year data from 4,820 patients ().Citation91
Table 4 Risk factors of stent thrombosis
Table 5 Clinical risk score for prediction of stent thrombosis and risk stratification as developed by Baran et al91
Finally, it is necessary to highlight that because of its relation with perioperative period, most authors agree that premature discontinuation of DAT was strongly associated with STCitation82,Citation90,Citation92 with a higher risk if clopidogrel is discontinued during the first 6 months after stenting. At 12 months, the cessation of clopidogrel does not appear to be related with a higher risk of ST.Citation93,Citation94
Management of APAs in the perioperative period
Around 5% of patients with a coronary stent are scheduled for noncardiac surgery within the first year after stent implantation. Citation94 Perioperative incidence of major adverse cardiac events and mortality in noncardiac surgery in these patients can reach a rate of 2.5%–21.4% in the case of ST.Citation95 This increased risk is strongly associated with the time elapsed from stenting to surgery and the premature discontinuation of DAT. Thus, this risk could be reduced by delaying surgery until the prescribed antiplatelet regimen is completed.Citation77,Citation96 Abrupt cessation of antiplatelet therapy may be followed by an increase of platelet reactivity or “rebound” effect in patients on chronic antiplatelet treatment. In addition, surgery itself creates a thrombogenic situation. The endocrine response to surgical stress may indirectly contribute to adverse cardiovascular events, such as increased vascular reactivity (vasospasm), platelet activation, increased clotting factors in plasma, and decreased fibrinolysis, in the perioperative period. Then, the risk of cardiac event in the perioperative period is not entirely prevented by continued DAT.
The decision to discontinue the antiplatelet therapy before surgery should be based on careful cardiovascular and thrombotic risk assessment of the patient and on the type of surgery and bleeding risk. In patients with coronary stent, it is essential to have specific information on the characteristics of coronary disease that has led to stent implantation, the date of procedure, the type of stent implanted, and the current antiplatelet regimen.Citation97
The recommendations about the perioperative management of antiplatelet therapy in these patients are not fully agreed, but they can be summarized as follows ():Citation7,Citation8,Citation71,Citation96–Citation100
Figure 2 Perioperative management of antiplatelet therapy in patients with drug-eluting stents (DES).
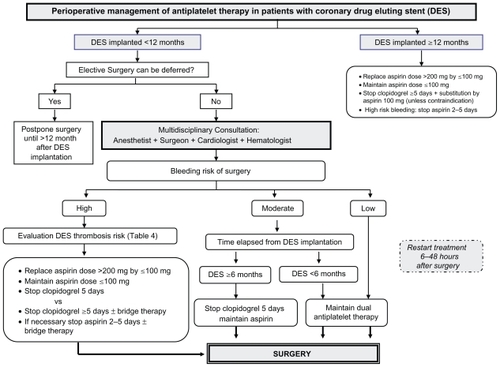
Whenever possible, antiplatelet therapy should be maintained during the high-risk period for as long as it is currently recommended: low-dose aspirin (75–100 mg) indefinitely, and clopidogrel 75 mg at least during 12 months after DES placement. Therefore, if the surgery is likely to cause little or no risk of bleeding, it is recommended to continue the antiplatelet therapy. However, regarding elective procedures in which there is a significant risk of perioperative bleeding, surgery should be delayed until patients have completed the DAT.
In all cases, it is recommended that a low-dose aspirin (75–100 mg) is maintained throughout the perioperative period, unless the risk of bleeding clearly outweighs thrombotic risk. To reduce the potential risk of bleeding, aspirin dose >200 mg should be replaced by 75 or 100 mg.
The treatment should be substituted by low-dose aspirin in the case of patients treated with clopidogrel as monotherapy, and where discontinuation is mandatory (unless contraindicated).
If antiplatelet therapy must be discontinued, it should be stopped the shortest time possible: 2 days for aspirin and 5 days for clopidogrel. Thereafter, treatment should be restarted as soon as possible following surgery after ensuring hemostasis, between 6 and 48 hours during the postoperative period. Depending on the withdrawal time and to accelerate antiplatelet response, LD administration may be indicated as follows: aspirin 250 mg, clopidogrel 300 mg.
Protocols involving the use of short-acting substances, such as IV administration unfractioned heparin and the infusion of either epifibatide or tirofiban, have been proposed to act as a bridge when the discontinuation of clopidogrel and/or aspirin is mandatory. It should be started 3 days before surgery and stopped 4–8 hours before surgery.Citation101,Citation102 However, currently there is no enough evidence to recommend their use.Citation71 Some authors defend that if aspirin is maintained, the short-term discontinuation of clopidogrel may be relatively safe without bridge therapy.Citation103
Management of the APAs in cases of emergency surgery
In the patients under the effect of any APA and undergoing emergency surgery, the key questions are the decision on the need to prevent or to treat bleeding, minimizing the risks of ischemia and ST, and whether the hemorrhagic risk may endanger the vital or functional prognosis of the intervention. The following strategies could be considered to decrease the consequences of hemorrhage.
Pharmacological treatment
The administration of desmopressin has been suggested to reduce excessive bleeding in patients scheduled for cardiac surgery under the effects of aspirin. However, consistent beneficial effects of this practice cannot be shown in large studies, and the drug cannot be recommended for current practice as an effective hemostatic agent in patients treated with aspirin.Citation104 The use of recombinant factor VII is controversial. It has been included “off-label” in some strategies or protocols to control a life-threatening hemorrhage because it could reverse the inhibitory effect of clopidogrel,Citation105 but there is not enough evidence to support its inclusion as a standard recommendation in urgent surgery for patients under the effect of an APA.
Platelet transfusion
As there is no drug to directly antagonize the antiplatelet effect of aspirin or clopidogrel, sometimes, platelet transfusion (1 U/5–10 kg) may be needed to stop bleeding. Citation106 Regular prophylactic transfusion of platelets should be discouraged and evaluated carefully on a case-by-case basis, considering the expected benefit gained from it and the possible risks of platelet transfusion. In patients who have received clopidogrel, despite the low immediate hemostatic effect, the preoperative transfusion of platelets could be a good alternative to facilitate postoperative hemostasis, only if the patients undergo surgery with high bleeding risk.Citation52,Citation107
The dilemma of whether to transfuse platelets before the beginning of clinical bleeding or only with the aim of treating a clinically significant hemostatic alteration depends in most cases on the kind of surgery and if the inherent bleeding could have a bad vital or functional implication.
Semiurgent surgery
What to do if surgery cannot be delayed and the patient is in a high-risk period for thrombosis? The management of APA should have minimizing the ST risk as priority, trying to expose the patient to the minimal bleeding risk. Several protocols have been proposed with a multidisciplinary approach, although none of them has been completely validated:Citation9,Citation106
Low bleeding risk: The recommendation is to maintain dual therapy until the day before surgery.
Moderate bleeding risk: Aspirin should be continued, and clopidogrel should be stopped 5 days before surgery. Some authors have proposed a bridging therapy, with the infusion of tirofiban and unfractionated heparin started 3 days before surgery and maintained until 6 hours before surgery, mainly if the patient has additional risk factors and very high thrombotic risk of DES,Citation101 or the infusion of tirofiban only started 4 days before surgery until 4 hours before surgery.Citation108
High bleeding risk: Aspirin and clopidogrel should be discontinued 5 days before surgery (considered to be the best option), or aspirin and clopidogrel should be stopped 10 days before surgery and a bridging therapy should be started as explained earlier.
Conclusions
Surgery after coronary stent placement is associated with a moderate to high risk of major adverse cardiac events, mostly caused by in-stent thrombosis due to the prothrombotic and proinflammatory states, which lead to hypercoagulability associated with surgical intervention. The interruption of antiplatelet therapy before surgery and/or through the perioperative period could have a rebound effect with an increase in the hypercoagulation and prothrombotic state and more risk for ST.
On the other hand, the maintenance of APA could increase the risk of bleeding in some interventions, with a wide range of implications: from no influence in the development of surgery and outcome to a vital risk associated with bleeding in closed spaces. So, the challenge for the surgical team is to use a therapy that can minimize both thrombotic and hemorrhagic risks to reach the better benefit for the patient.
Although there is no valid algorithm for all situations, current trend is to delay all surgery that is not life-threatening if the stent has high thrombotic risk and, if not possible, to give optimal protection to the patients with BMS or DES with the maintenance of at least an APA, or running an alternative protocol with anticoagulants and aspirin. In all other patients, aspirin should be maintained throughout the perioperative period if possible, balancing individual benefit/risk ratio of the withdrawal of APA.
Disclosure
The authors report no conflicts of interest in this work.
References
- NewsomeLTKutcherMARoysterRLCoronary artery stents, Part I: Evolution of percutaneous coronary interventionAnesth Analg200810755256918633035
- SharmaRKReddyHKSinghVNAspirin and clopidogrel hyporesponsiveness and nonresponsiveness in patients with coronary artery stentingVasc Health Risk Manag2009596597219997577
- GasparyanAYAspirin and clopidogrel resistance: methodological challenges and opportunitiesVasc Health Risk Manag20106109101220448796
- FerrandisRLlauJVMugarraAPerioperative management of antiplatelet-drugs in cardiac surgeryCurr Cardiol Rev2009512513220436853
- SpahnDRHowellSJDelabaysAChassotPGCoronary stents and perioperative antiplatelet regimen: dilemma of bleeding and stent thrombosisBr J Anaesth20069667567716698866
- RabbitsJANuttallGABrownMJCardiac risk of noncardiac surgery after percutaneous coronary intervention with drug-eluting stentsAnesthesiology200810959660418813037
- LlauJVLópez-ForteCSapenaMLFerrandisRPerioperative management of antiplatelet agents in noncardiac surgeryEur J Anaesthesiol20092618118719244686
- ChassotPGDelabaysASpanhDRPerioperative antiplatelet therapy: the case for continuing therapy in patients at risk of myocardial infarctionBr J Anaesth20079931632817650517
- PatronoCBaigentCHirshJRothGAntiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition)Chest2008133Suppl 6199S233S18574266
- AngiolilloDFerreiroJLPlatelet adenosin diphosphate P2Y12 receptor antagonism: benefit and limitations of current treatment strategies and future directionsRev Esp Cardiol201063607620089227
- VaneJRBottingRMThe mechanism of action of aspirinThromb Res200311025525814592543
- CoxDMareeAODooleyMConroyRByrneMFFitzgeraldDJEffect of enteric coating on antiplatelet activity of low-dose aspirin in healthy volunteersStroke2006372153215816794200
- JimenezAHStubbsMEToflerGHWintherKWilliamsGHMullerJERapidity and duration of platelet suppression by enteric-coated aspirin in healthy young menAm J Cardiol1992692582621731469
- DienerHCCunhaLForbesCSiveniusJSmetsPLowenthalAEuropean Stroke Prevention Study, Part II: Dipyridamole and acetylsalicylic acid in the secondary prevention of strokeJ Neurol Sci19961431138981292
- International Stroke Trial Collaborative GroupThe International Stroke Trial (IST): a randomized trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischemic strokeLancet19973499065156915819174558
- ISIS-2 Collaborative GroupRandomized trial of intravenous streptokinase, oral aspirin, both or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2Lancet1988286073493602899772
- Antithrombotic Trialists CollaborationCollaborative meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high-risk patientsBMJ20023247329718611786451
- LandolfiRMarchioliRKuttiJEfficacy and safety of low-dose aspirin in polycythemia veraN Engl J Med200435011412414711910
- LorenzRLSchackyCVWeberMImproved aortocoronary bypass patency by low-dose aspirin (100 mg daily): effects on platelet aggregation and thromboxane formationLancet198418389126112646144975
- Juul-MöllerSEdvardssonNJahnmatzBRosénASørensenSOmblusRDouble-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectorisLancet19923408833142114251360557
- GasparyanAYWatsonTLipGYThe role of aspirin in cardiovascular prevention: implications of aspirin resistanceJ Am Coll Cardiol2008511829184318466797
- BonviniRFRenyJLMachFAcute coronary syndrome and its antithrombotic treatment: focus on aspirin and clopidogrel resistanceCurr Vasc Pharmacol2009719820819356003
- GachetCThe platelet P2 receptors as molecular targets for old and new antiplatelet drugsPharmacol Ther200510818019215955565
- BoeynaemsJMvan GiezenHSaviPHerbertJMP2Y12 receptor antagonists in thrombosisCurr Opin Investig Drugs20056275282
- CAPRIE Steering CommitteeA randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE)Lancet19963489038132913398918275
- SudlowCLMasonGMauriceJBWedderburnCJHankeyGJThienopyridine derivatives versus aspirin for preventing stroke and other serious vascular events in high vascular risk patientsCochrane Database Syst Rev20094CD00124619821273
- MehtaSRYusufSClopidogrel in Unstable angina to prevent Recurrent Events (CURE) Study InvestigatorsThe Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial programme; rationale, design and baseline characteristics including a meta-analysis of the effects of thienopyridines in vascular diseaseEur Heart J2000212033204111102254
- BowryADBrookhartMAChoudhryNKMeta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular eventsAm J Cardiol200810196096618359315
- De LucaGCassettiEMarinoPImpact of duration of clopidogrel prescription on outcome of DES as compared to BMS in primary angioplasty: a meta-regression analysis of randomized trialsJ Thromb Thrombolysis20092736537818498003
- AntmanEMAnbeDTArmstrongPWACC/AHA guidelines for the management of patients with ST-elevation myocardial infarctionJ Am Coll Cardiol20044467171915358045
- SilberSAlbertssonPAvilésFFGuidelines for percutaneous coronary interventionsEur Heart J20052680484715769784
- DugganSTKeatingGMPrasugrel: a review of its use in patients with acute coronary syndromes undergoing percutaneous coronary interventionDrugs2009691707172619678718
- MarzotFPengoVPrasugrel for the treatment of patients with acute coronary syndromeVasc Health Risk Manag2009532132419436677
- MotovskaZWidimskyPImproving outcomes in patients undergoing percutaneous coronary intervention: role of prasugrelVasc Health Riak Manag20095475481
- NorgardNBAbu-FadelMComparison of prasugrel and clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary interventionVasc Health Riak Manag20095873882
- SilvaMADonovanJLGandhiPJPlatelet inhibitors in non-ST-segment elevation acute coronary syndromes and percutaneous coronary intervention: glycoprotein IIb/IIIa inhibitors, clopidogrel, or bothVasc Health Risk Manag20062394817319468
- TchengJEEllisSGGeorgeBSPharmacodynamics of chimeric glycoprotein IIb/IIIa integrin antiplatelet antibody Fab 7E3 in high-risk coronary angioplastyCirculation199490175717647923659
- EPIC InvestigatorsUse of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplastyN Engl J Med19943309569618121459
- EPILOG InvestigatorsPlatelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularizationN Engl J Med1997336168916969182212
- EPISTENT InvestigatorsRandomized placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockadeLancet1998352912287929672272
- TopolEJLincoffAMKereiakesDJMulti-year follow-up of abciximab therapy in three randomized, placebo-controlled trials of percutaneous coronary revascularizationAm J Med20021131612106616
- BisharaAIHagmeyerKOAcute profound thrombocytopenia following abciximab therapyAnn Pharmacother20003492493010928405
- BarrettJSMurphyGPeerlinckKPharmacokinetics and pharmacodynamics of MK-383, a selective non-peptide platelet glycoprotein-IIb/IIIa receptor antagonist, in healthy menClin Pharmacol Ther1994563773887955799
- Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Study InvestigatorsA comparison of aspirin plus tirofiban with aspirin plus heparin for unstable anginaN Engl J Med1998338149815059599104
- RESTORE InvestigatorsEffects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplastyCirculation199796144514539315530
- HarringtonRAKleimanNSKottke-MarchantKImmediate and reversible platelet inhibition after intravenous administration of a peptide glycoprotein IIb/IIIa inhibitor during percutaneous interventionAm J Cardiol199576122212277503000
- TchengJEHarringtonRAKottke-MarchantKMulticenter, randomized, double-blind, placebo-controlled trial of the platelet integrin glycoprotein IIb/IIIa blocker integrelin in elective coronary interventionCirculation199591215121577697843
- PURSUIT Trial InvestigatorsInhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromesN Engl J Med19983394364439705684
- MichelsonADMethods for the measurement of platelet functionAm J Cardiol2009103Suppl 320A26A
- ChristiaensLMacchiLMonitoring of the antiplatelet drugs effect in patients with coronary artery disease: what is the real clinical impactCurr Vasc Pharmacol2007529330117979795
- GibbsNMPoint-of-care assessment of antiplatelet agents in the perioperative period: a reviewAnaesth Intensive Care20093735436919499855
- Howard-AlpeGMde BonoJHudsmithLCoronary artery stents and non-cardiac surgeryBr J Anaesth20079856057417456488
- CraftRMChavezJJSniderCCComparison of modified thrombelastograph and Plateletworks whole blood assays to optical platelet aggregation for monitoring reversal of clopidogrel inhibition in elective surgery patientsJ Lab Clin Med200514530931515976759
- LennonMJGibbsNMWeigthmanWMA comparison of Plateletworks™ and platelet aggregometry for the assessment of aspirin- related platelet dysfunction in cardiac surgical patientsJ Cardiothorac Vasc Anesth20041813614015073699
- MalininASperglingMMuhlesteinBAssessing aspirin responsiveness in subjects with multiple risk factors for vascular disease with a rapid platelet function analyzerBlood Coagul Fibrinolysis20041529530115166914
- RoyPOkabeTPinto SlottowTLCorrelates of clinical restenosis following intracoronary implantation of drug-eluting stentsAm J Cardiol200710096596917826379
- StoneGWMosesJWEllisSGSafety and efficacy of sirolimus-and paclitaxel-eluting coronary stentsN Eng J Med20073569981008
- DoostzadehJClarkLNBezenekSPiersonWSoodPRSudhirKRecent progress in percutaneous coronary intervention: evolution of the drug-eluting stents, focus on the XIENCE V drug-eluting stentCoron Artery Dis201021465619952925
- Pinto SlottowTLWaksmanRDrug-eluting stent safetyAm J Cardiol2007100Suppl 210M17M
- AwataMKotaniJUematsuMSerial angioscopic evidence of incomplete neointimal coverage after sirolimus-eluting stent implantation: comparison with bare-metal stentsCirculation200711691091617684153
- SpertusJAKettelkampRVanceCPrevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registryCirculation20061132803280916769908
- KingSBSmithSCHirshfeldJWJacobsAKMorrisonDAWilliamsDO2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing CommitteeCirculation200811726129518079354
- CohenMAntiplatelet therapy in percutaneous coronary intervention: a critical review of the 2007 AH/ACC/SCAI guidelines and beyondCatheter Cardiovasc Interv20097457959719472347
- CuissetTFrereCQuiliciJBenefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stentingJ Am Coll Cardiol2006481339134517010792
- YongGRankinJFergusonLRandomized trial comparing 600- with 300-mg loading dose of clopidogrel in patients with non- ST elevation acute coronary syndrome undergoing percutaneous coronary intervention: results of the Platelet Responsiveness to Aspirin and Clopidogrel and Troponin Increment after Coronary Intervention in Acute Coronary Lesions (PRACTICAL) TrialAm Heart J200915760.e160.e919081397
- MehtaSRYusufSPetersRJEffects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE studyLancet2001358928152753311520521
- BeckerRCMeadeTWBergerPBThe primary and secondary prevention of coronary artery disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition)Chest2008133Suppl 6776S814S18574278
- EisensteinELAnstromKJKongDFClopidogrel use and longterm clinical outcomes after drug-eluting stent implantationJAMA200729715916817148711
- JenningsDLKalusJSAddition of cilostazol to aspirin and a thienopyridine for prevention of restenosis after coronary artery stenting: a meta-analysisJ Clin Pharmacol201050441542120081227
- AlbaladejoPMarretEPiriouVSamamaCMPerioperative management of antiplatelet agents in patients with coronary stents: recommendations of a French Task ForceBr J Anaesth20069758058216956897
- DouketisJDBergerPBDunnASThe perioperative management of antithrombotic therapyChest2008133299S339S18574269
- LecompteTHardyJFAntiplatelet agents and perioperative bleedingCan J Anesth200653S103S11216766783
- BurgerWChemnitiusJMKneisslGDRückerGLow-dose aspirin for secondary cardiovascular prevention: cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation – review and meta-analysisJ Intern Med200525739941415836656
- HelfriedMFlorianPMünchAPremature discontinuation of antiplatelet drug therapy in cardiovascular risk patients: a preliminary study on the role of P2Y12 receptor monitoringEur J Anaesthesiol20102713814519593147
- SamamaCMBastienOForestierFthe expert groupAntiplatelet agents in the perioperative period: expert recommendations of the French Society of Anesthesiology and Intensive Care (SFAR) 2001 – summary statementCan J Anesth200249S26S3512557412
- SabatéSMayoralMVernettaDHernandoDBaxariasPIntraoperative blood loss and transfusion requirements for radical laparoscopic vs radical retropubic prostatectomyEur J Anaesthesiol200825Suppl 4490
- SchoutenOvan DomburgRTBaxJJNoncardiac surgery after coronary stenting: early surgery and interruption of antiplatelet therapy are associated with an increase in major adverse cardiac eventsJ Am Coll Cardiol20074912212417207733
- FoxKAAMehtaSRPetersRBenefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) TrialCirculation20041101202120815313956
- WenaweserPReyCEberliFRStent thrombosis following bare-metal stent implantation: success of emergency percutaneous coronary intervention and predictors of adverse outcomeEur Heart J2005261180118715728650
- IakovouISchmidtTBonizzoniEIncidence, predictors, and outcomes of thrombosis after successful implantation of drug-eluting stentsJAMA20052932126213015870416
- Balaguer-MalfagónJRPomar-DomingoFVilar-HerreroJVPlanas-del ViejoAMPérez-FernándezETrombosis del stent en la era moderna: incidencia, consecuencias y factores predictores (Spanish)Rev Esp Cardiol20065984284516938235
- De la Torre-HernándezJMAlfonsoFHernándezFDrug- eluting stent thrombosis: results from the multicenter Spanish Registry ESTROFA (Estudio EsSpanol sobre TROmbosis de stents FArmacoactivos)J Am Coll Cardiol20085198699018325436
- CaixetaALeonMBLanskyAJ5-year clinical outcomes after sirolimus-eluting stent implantation insights from a patient-level pooled analysis of 4 randomized trials comparing sirolimus-eluting stents with bare-metal stentsJ Am Coll Cardiol20095489490219712798
- CutlipDEWindeckerSMehranRClinical end points in coronary stent trials: a case for standardized definitionsCirculation20071152344235117470709
- CamenzindEStegPGWijnsWStent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concernCirculation20071151440145517344324
- GrinesCLBonowROCaseyDEPrevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stentsJ Am Coll Cardiol20074973473917291948
- CarlssonJJamesSKLindbäckJOutcome of drug-eluting versus bare-metal stenting used according to on- and off-label criteriaJ Am Coll Cardiol2009531389139819371822
- KirtaneAJGuptaAIyengarSSafety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studiesCirculation20091193198320619528338
- LagerqvistBCarlssonJFröbertOStent thrombosis in Sweden: a report from the Swedish Coronary Angiography and Angioplasty RegistryCirc Cardiovasc Interv2009240140820031749
- PopescuWMPerioperative management of the patient with a coronary stentCurr Opin Anaesthesiol20102310911519901828
- BaranKWLasalaJMCoxDAA clinical risk score for prediction of stent thrombosisAm J Cardiol200810254154518721509
- van WerkumJWHeestermansAAZomerACPredictors of coronary stent thrombosis: the Dutch Stent Thrombosis RegistryJ Am Coll Cardiol2009531399140919371823
- RoyPBonelloLTorgusonRTemporal relation between clopidogrel cessation and stent thrombosis after drug-eluting stent implantationAm J Cardiol200910380180519268735
- KimuraTMorimotoTNakagawaYAntiplatelet therapy and stent thrombosis after sirolimus-eluting stent implantationCirculation200911998799519204304
- VincenziMNMeislitzerTHeitzingerBHalajMFleisherLAMetzlerHCoronary artery stenting and non-cardiac surgery. A prospective outcome studyBr J Anaesth20069668669316670113
- NewsomeLTWellerRSGerancherJCKutcherMARoysterRLCoronary artery stents, Part II: Perioperative considerations and managementAnesth Analg200810757059018633036
- SierraPTormosPUnzuetaMCSabatéMMonsalveCSabatéSManejo perioperatorio de la antiagregación en pacientes portadores de stent coronario (Spanish)Rev Esp Anestesiol Reanim200855Suppl 111418333379
- LehotJStaatPFfrenchPVichovaZCannessonMCoronary stents and anaesthesiaAnn Fr Anesth Reanim200726978479017692497
- KingSBSmithSCHirshfeldJW2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelinesJ Am Coll Cardiol20085117220918191745
- PoldermansDBaxJJBoersmaEGuidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgeryEur Heart J2009302769281219713421
- BroadLLeeTConroyMSuccessful management of patients with a drug-eluting coronary stent presenting for elective, non-cardiac surgeryBr J Anaesth200798192217124186
- BigalkeBSeizerPGeislerTLindemannSGawazMMayAEPerioperative antiplatelet therapy in patients at risk for coronary stent thrombosis undergoing noncardiac surgeryClin Res Cardiol200998533533919283333
- EisenbergMJRichardPRLibersanDFilionKBSafety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stentsCirculation20091191634164219289638
- DespotisGEbyCLublinDMA review of transfusion risks and optimal management of perioperative bleeding with cardiac surgeryTransfusion2008482S30S18302579
- LauritzenBTranholmMEzbanMrFVIIa and a new enhanced rFVIIa 10analogue, NN1731, reduce bleeding in clopidogrel-treated and in thrombocytopenic ratsJ Thromb Haemost2009765165719175492
- BornemannHPrüllerFMetzlerHThe patient with coronary stents and antiplatelet agents: what to do and how to deal?Eur J Anaesthesiol 201027540641010.1097/EJA.0b013e328225b28420009935
- VilahurGChoiBGZafarMUNormalization of platelet reactivity in clopidogrel-treated subjectsJ Thromb Haemost20075829017239165
- SavonitoSD’UrbanoMCaraccioloMUrgent surgery in patients with recently implanted coronary drug-eluting stent: a phase II study of “bridging” antiplatelet therapy with tirofiban during temporary withdrawal of clopidogrelBr J Anaesth2010104328529120047898