Abstract
The field of mechanical circulatory support has made great strides in the preceding 2 decades. Although pediatric mechanical circulatory support has lagged behind that of adults, the gap between them is expected to close soon. The only device currently approved by the US Food and Drug Administration for use in children is the Berlin Heart EXCOR ventricular assist device (VAD). The prospective Berlin Heart Investigational Device Exemption Trial demonstrated good outcomes, such as bridge to transplantation or recovery, in ~90% of children supported with this device. However, a high incidence of hemorrhagic and thrombotic complications was also noted. As a result, pediatric centers have just started implanting adult intracorporeal continuous-flow devices in children. This paradigm shift has opened a new era in pediatric mechanical circulatory support. Whereas children on VAD were previously managed exclusively in hospital, therapeutic options such as outpatient management and even destination therapy have been becoming a reality. With continued miniaturization and technological refinements, devices currently in development will broaden the range of options available to children. The HeartMate 3 and HeartWare MVAD are two such compact VADs, which are anticipated to have great potential for pediatric use. Additionally, a pediatric-specific continuous-flow VAD, the newly redesigned Jarvik Infant 2015, is currently undergoing preclinical testing and is expected to undergo a randomized clinical trial in the near future. This review aims to discuss the challenges posed by the use of intracorporeal adult continuous-flow devices in children, as well as to provide our perspective on the future prospects of the field of pediatric VADs.
Introduction
The advent of mechanical circulatory support has had a significant impact on the management of heart failure. In particular, adult mechanical circulatory support has rapidly evolved over the past 2 decades. Continued improvements in ventricular assist device (VAD) technology, as well as clinical management, have produced superior outcomes. The introduction of implantable continuous-flow devices with favorable complication profiles, such as the HeartMate II (St. Jude Medical, Inc., St Paul, MN, USA; ) and HeartWare HVAD (HeartWare, Inc., Framingham, MA, USA; ), has led to adult physicians favoring VAD support over escalation of medical therapy. As a result, VAD therapy is fast becoming the standard of care for intractable heart failure. Indeed, VAD support may become the primary alternative to heart transplantation in the future.Citation1,Citation2
Figure 1 HeartMate II™ Left Ventricular Assist Device (LVAD).

In contrast, despite being a critically unmet need, the development of pediatric mechanical circulatory support continues to lag behind that of adult VADs. Although reasons for this are multifactorial, certain elements, such as patient heterogeneity, smaller consumer numbers, and regulatory challenges, may have disincentivized the development of pediatric devices.Citation3,Citation4 The number of children awaiting transplantation is steadily risingCitation5 in the setting of static supply of pediatric donor hearts numbering ~500 per annum worldwide.Citation6 Improved surgical palliation has allowed an increased proportion of young children with complex congenital heart diseases to survive longer; in many cases, the palliated heart ultimately fails, culminating in the need for transplantation. Pediatric patients awaiting heart transplantation carry the highest wait-list mortality risk of all solid organs.Citation7 The ensuing rise in the proportion of sicker children with end-stage heart failure has reinvigorated interest in pediatric mechanical circulatory support. Through this article, the authors aim to present an overview of current challenges facing the realm of pediatric VADs, as well as its future prospects.
Current challenges
Challenges in patient selection
In principle, VAD therapy should be considered when presented with a child with end-stage heart failure in whom medical therapy is failing. However, in order to achieve the best possible outcomes, it is imperative to discern the subset of patients who are poor candidates for VAD support. Selected examples of challenging populations are described subsequently.
Small infants
Lying at one extreme in the spectrum in pediatric VAD candidates, small infants (<5 kg) comprise a cohort that presents a unique set of challenges. In a multicenter study, Almond et alCitation8 reviewed outcomes of 204 children implanted with the Berlin EXCOR VAD (Berlin Heart, Inc., The Woodlands, TX, USA; ) at 47 centers between 2007 and 2010. Notably, they found that approximately two thirds of patients weighing <5 kg died while on Berlin EXCOR support.Citation8 Factors implicated in contributing to such poor outcomes include underlying etiology and potential patient–device size mismatch. In conventional left ventricular assist device (LVAD) physiology, the pump rate is determined by cardiac output from the native right ventricle. In a very small child, therefore, a pump rate may become too low even with the smallest (10 mL) pump, which is a recognized risk factor for pump thrombosis. For example, if a 3 kg baby has a cardiac output of 150 mL/kg/min, the pump rate will be 45 beats/min (150 mL/kg/min × 3 kg divided by 10 mL) assuming the pump to be fully filled and fully ejected without significant aortic insufficiency. In order to run a pump at a reasonably high rate (ie, 70 beats/min or 80 beats/min), addition of a right ventricular assist device (RVAD) may be necessary in small infants to maintain cardiac output. That said, another study examining 204 Berlin EXCOR VAD implantations in North America between 2007 and 2010 has suggested that some children undergoing durable BiVAD support might have done better with LVADs alone.Citation9 To mitigate the risk of pump thrombosis, the use of a centrifugal pump, such as PediMag (St. Jude Medical, Inc.) and Rotaflow (Maquet Cardiovascular LLC, Wayne, NJ, USA), may be considered instead of the Berlin EXCOR pulsatile pump;Citation10 the efficacy of this approach in small children has not been well studied to date.
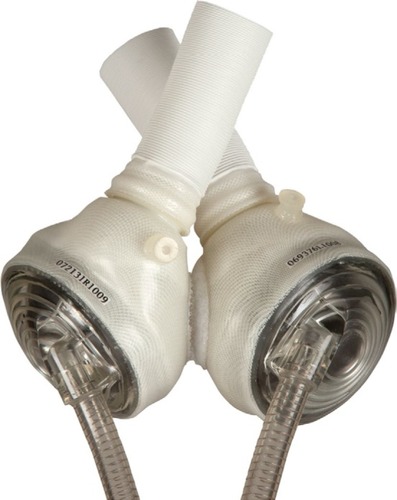
Figure 3 Berlin Heart EXCOR VAD.
Abbreviation: VAD, ventricular assist device.
Undoubtedly, there is a definitive need for other methods to support the failing heart in this high-risk subset of patients. A potential alternative is pulmonary artery banding (PAB). Schranz et alCitation11 reported their experience with employing this strategy in 12 patients (ten infants and two toddlers) with dilated cardiomyopathy referred for heart transplantation. The authors described an improvement in left ventricular (LV) function by echocardiographic measures (improved LV ejection fraction, decreased LV cavitary volume, and mitral regurgitation), as well as reduced serum brain natriuretic peptide. There were two deaths, and ten patients remained delisted at a mean follow-up of 2 years. The exact reason why PAB improves hemodynamics in children with dilated cardiomyopathy is not well understood. It has been suggested that leftward shift of the interventricular septum by increasing right ventricular afterload may diminish mitral regurgitation, which could improve overall cardiac output. This theory sounds plausible but is not necessarily consistent with our experience.Citation12 In a 6-week-old infant (weight, 4 kg) with dilated cardiomyopathy in impending need of a VAD, we observed significant hemodynamic improvement with PAB even with minimal change in septal configuration and mitral regurgitation. For a better understanding of PAB physiology, we monitor pressures of all four cardiac chambers during PAB placement, namely both atria and right ventricle directly and LV (using the arterial line pressure as a surrogate). With gradual tightening of the band, we observed a reduction in left atrial pressure, whereas right atrial pressure changed very little. Although more studies are necessary to confirm this hypothesis, it seems that PAB, in the setting of dilated cardiomyopathy, facilitates a redistribution of hemodynamic stresses exerted by the failing LV to a relatively preserved right ventricle. In selected patients, PAB may reportedly induce cardiac recoveryCitation11 or may serve as a temporizing measure until a suitable organ becomes available for heart transplantation. Even if the patient deteriorates after PAB placement, the possibility of delaying VAD implantation can be beneficial if somatic growth is achieved on PAB, as VAD outcomes are significantly better in patients weighing >5 kg (25% mortality) compared with those weighing <5 kg (64% mortality), as described in a study by Conway et al.Citation13 This significant difference in mortality, however, must be interpreted with caution since the proportion of congenital heart disease was significantly higher in smaller children. In this study, congenital heart disease was identified as an independent risk factor for mortality by a multivariate analysis.Citation13 PAB may also condition the RV, which would be favorable for LVAD physiology.Citation12
Single-ventricle congenital heart diseas
The decision to provide VAD support in patients with complex single-ventricle physiology warrants careful consideration. In reviewing the outcomes of Berlin EXCOR VAD in the single-ventricle cohort during the Food and Drug Administration Investigational Device Exemption trial, Weinstein et alCitation14 found that survival was significantly worse compared with biventricular physiology (42% vs 73%). These data certainly highlight the challenging nature of VAD support in this patient population. A striking finding in their study was the dramatic difference in survival depending on the stage of single-ventricle palliation at which the Berlin Heart EXCOR VAD was implanted. Patients after Stage I palliation had extremely poor outcomes (11% survival), whereas those after Stage II and Stage III did significantly better, with survival reaching 60%. Survival rates with VAD support for Stage II (58%) and Stage III (60%) are still better than previously reported outcomes for ECMO support in single-ventricle physiology (Stage II [41%]Citation15 and Stage III [35%]Citation16). The type of VAD used for single-ventricle physiology may also have an important clinical impact on outcome. Horne et alCitation17 have described the superiority of continuous-flow VAD over pulsatile VAD. The fact that pulsatile VAD decompresses the failing systemic ventricle only during pump diastole could be deleterious to pulmonary circulation that lacks a pumping chamber (ie, subpulmonary ventricle). There have been several reports describing successful use of an implantable continuous-flow VAD in a failing Fontan circulation (ie, Stage III).Citation18–Citation20 Fontan circulations can fail for multiple reasons, including systolic or diastolic dysfunction of the heart, elevated pulmonary vascular resistance, or a combination of these.Citation21 In contrast, the use of an implantable continuous-flow VAD in failing Glenn circulation (ie, Stage II) is severely limited, likely due to the smaller size of the patients in this group. To our knowledge, the report we have published recently is the only such case in reported literature.Citation22 With ongoing miniaturization of devices, nonetheless, implantable continuous-flow VAD will soon become the mainstay of VAD support for failing Glenn physiology.
Chronic graft dysfunction
Three decades have passed since pediatric heart transplantation became a clinical reality.Citation23 In present days, many of previous transplant recipients, now older children, adolescents, and young adults, return with medically resistant chronic graft dysfunction. This represents a particularly difficult population to manage. Some require relisting for heart transplantation; yet, due to relatively preserved systolic function in this subgroup, their listing status is typically not high enough to expect a timely offer. Rather, the underlying process is primarily diastolic dysfunction in the setting of coronary ischemia. As such, preserved systolic cardiac function with nondilated LV cavity creates technical challenges in placing a VAD inflow cannula if attempted. In addition, patients’ immunocompromised state increases the risk of infectious complications with VAD support.Citation24 The Total Artificial Heart (Syncardia Systems, Inc., Tucson, AZ, USA; ) presents an alternate option in these circumstances since cardiectomy, as a part of the implantation procedure, permits discontinuation of immunosuppression. Due to size constraints with the existing 70 mL pump, pediatric patients are unlikely to avail this option.Citation25 The development of smaller pumps (50 mL) may widen its indication to some degree. A clinical trial with the 50 mL pump in the pediatric population is to begin.Citation26
Challenges associated with optimal timing of VAD support
Determining not only whom but also when to initiate VAD support is also challenging. While waiting too long to initiate VAD support sharply increases the risk of mortality and morbidity, implanting a VAD too early also predisposes the patient to risks of device-related complications. Therefore, ascertaining the optimal timeframe within which to start VAD therapy is crucial to securing good outcomes. In the early days of device evolution, an exceedingly high complication profile of VAD support essentially restricted offering mechanical support to patients who were critically unwell, that is INTERMACS Stage I (critical cardiogenic shock with evidence of end-organ injury).Citation27 Accumulated VAD experience has demonstrated poor outcomes with this approach.Citation1 Subsequently, particularly in the adult patient population, the paradigm has shifted in favor of instituting VAD support earlier, that is, when the patient is more stable, with a significant resultant improvement in morbidity and mortality.Citation28 Practices in pediatric VAD management are often extrapolated from observed trends within existing adult data. However, the aforementioned trend of earlier VAD implantation in adults has not yet been adopted in pediatric settings. Pediatric physicians traditionally err on the conservative side with respect to initiating VAD support. To date, most children with heart failure undergo VAD placement at INTERMACS Stage I or Stage II, especially small children who can be supported only with a pulsatile VAD. In the entire North American experience with the EXCOR, >50% of patients were at INTERMACS Stage I and 40% were on ECMO support at the time of VAD implantation.Citation6 Additionally, there has been a clear trend toward longer duration of VAD support in the recent era.Citation29 Considering the fact that risks of device-related complications, such as embolic stroke, increase proportionately with the length of support duration, it stands to reason that the burden of such risks may increase further. The window for instituting VAD support in children with rapidly deteriorating hemodynamics is small, leaving little room for error; it is thus essential to strike a balance between reaping the intended benefits of VAD support and avoiding exposure to risk of complications prematurely, if best outcomes are to be ensured.
Challenges with influencing risk profiles of existing devices
Continuous-flow VAD in adolescents
As described earlier, in order to take advantage of predictable complication profiles while simultaneously circumventing the risk of morbidity associated with pulsatile devices, pediatric centers are starting to favor implanting adult continuous-flow devices in children. Continuous-flow devices are anticipated to be increasingly used in the pediatric population, driven by theoretically better complication profiles and the potential for home discharge. Although it is premature to conclude that the use of continuous-flow VADs in children is a reasonably safe option, preliminary experiences are encouraging.
The most widely used implantable continuous-flow devices in the adult population is the HeartMate II. Its popularity is driven by a lower incidence of thromboembolic complications compared with previous VADs.Citation30 According to the Seventh INTERMACS report, the rate of strokes in the first year after continuous-flow VAD implant in adults in the recent era (2012–2014) stands at 1.61 events per 100 patient months of support.Citation28 The HeartMate II presents an excellent option for older children and adolescents with a body surface area of >1.3m2. Reported outcomes with the device in appropriately selected patients are good, with >90% of children implanted with a HeartMate II surviving to transplant, explanted for recovery, or continuing on support at 6 months follow-up.Citation31 The use of this device has largely negated the need for Berlin EXCOR 50 mL and 60 mL pumps in adolescents. Compact continuous-flow VADs, such as the HVAD, may be implanted in both right and left ventricles to provide hemodynamic support in appropriately sized adolescents with biventricular failure.Citation32 From a viewpoint of logistics surrounding pediatric VAD programs, the most significant impact the HeartMate II has had is that pediatric centers have learned how to manage their patients on VAD support on an outpatient basis. In the previous era where the pulsatile pump was the sole option for children, virtually all the patients with VAD support were managed as in-patients. In a recent multi-institutional study, we have demonstrated the feasibility of discharging majority of children on HVAD support, who may then go on to resume regular activities.Citation33 However, recent studies (including the PediMACS registry data) show that about half of the patients supported by continuous-flow VADs are still managed in hospital.Citation34–Citation36 As the experience with continuous-flow VAD in children advances, the home discharge rate will steadily increase. At our institution, nearly all children implanted with continuous-flow VADs are discharged home. The fact that the pediatric community has learned what is prerequisite to discharging children with ongoing VAD support (ie, education of primary caregivers and school teachers, etc) has made it possible to move the pediatric VAD programs to the next stage where a miniaturized continuous-flow VAD is offered even to small children.
Continuous-flow VAD in small children
The emergence of a smaller continuous-flow device further accelerated the trend of favoring continuous-flow VAD in children. The HVAD is now gaining wider attention from pediatric centers worldwide due to its compact design. Debate is ongoing with respect to how young and how small an HVAD recipient can be. Anecdotal experience with the HVAD suggests that children with a body surface area as low as 0.6 m2 may be candidates for HVAD support.Citation35 This is consistent with our experience. The smallest patient in our series was a 4-year-old boy weighing 13 kg with a body surface area of 0.6 m2 at HVAD implantation. He has been supported well (>600 days of ongoing support) without any neurologic events. These anecdotal experiences clearly demonstrate the potential feasibility of continuous-flow VAD support even in small children.Citation20,Citation30,Citation31,Citation35,Citation37
Care must be taken, however, when applying adult devices in smaller children since this will, by definition, inevitably result in patient–device size mismatch. For example, an inflow cannula may be too large for a child’s intraventricular cavitary volume. When adult-sized pulsatile pumps were used in children, the most significant risk factor for poor outcome was the size of patients;Citation38 the smaller the child, the worse the survival. In other words, the degree of patient–device size mismatch has a direct impact on outcome. Having realized the difference between pulsatile and continuous-flow devices, however, it would be still prudent to assume that size mismatch remains a risk in the current era of the use of continuous-flow VAD.
Surgical modifications to mitigate patient–device size mismatch
Considering the inevitable dilemma of patient–device size mismatch, avenues to improve outcomes must be found until pediatric-specific, age-appropriate devices become available. We have found potential areas of improvement in making the HVAD work efficiently in children. Foremost, the standard implantation technique that has proven effective in the adult population may not be the best option for pediatric patients.
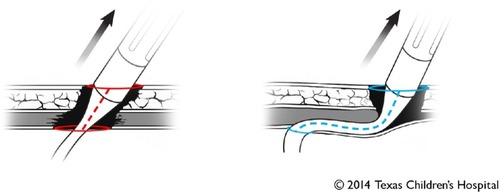
In our opinion, the most critical aspect is the angle of the inflow cannula relative to the interventricular septum. In this regard, the standard technique, so-called “intrapericardial placement”, is suboptimal. A concern with the standard intra-pericardial placement is that the pump inflow typically lies in a horizontal plane (ie, perpendicular to the interventricular septum).Citation39,Citation40 One of the most important lessons learned from the large experience (>20,000 implants worldwide) with the HeartMate II is that such a perpendicular orientation of the inflow cannula relative to the septum may predispose to pump thrombosis or suction events.Citation41 Although one can argue that HVAD is not exactly the same as the HeartMate II, the fundamental principle of the inflow cannula configuration should be the same: the inflow cannula needs to be parallel, rather than perpendicular, to the interventricular septum. The concern for inflow angle with the standard technique was first raised by the team at Texas Heart Institute, led by OH Frazier. His group places the inflow cannula through the diaphragmatic surface of the LV, rather than the apex.Citation39 While this approach is an attractive option in adults who have comparatively large ventricular cavities, it may be less than ideal in those with smaller hearts. With the posterior descending coronary artery running in close proximity to the proposed implantation site, myocardial perfusion may be jeopardized by the occlusive effects of the sewing ring. Our preference is to use the LV apex as an insertion site, consistent with the standard approach. However, we place the pump in a pocket created by dividing the left hemidiaphragm.Citation40 The cardiac apex is thus oriented more caudomedially, aligning the inflow cannula in a vertical orientation (ie, parallel to the interventricular septum). The key concept of our approach is that by affixing the pump housing within the pocket, the surgeon can determine the angle of the inflow cannula, rather than letting the pump “free-float” within the pericardial sac. Although there is no contemporary data to prove the superiority of this approach over the conventional one in small children, we believe the relationship of the inflow cannula relative to the interventricular septum is important. A caveat remains in that the smaller the LV, the lower the tolerance for technical imperfections. Miera et alCitation35 recently published their experience with the use of HVAD in small children with a body surface area of ≤1.0 m2. In this multicenter retrospective study, four patients (33%) out of 12, excluding one who died early postoperatively, experienced pump thrombosis. In our experience of 19 HVAD implants at Texas Children’s Hospital, we have not had any pump thrombosis events despite a lower target international normalized ratio (INR) (2.0–2.5) compared with relatively high target (>2.5 up to 3.5) in the multicenter study. Further discussion is necessary to clarify what would be the best implantation technique for small children.
A driveline is the Achilles’ heel of implantable VADs. In our opinion, special care also must be taken when tunneling the driveline through a comparatively less developed abdominal wall in sick children. Goldstein et alCitation42 reported interesting findings using the INTERMACS registry. The authors demonstrated that the younger the patient (among the adult age group), the higher the risk of driveline infection.Citation42 Due to its retrospective nature using registry data, reasons behind this finding are unclear. One may assume that younger patients are more active, and therefore the driveline exit site is subjected to a greater degree of stress, an identified risk factor for developing driveline site infections. In our experience, pediatric patients are prone to driveline site issues due to vigorous physical activity, as can be expected in children, and inattentiveness to the drive-line. It stands to reason, then, that the risk of developing this complication is reportedly high in children, with up to 80% of VAD-specific infections involving the driveline.Citation43 The problem with the standard tunneling technique for the HVAD driveline is that the integrity of the abdominal muscle layer, which is the primary supporting mechanism of the driveline, is destroyed by passing the large connector part (12 mm in diameter; triple the size of the cable; ). Surgical modifications such as ours that maintain the abdominal wall integrity may solve issues related to driveline in active children.Citation44
Anticoagulation strategies
Strategies surrounding anticoagulation and its surveillance vary considerably between institutions. Durable VAD support requires chronic forms of anticoagulation, ie, warfarin and antiplatelet agents, often requiring a heparin bridge. As desirable as it may be, achieving optimal anti-coagulation in children on VAD support is challenging on account of certain developmental differences in hemostasis (eg, pharmacokinetics/pharmacodynamics of anticoagulants, decreased thrombin generation and fibrinolytic capacity, and increased inhibitory capacity).Citation45 As a result, children on VADs may either be overanticoagulated (manifested as hemorrhagic complications) or underanticoagulated (manifested as thrombotic complications). Anticoagulation is also impacted by the type of VAD selected: balancing anticoagulation on the pulsatile Berlin EXCOR VAD is more challenging due to its inherent tendency for fibrin/thrombus formation. Although the standard anticoagulation regimen for the Berlin EXCOR VAD (known as the Edmonton protocol) calls for low-molecular weight heparin in infants, our institutional preference is to administer warfarin to all age groups. With this particular device, the narrow gap between two related complications, namely hemorrhage and thrombosis, often results in the clinician tolerating over- or underanticoagulation, depending on the patient’s current clinical state. Presence of concomitant systemic inflammation, often associated with hypercoagulability, further complicates matters by requiring extremely high doses of anticoagulants in order to attain therapeutic levels. As such, it is our policy that if anticoagulation cannot be achieved without excessive anti-coagulation, the clinician may need to accept subtherapeutic anticoagulation while maintaining a very low threshold to exchange the pump for fibrin deposition/thrombosis. This “permissive under-anticoagulation” strategy is particularly important in the early postoperative period when the risk of bleeding exceeds the risk of thrombotic complications.Citation46
Future prospects
Driven by the continued demand for device miniaturization, small-sized adult devices are currently in development, and may pave the way for greater adoption in pediatric cohort. The wave of implantable continuous-flow VADs may finally reach small children.
Compared to its larger predecessor, the HeartMate 3 (St. Jude Medical, Inc., ) is a device on the horizon, which has potential for use in children. At full range of operation, it is able to provide flows between 2.5 L/min and 10 L/min, as well as an artificial pulse by alternating the pump rotor speed once every other second. In October 2015, the device received the Conformité Européenne Mark approval. The trial enrolled 50 patients in six European hospitals. Six-month survival was 92%. There were no pump thrombosis events, and adverse event rates were lower than or consistent with expectations for severely ill and complex patients requiring LVAD support. The MOMENTUM 3 Investigational Device Exemption trial is ongoing in the US.Citation47
Figure 6 HeartMate 3™ Left Ventricular Assist Device (LVAD).

The MVAD (HeartWare, Inc.; ) is a device, which is currently undergoing testing. It has a displacement volume of 20 mL and weighs <100 g. Its speed ranges from 8,000 rpm to 18,000 rpm and is capable of delivering blood flow between 1 L/min and 7 L/min at a pressure of 75 mmHg. Moreover, unique features such as an adjustable inflow angle relative to the sewing ring (±10°) and an adjustable inflow cannula depth allow customizable configuration to ensure a good fit. The Conformité Européenne Mark trial commenced in July 2015 with first implants performed in the UK and Austria. Simultaneously, HeartWare had applied to the Food and Drug Administration for an Investigational Device Exemption to commence clinical trials in the US.Citation48 Given the size of the device, the MVAD may have the potential to completely change the outlook of pediatric VAD support.
Figure 7 HeartWare® MVAD.

Finally, the Jarvik Infant (Jarvik Heart, Inc., New York, NY, USA; ) is an implantable continuous-flow VAD specifically designed for small children. It had previously experienced setbacks due to hemolysis, resulting in disapproval of Investigational Device Exemption applications. Recently, though, it has undergone significant design overhaul. In the latest iteration, the Jarvik Infant 2015, the size of inflow cannula has increased from 11 mm to 15 mm. This long-awaited device is currently undergoing preclinical testing as collaboration between our center and the Texas Heart Institute. Once successfully completed, this device will be evaluated in the PumpKIN trial (pumps for kids, infants, and neonates) funded by the National Heart, Lung, and Blood Institute, a subsidiary of the National Institutes of Health. This trial is intended to be a two-arm prospective randomized trial where patients will be randomly allocated to receive either the Berlin EXCOR or the Jarvik Infant 2015 device, with each group consisting of 44 patients.Citation49 This trial may open the era of the use of implantable continuous-flow VADs in small children.
Conclusion
To summarize, development of pediatric VADs has not kept pace with adult devices, yet this gap is expected to soon close as overall experience with VADs evolves and the number of potential pediatric candidates for VAD support rises. The contemporary field of pediatric VAD therapy poses many challenges including determining which candidate would derive most benefit from VAD support and selecting the best device for the patient considering their individual clinical circumstances and inherent device complication profiles. Owing to their favorable complication profiles, continuous-flow VADs previously reserved for adults are now being used more frequently in children, predisposing to patient–device size mismatch. The scope of employing continuous-flow VADs in children has widened as solutions to mitigating consequences of patient–device size mismatch are found. Future prospects of pediatric VADs are certainly encouraging: with the continued demand for technological adaptations, such as device miniaturization, it is anticipated that VAD therapy will play a more prominent role in successfully managing children with end-stage heart failure.
Disclosure
Iki Adachi, MD, serves as a consultant and proctor for the Berlin Heart Inc. and the HeartWare Inc. Dr Adachi receives a salary support for his role as a consultant with the New England Research Institute related to the Pump-KIN trial. The authors report no other conflicts of interest in this work.
References
- KirklinJKNaftelDCKormosRLFifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patientsJ Heart Lung Transplant201332214115623352390
- BirksEJThe comparative use of ventricular assist devices: differences between Europe and the United StatesTex Heart Inst J201037556556720978570
- BaldwinJTBorovetzHSDuncanBWGartnerMJJarvikRKWeissWJThe national heart, lung, and blood institute pediatric circulatory support program: a summary of the 5-year experienceCirculation2011123111233124021422399
- AlmondCSChenEABermanMRHigh-risk medical devices, children and the FDA: regulatory challenges facing pediatric mechanical circulatory support devicesASAIO J20075314717237642
- RossanoJWKimJJDeckerJAPrevalence, morbidity, and mortality of heart failure-related hospitalizations in children in the United States: a population-based studyJ Card Fail201218645947022633303
- DipchandAIRossanoJWEdwardsLBThe Registry of the International Society for Heart and Lung Transplantation: eighteenth official pediatric heart transplantation report? 2015; Focus theme: early graft failureJ Heart Lung Transplant201534101233124326454737
- AlmondCSThiagarajanRRPierceyGEWaiting list mortality among children listed for heart transplantation in the United StatesCirculation2009119571772719171850
- AlmondCSMoralesDLBlackstoneEHBerlin heart EXCOR pediatric ventricular assist device for bridge to heart transplantation in US ChildrenCirculation2013127161702171123538380
- ZafarFJefferiesJLTjossemCJBiventricular Berlin heart EXCOR pediatric use across the United StatesAnn Thorac Surg20159941328133425704863
- MaatAPvan ThielRJDalinghausMBogersAJConnecting the Centrimag Levitronix pump to Berlin Heart Excor cannulae; a new approach to bridge to bridgeJ Heart Lung Transplant200827111211518187096
- SchranzDRuppSMullerMPulmonary artery banding in infants and young children with left ventricular dilated cardiomyopathy: a novel therapeutic strategy before heart transplantationJ Heart Lung Transplant201332547548123410738
- GoldbergJFVeselTPJeewaAAdachiIPulmonary artery band reduces left atrial pressure in dilated cardiomyopathyAnn Thorac Surg20151002e35e3626234878
- ConwayJStLouisJMoralesDLSLawSTjossemCHumplTDelineating survival outcomes in children <10 kg bridged to transplant or recovery with the Berlin heart EXCOR ventricular assist deviceJACC Heart Fail201531707725454394
- WeinsteinSBelloRPizarroCThe use of the Berlin Heart EXCOR in patients with functional single ventricleJ Thorac Cardiovasc Surg20141472697704 discussion 704–70524290716
- JolleyMThiagarajanRRBarrettCSExtracorporeal membrane oxygenation in patients undergoing superior cavopulmonary anastomosisJ Thorac Cardiovasc Surg201414841512151824951018
- RoodKLTeeleSABarrettCSExtracorporeal membrane oxygenation support after the Fontan operationJ Thorac Cardiovasc Surg2011142350451021353253
- HorneDConwayJRebeykaIMBuchholzHMechanical circulatory support in univentricular hearts: current managementSemin Thorac Cardiovasc Surg Pediatr Card Surg Annu2015181172425939838
- MoralesDLAdachiIHeinleJSFraserCDJrA new era: use of an intracorporeal systemic ventricular assist device to support a patient with a failing Fontan circulationJ Thorac Cardiovasc Surg20111423e138e14021762934
- NieblerRAGhanayemNSShahTKUse of a HeartWare ventricular assist device in a patient with failed Fontan circulationAnn Thorac Surg2014974e115e11624694452
- MieraOPotapovEVRedlinMFirst experiences with the Heart-Ware ventricular assist system in childrenAnn Thorac Surg20119141256126021440155
- VanderPluymCUrschelSBuchholzHAdvanced therapies for congenital heart disease: ventricular assist devices and heart transplantationCan J Cardiol201329779680223683470
- AdachiIJeewaABurkiSMcKenzieEDFraserCDJrOutpatient management of a child with bidirectional Glenn shunts supported with implantable continuous-flow ventricular assist deviceJ Heart Lung Transplant201635568869027056613
- ChinnockREBaileyLLHeart transplantation for congenital heart disease in the first year of lifeCurr Cardiol Rev201172728422548030
- AdachiIGuzman-PrunedaFAKhanMSMcKenzieEDFraserCDJrVentricular assist device in children with cardiac graft failureASAIO J201561672973026366683
- RyanTDJefferiesJLZafarFLortsAMoralesDLThe evolving role of the total artificial heart in the management of end-stage congenital heart disease and adolescentsASAIO J201561181425248044
- SynCardia [webpage on the Internet]FDA Approves Investigational Study for New Smaller SynCardia Total Artificial Heart Available from: http://www.syncardia.com/2015-multimedia-releases/fda-approves-investigational-study-for-smaller-syncardia-total-artificial-heart/itemid-1750.htmlAccessed June 25, 2016
- StevensonLWPaganiFDYoungJBINTERMACS profiles of advanced heart failure: the current pictureJ Heart Lung Transplant200928653554119481012
- KirklinJKNaftelDCPaganiFDSeventh INTERMACS annual report: 15,000 patients and countingJ Heart Lung Transplant201534121495150426520247
- AdachiIKhanMSGuzman-PrunedaFAEvolution and impact of ventricular assist device program on children awaiting heart transplantationAnn Thorac Surg201599263564025530089
- PaganiFDMillerLWRussellSDHeartMate II InvestigatorsExtended mechanical circulatory support with a continuous-flow rotary left ventricular assist deviceJ Am Coll Cardiol200954431232119608028
- CabreraAGSundareswaranKSSamayoaAXOutcomes of pediatric patients supported by the HeartMate II left ventricular assist device in the United StatesJ Heart Lung Transplant201332111107111324002006
- SteinMLYehJReinhartzOHeartWare HVAD for biventricular support in children and adolescents: The Stanford experienceASAIO J Epub2016225
- SchweigerMVanderpluymCJeewaAOutpatient management of intra-corporeal left ventricular assist device system in children: a multi-center experienceAm J Transplant201515245346025612114
- RossanoJWLortsAVanderPluymCJOutcomes of pediatric patients supported with continuous-flow ventricular assist devices: a report from the Pediatric Interagency Registry for Mechanical Circulatory Support (PediMACS)J Heart Lung Transplant201635558559027056612
- MieraOKirkRBuchholzHA multicenter study of the Heart-Ware HVAD ventricular assist device in small childrenJ Heart Lung Transplant201635567968126922273
- PengEKirkRWrightsonNAn extended role of continuous flow device in pediatric mechanical circulatory supportAnn Thorac Surg Epub2016426
- PadalinoMABottioTTarziaVHeartWare ventricular assist device as bridge to transplant in children and adolescentsArtif Organs201438541842224117521
- BlumeEDNaftelDCBastardiHJPediatric Heart Transplant Study InvestigatorsOutcomes of children bridged to heart transplantation with ventricular assist devices: a multi-institutional studyCirculation2006113192313231916702487
- GregoricIDCohnWEFrazierOHDiaphragmatic implantation of the HeartWare ventricular assist deviceJ Heart Lung Transplant201130446747021211994
- AdachiIGuzman-PrunedaFAJeewaAFraserCDJrDean McKenzieEA modified implantation technique of the HeartWare ventricular assist device for pediatric patientsJ Heart Lung Transplant201534113413625447570
- TaghaviSWardCJayarajanSNGaughanJWilsonLMMangiAASurgical technique influences HeartMate II left ventricular assist device thrombosisAnn Thorac Surg20139641259126523968757
- GoldsteinDJNaftelDHolmanWContinuous-flow devices and percutaneous site infections: clinical outcomesJ Heart Lung Transplant201231111151115722766022
- CabreraAGKhanMSMoralesDLSInfectious complications and outcomes in children supported with left ventricular assist devicesJ Heart Lung Transplant201332551852423489988
- AsakiSYDean McKenzieEEliasBAdachiIRectus-sparing technique for driveline insertion of ventricular assist deviceAnn Thorac Surg201510051920192226522548
- MassicotteMPBaumanMEMurrayJAlmondCSAntithrombotic therapy for ventricular assist devices in children: do we really know what to do?J Thromb Haemost201513Suppl 1S343S35026149046
- ByrnesJWFrazierETangXHemorrhage requiring surgical intervention among children on pulsatile ventricular assist device supportPediatr Transplant201418438539224802345
- THORATEC [webpage on the Internet]St. Jude Medical Announces CE Mark Approval for the HeartMate 3 Left Ventricular Assist System Available from: https://www.thoratec.com/medical-professionals/vad-product-information/heartmate3/HeartMate3-CE-Mark-Press-Release.pdfAccessed June 25, 2016
- HeartWare [webpage on the Internet]HeartWare International Announces First Human Implants Of The MVAD® System In CE Mark International Clinical Trial Available from: http://ir.heartware.com/phoenix.zhtml?c=187755&p=irol-newsArticle_print&ID=2068959Accessed June 25, 2016
- ScheelJNNews from the PumpKIN PatchISHLT Links201463