?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
As the Thoroughbreds race for the final stretch, 44 hooves flash and thunder creating a cacophony of tortured air and turf. Orchestrated by selective breeding for physiology and biomechanics, expressed as speed, the millennia-old symphony of man and beast reaches its climax. At nearly 73 kilometers per hour (45 mph) over half a ton of flesh and bone dwarfs its limpet-like jockey as, eyes wild and nostrils flaring, their necks stretch for glory. Beneath each resplendent livery-adorned, latherin-splattered coat hides a monstrous heart trilling at 4 beats per second, and each minute, driving over 400 L (105 gallons) of oxygen-rich blood from lungs to muscles. Matching breath to stride frequency, those lungs will inhale 16 L (4 gallons) of air each stride moving >1,000 L/min in and out of each nostril – and yet failing. Engorged with blood and stretched to breaking point, those lungs can no longer redden the arterial blood but leave it dusky and cyanotic. Their exquisitely thin blood–gas barrier, a mere 10.5 μm thick (1/50,000 of an inch), ruptures, and red cells invade the lungs. After the race is won and lost, long after the frenetic crowd has quieted and gone, that blood will clog and inflame the airways. For a few horses, those who bleed extensively, it will overflow their lungs and spray from their nostrils incarnadining the walls of their stall: a horrifically poignant canvas that strikes at horse racing’s very core. That exercise-induced pulmonary hemorrhage (EIPH) occurs is a medical and physiological reality. That every reasonable exigency is not taken to reduce/prevent it would be a travesty. This review is not intended to provide an exhaustive coverage of EIPH for which the reader is referred to recent reviews, rather, after a brief reminder of its physiologic and pathologic bases, focus is brought on the latest developments in EIPH discovery as this informs state-of-the-art knowledge, the implementation of that knowledge and recommendations for future research and treatment.
Background
Exercise-induced pulmonary hemorrhage (EIPH) is diagnosed most commonly in the racehorse,Citation1–Citation7 but also occurs in GreyhoundsCitation8 and healthy human athletesCitation9 as well as in patients with diseases such as left heart failure which elevate pulmonary vascular pressures.Citation5 Other human endeavors such as prolonged saxophone playing may also induce alveolar hemorrhage.Citation10 In horses, the 2015 American College of Veterinary Internal Medicine Consensus Statement made the strong recommendation that EIPH be considered as a disease rather than a “condition”.Citation7 The necessary conditions for EIPH in the healthy exercising horse consist of high pulmonary capillary transmural pressures, the product of a prodigious cardiac output, combined with very negative alveolar pressures that summate across the exquisitely thin intervening blood–gas barrier. EIPH occurs primarily in Thoroughbreds, Quarter Horses and Standardbreds and during sprint racing. EIPH is also apparent across other high-performance equine athletes, including cutting, reining, barrel, roping, polo, cross-country and 3-day event, show jumping, hunter-jumper, steeplechase, dressage and draft horses.Citation6,Citation7,Citation11 Indeed, even sustained submaximal exercise induces EIPH.Citation12 EIPH is a substantial problem for the horse racing industry for multiple reasons that include decreased performance,Citation6 lost training days, necessity for pre-race medication, banning of horses from racing, occasional death,Citation13,Citation14 and public perception.Citation1,Citation6,Citation15 Moreover, EIPH constitutes a critical basis for exercise intolerance and progressively damages a horse’s lungs in proportion to its lifetime starts and training history.Citation1,Citation16–Citation19 Horses with either no or mild bleeding have a 4-fold higher chance of winning a race as their counterparts with moderate or severe EIPH.Citation6,Citation15,Citation19
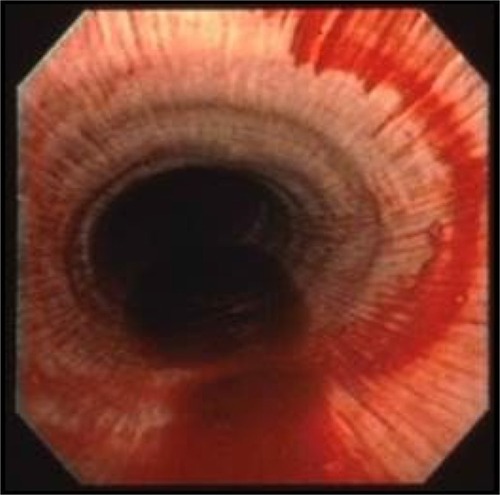
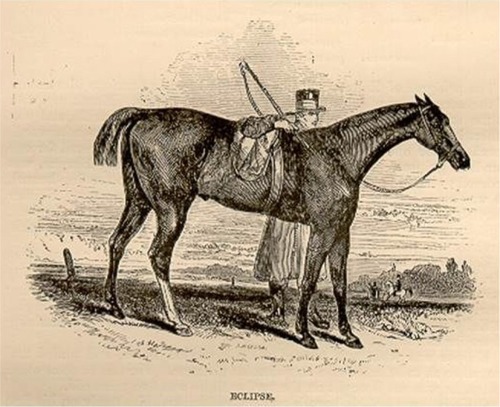
Epistaxis, bleeding from the nostrils, is the most visible form of EIPH (). This condition has been recognized at least since Elizabethan times. For instance, Gervase Markham’s “Masterpiece” described bleeding after exercise in 1688,Citation20–Citation22 and elite racehorses certainly have epistaxis in their ancestry. For instance, Bartlett’s Childers was renamed from Bleeding Childers (b. 1716), and was the great Grand-sire of the famous racehorse Eclipse (1764–1789), who never lost a race (). Eclipse was so dominant that on October 18, 1770, in the King’s Plate at Newmarket, his reputation so scared other owners that they withdrew their horses, and he simply walked the course unopposed! He went on to sire 334 winners. This case report demonstrates that horses with severe EIPH/epistaxis (Bleeding Childers not Eclipse) can perform at the highest levels, despite the epidemiological evidence that horses with the most severe EIPH do not perform as well as their less-affected counterparts.Citation6,Citation15,Citation23–Citation25 It is also true that there are genetic (as well as nongenetic) links to EIPH/epistaxis.Citation26 However, it is pertinent that such research is extremely challenging, and therefore, conclusions must be tempered with this reality in mind. There is also a rather controversial retrospective study reporting greater career longevity for horses with EIPH!Citation27
Figure 1 Epistaxis, bleeding from the nostrils, is the most visible form of exercise-induced pulmonary hemorrhage.

Figure 2 The acclaimed racehorse Eclipse, whose great Grandsire was Bleeding (Bartlett’s) Childers (1764–1789).
Brief history of discoveries key to our understanding of EIPH
Understanding the pathogenesis of EIPH has necessitated substantial progress in pulmonary and cardiovascular anatomy and physiology, and it is instructive to follow key elements of that progress. Whereas Galen (129 CE-c. 200/c.216) considered that most blood went through the lungs, he did hypothesize the existence of tiny pores through which blood passed from the right to the left ventricle.Citation5 William Harvey (1578–1657) subsequently placed his “invisible porosities” within the lungs themselves and surmised that they were actually small blood vessels.Citation28 However, Harvey and his contemporaries considered the lungs to comprise solid material as for the liver. It was only in the early 1660s, with the use of hand lenses and very early compound microscopes, that Marcello Malpighi (1628–1694) simultaneously discovered the pulmonary capillaries and alveoli in the lungs of the living frog ().Citation29,Citation30 Albert von Kölliker (1817–1905) in 1881 first described the Type I and II pneumocytesCitation5 that we now know constitute the “thin” portion of the blood–gas barrier for gas exchange and synthesize pulmonary surfactant essential for reducing surface tension and setting the extraordinary compliance of the lung, respectively. But it was only with the development of the electron microscope and its application to the pulmonary capillaries that Frank Low (1911–1998) visualized the exquisitely fine structure of the blood–gas barrier. His electron micrographs identified its tripartite structure consisting of the capillary endothelium, the alveolar epithelium and the intervening basement membrane with an in toto thickness of <0.5 μm in 2 seminal papers published in 1952 and 1953.Citation31,Citation32 Because gases diffuse far more slowly through fluids than air (>10−5) and exercise, especially in highly aerobic mammals such as the horse and dog, demands extremely high rates of oxygen and carbon dioxide transport across the blood–gas barrier, there has been strong selective pressure to ensure its thinness. It is worth remembering that the capacity for the lung to exchange oxygen and carbon dioxide is dependent upon the ratio of its functional surface area formed by Type I cells, some 2,800 m2 in the racehorse, to the thickness of the blood–gas barrier (~0.5 μm). To grasp the true enormity of this ratio, if we increased the blood–gas barrier thickness to 1 inch (ie, ×56,000) and did the same to the surface area, its 144 km2 would more than cover the world’s 5 smallest countries (San Marino, Tuvalu, Nauru, Monaco and Vatican City)!
Figure 3 Left: Marcello Malpighi, professor of medicine and extraordinary anatomist and physiologist (1628–1694). Right: Malpighi’s depiction of the pulmonary capillaries and alveoli. I: 2 lungs with the alveoli on the left and the capillaries on the right. II: pulmonary capillaries in the alveolus dissected open reveal the incredible density of capillaries that form the blood–gas barrier.

Despite recognition of the thinness of the blood–gas barrier and the awareness that blood erupted into the alveolar space in diseases such as mitral stenosis which are characterized by high pulmonary vascular pressures,Citation33,Citation34 the source of the blood in EIPH was curiously attributed to the mucosa of the terminal bronchioles (ie, larger systemic circulation vessels). This situation persisted for 4 decades, and West considers 3 reasons for this blind spot in understanding how the pulmonary capillaries can rupture:Citation5 1) Misunderstanding of Laplace’s equation. Specifically, the capillary wall stress (S) is calculated as S = (P×r)/t, where P is transmural pressure, r is radius and t is wall thickness. As the strength of the blood–gas barrier comes from the 0.05 μm (ie, 50 nm) Type IV collagen of the basement membrane, S becomes very high despite r being only 3–5 μm. 2) Lack of capillary rupture in other tissues. In stark contrast to the gas surrounding the pulmonary capillaries, their counterparts in muscle and other tissues are supported by the adjacent tissue matrix and also collagen strutsCitation35,Citation36 such that their transmural pressures remain very low. They are thus not subject to catastrophic failure. 3) Lack of appreciation of elevated pulmonary artery pressures during exercise. Because the human pulmonary artery mean pressure at rest is low in comparison to the systemic (ie, ~15 versus ~93 mmHg), and it was not considered to be much elevated during exercise, high capillary transmural pressures were not suspected in healthy individuals. However, in 1986, Wagner et al measured elevated mean pulmonary artery and wedge pressures (estimate of venous and capillary pressures) in healthy humans during heavy but submaximal cycle ergometer exercise.Citation37 Pulmonary artery pressure increased from 15 to 37 mmHg and wedge pressure from 14 to 21 mmHg demonstrating that pulmonary capillary pressures can become elevated during exercise.
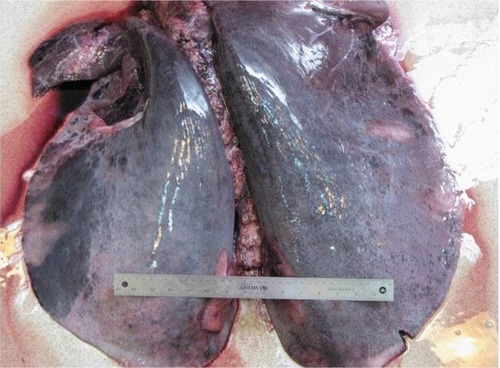
In 1981, endoscopy revealed that ~70% of horses post-race had significant blood in their airways,Citation38 and subsequently hemosiderin-laden macrophages were recovered in tracheal washings from Thoroughbred horses in training.Citation39 It is now recognized that the vast majority, if not all, performance horses experience EIPH (),Citation2,Citation6 but fatalities attributed to EIPH are uncommon ().
Figure 5 Lungs excised from a horse with a rare fatal instance of exercise-induced pulmonary hemorrhage.
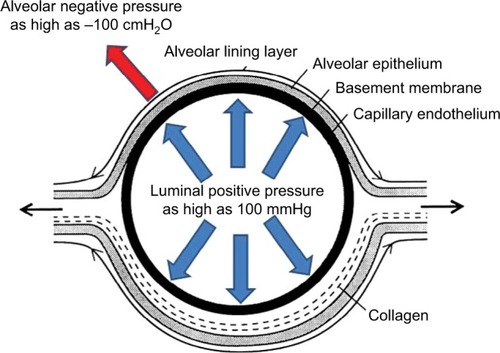
Thoroughbred horses have been bred for speed which has selected preferentially for a heart that can be up to 2% of their body mass compared with 0.5–1% for other mammalian species.Citation40 This has resulted in horses capable of generating cardiac outputs in excess of 400 L/min and achieving maximal oxygen uptakes well over 80 L/min – the highest measured to date in any species. To achieve these prodigious blood and oxygen fluxes, left ventricular filling pressures must be extraordinarily high and elevate pulmonary venous and capillary intraluminal pressures. In 1992, the first peer-reviewed paper was published demonstrating prodigiously high pulmonary artery pressures in horses during high-intensity treadmill running.Citation41–Citation44 These groundbreaking studies demonstrated that, simultaneously with mean systemic arterial pressures of 240 mmHg, mean pulmonary artery pressures reached ~120 mmHg, and directly measured left atrial pressure peaked at 70 mmHg. From such measurements, capillary intraluminal pressures in the region of 100 mmHg have been estimated ().
Figure 6 Schematic depicting cross-section of a pulmonary capillary.
The “other” side of the blood–gas barrier
Notwithstanding the extraordinary dimensions of racehorse lung surface area, the physical constraints of the chest wall have resulted in a structure that is underbuilt in comparison with the heart. In addition, the necessity for long extrathoracic airways (increasing dead space and airway(s) resistance) and the presence of collapsible nasal passageways provide additional impediments to pulmonary function.Citation40,Citation45,Citation46 For an elite horse achieving 90 L/min oxygen uptake during the gallop with a 3 L dead space and a respiratory frequency of 130 breaths per minute, controlling its arterial CO2 pressure at resting values of 40 mmHg would necessitate ~2,400 L/min total ventilation. Whereas humans typically hyperventilate driving arterial CO2 pressures down to 30 mmHg or so to help constrain the exercise-induced acidemia, the horse actually hypoventilates and allows its arterial CO2 to rise to ~60 mmHg.Citation42 Although this situation exacerbates the arterial hypoxemia – the primary cause being the very short red blood cell (RBC) transit times in the pulmonary capillaries – it lowers the ventilatory demands of the exercise to an achievable ~1,700 L/min. Even so, attaining the required peak flow rates approaching 120 L/min necessitates extremely low airway, and especially, alveolar negative pressures that may exceed –100 cmH2O. It is this negative pressure that sum-mates with the positive intraluminal pressure across the thin blood–gas barrier rupturing it and causing EIPH.
The most compelling evidence that the pulmonary capillaries constitute the unequivocal site of EIPH includes the following:
Only microspheres (10 and 15 μm diameter, differentially colored) injected into the jugular vein appear in the airways during a bout of EIPH. Those injected into the left atrium do not.Citation43
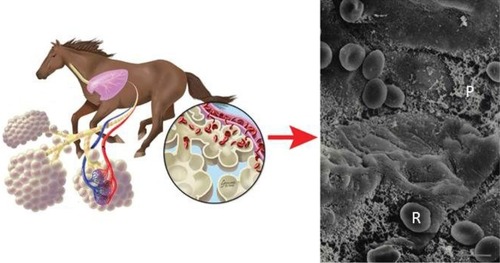
There is irrefutable electron micrographic evidence for catastrophic pulmonary capillary rupture and RBCs escaping into the alveolar space in equids following heavy-/severe-intensity running ().Citation5,Citation16,Citation43
Although there are exceptions, as discussed in the “EIPH causes and mechanisms” section, the majority of conditions that reduce the transmural pressure across the pulmonary capillary (eg, Lasix, nasal strip, correction of laryngeal hemiplegia) reduce EIPH, whereas elevation of capillary transmural pressures increases EIPH (eg, L-nitroarginine methyl ester [l-NAME] -induced pulmonary hypertension, inclined running, laryngeal hemiplegia/experimental increase of upper airway resistance).Citation2,Citation5
Figure 7 Left: Schematic showing exercise-induced pulmonary hemorrhage in the horse. Right: Scanning electron micrograph of the cranial lobe of an exercised pony reveals red blood cells (R) and proteinaceous material (P) in the alveoli. Bar =5 μm.
Consequently, the most effective approaches to reducing EIPH have focused on lowering either pulmonary capillary intraluminal pressures (furosemide/Lasix) or alveolar negative pressures (nasal strip, long-term management of airway health) as discussed in the “Prevention and treatment of EIPH” section.
Diagnosis and prevalence
Epistaxis
Visible epistaxis, as seen in , was the original method of diagnosing EIPH, and although considered to be a serious problem, its relatively rare occurrence (0.25–13% of all sprinting horses)Citation47–Citation49 mitigated concern. A comprehensive analysis of 250,000 race starts published in 2001 reported the incidence of overt epistaxis to be only 0.15%.Citation50 That investigation and subsequent work (some using endoscopy) noted that epistaxis/EIPH was most prevalent for the following:
shorter higher intensity races (greater pulmonary intravascular [positive] and extravascular [negative] pressures),
steeplechase compared with flat races (greater tidal volumes and respiratory/impact forces),Citation50–Citation52
older in preference to younger (~2-year olds) horses (more race starts),Citation53
males rather than females (latter may retire earlier to breed),Citation6 and
cool ambient temperatures.Citation53,Citation54
Bronchoalveolar lavage (BAL)
BAL when feasible is considered to provide the most sensitive determination of EIPH severity compared with tracheal lavage or endoscopy.Citation55–Citation59 Performed ideally 30 min post-race, the procedure consists of inserting either an endoscope or preferably a long, soft flexible tube into the naris which invariably wedges in a subsegmental bronchus subserving the dorsocaudal lobe.Citation45,Citation60 Then, 300 mL of phosphate-buffered saline (pH 7.4, 300 mosm) is infused in 50 mL aliquots, and after 2 breaths, aspirated. Fluid recovery of ≥55% is acceptable, and that fluid is then analyzed for the cell populations of interest. BAL facilitates an accurate analysis of the cytological population in the terminal airways and alveolar spaces. Investigations using BAL indicate that hemorrhage occurs in almost all horses in racing or training,Citation2,Citation60,Citation61 and BAL cytological findings cohere closely with clinical disease, pulmonary histopathology and endoscopic evidence of EIPH.Citation57–Citation60 Concerns regarding BAL most often include the need for rapid access to the horse post-race, the need for sedation (eg, 10–20 μg/kg intravenous detomidine), whether the entire lung volume affected by EIPH is sampled and the potential for heterogeneity of EIPH between lungs.Citation62 To date, many of the incisive investigations into the impact of furosemide, the nasal strip, immunomodulation and other therapeutic strategies on EIPH have successfully utilized BAL.Citation1,Citation3,Citation45,Citation55,Citation59–Citation61,Citation63,Citation65–Citation73 By comparison, endoscopy (vide infra) evaluation is extremely crude ()Citation75 and also depends on transduction of blood into the trachea which may be a time- and posture-dependent process.
Endoscopy
Use of a rigid endoscope enabled Cook in 1974 to recognize that the lungs, rather than extrathoracic airways or other putative sites, were the source of hemorrhage.Citation47 Endoscopy increased the detection of post-race EIPH to up to over 60% in Standardbreds,Citation76,Citation77 Quarter HorsesCitation78 and Thoroughbreds.Citation49,Citation79–Citation82 Repeated examination of individual horses raised the detected incidence of EIPH to ~95%.Citation78,Citation80,Citation81
Tracheal lavage
Whereas tracheal lavage can detect EIPH from the presence of hemosiderophages in the fluid aspirated, defining the time course of the hemorrhage is challenging. Moreover, the relationship between tracheal wash cytology and lung histopathology is weak.Citation39,Citation83,Citation84 It is also concerning that the cell population in the tracheal fluid is markedly different from that in the lower respiratory tract.Citation83,Citation84
Radiography
EIPH is only vaguely discernible by radiography as an increase in interstitial density in the dorsocaudal lobe of the lungCitation85–Citation88 and is therefore of limited value. Potentially in horses with severe, repeated EIPH episodes, radiography may be of use in documenting EIPH progression.
Echocardiography
Echocardiography may reveal a higher incidence of spontaneous contrast at rest in horses with a history of EIPH, but it is not quantitative.Citation89–Citation91
Nuclear scintigraphy
Small amounts of hemorrhage may potentially be detected with scintigraphy, but this is contingent upon the level of lung background radioactivityCitation92 and results have generally been disappointing.Citation2,Citation85
There is no doubt that scientific progress into understanding EIPH mechanisms and treatment is dependent on evaluating bleeding severity acutely, as with BAL. But it is also true that assessment of the long-term impact of successive EIPH episodes will inform putative therapeutic strategies including race–race interval, immunomodulation, pre-race interventions, training regimens and possibly diet and breeding programs. Thus, development of an effective technique to assess pulmonary venous remodelingCitation93,Citation94 and the mechanical sequelae to repeated EIPH episodes in vivo would be extremely valuable.
EIPH causes and mechanisms
A variety of causes and pathophysiologic mechanisms have been postulated for EIPH. The experimental evidence indicates that a given instance of EIPH may be caused or exacerbated by multiple factors acting in concert.Citation2 These can be grouped broadly into 3 primary categories that involve intravascular pressures (capillary, positive), extravascular pressures (alveolar, negative) and properties of the blood–gas barrier (strength/fragility) and lung parenchyma (reduced compliance/fibrosis).
High pulmonary capillary intravascular pressures
Pulmonary artery pressure is set by the product of cardiac output and pulmonary vascular resistance. As mentioned earlier, the immense heart size of the racehorse results in a huge stroke volume which, combined with maximal heart rates of ~240 beats per minute, results in extraordinary pulmonary blood flows. The pulmonary vascular resistance will in turn be set by the cross-sectional area of the resistance vessels and hemorheological properties (ie, apparent viscosity) of the blood at a hematocrit of ≥65%. Thus, the driving pressure in the pulmonary artery will increase to achieve the required flow irrespective of downstream venous pressures, and those pressures are elevated as the left ventricle becomes stiff (rate of relaxation is inadequate) requiring an elevated filling (left atrial) pressure. The pulmonary capillaries are thus caught between extremely high pulmonary artery pressures of ~120 mmHg and left atrial pressures that may rise to ≥70 mmHg such that mean pulmonary capillary pressures of 100 mmHg are feasible (). Two additional factors have been considered to also play a role in the elevated pulmonary vascular pressures: the resistance provided by the comparatively small cross-sectional area of the atrioventricular valves and regurgitation through these valves during ventricular systole when left ventricular pressures may be ≥240 mmHg.Citation40
Pulmonary blood flow in the equine lung is neither homogeneous nor distributed according to gravitational control.Citation95 Rather, arteriolar vasomotor control favors a preferential vasodilation toward the dorsal aspect of the lung such that blood flow increases from ventral–dorsal regions (ie, opposite to that expected from gravitational effects) and specifically is highest toward the dorsocaudal region that is most impacted by EIPH.Citation95–Citation99
Very high negative alveolar pressures
Generating peak inspiratory flow rates of ~120 L/s against the resistance provided by the horse’s long extrathoracic airways demands a steep pressure gradient and hence extremely negative alveolar pressures ().Citation45,Citation100,Citation101 Anything that reduces airway(s) cross-sectional area will either compromise airflow or require an elevated driving pressure to maintain the same flow. Because the bulk of the pulmonary resistance especially during inspiration is located in the large extrathoracic airways,Citation88,Citation101 upper airway obstruction, as caused by sucking in the soft tissue overlying the nasal passageways just rostral to the nasoincisive notch, profoundly increases airway resistance.Citation1,Citation70,Citation100,Citation102,Citation103 This phenomenon requires even more negative alveolar pressures and is expected to exacerbate capillary rupture. Laryngeal hemiplegia, often identified as a pronounced roaring on inhalation, is also a cause of elevated large airway resistance and hence more negative alveolar pressures.Citation104,Citation105 Because the small airways constitute relatively little to total airways resistance owing to their far greater total (combined) cross-sectional area, their potential to increase total inspiratory resistance is far less. However, given its high prevalence,Citation106 extensive small airways disease has certainly been considered as a potential contributing factor to EIPH, and even airway disease that is subclinical at rest may become important during maximal exercise. It is pertinent that there is a demonstrable association between lower airway disease and EIPHCitation107,Citation108 and also that experimentally induced inflammation, consequent to instillation of dilute acetic acid into the distal bronchi, increases EIPH severity.Citation109 Instillation of blood into the airways also causes a transient airway inflammation that resolves within 2 weeks,Citation110 but the fibrotic lesions characteristic of the lungs of horses with a prolonged history of EIPH may well require hemosiderin within the interstitial space.Citation111 That not all horses with EIPH have any evidence of lower airway inflammation supports that this condition may exacerbate EIPH, but it by no means is a primary cause.Citation6
Chronic lung damage
EIPH is associated with edema and blood in the alveolar space, small airways and interstitium. This produces airway irritation and inflammation, and with repeated strenuous exercise in either training or actual competition, the hemorrhage results in fibrosis/scarring, a weakened blood–gas barrier and sustained inflammation. The blood within the alveoli may adversely affect lung health and exercise capacity by interfering with gas exchange. Because chronic scarification changes the compliance properties of the lung and progresses as a “front” between 2 regions, diseased and healthy, there may be substantial shearing forces at the junction of these regions which increases capillary rupture and EIPH particularly upon forceful inhalation. There will also be the progression of veno-occlusive disease with repeated bouts of EIPH that further elevates pulmonary venous and capillary pressures. Specifically, repeated instances of hemosiderin accumulation, and possibly their inflammatory consequences, induce veno-occlusive remodeling of small-diameter (100–200 μm) pulmonary veins primarily in the dorsocaudal region and fibrosis of the alveolar interstitium, bronchovascular bundle, septa and pleura.Citation19,Citation93,Citation94,Citation97–Citation99,Citation112 Both of these factors help explain how chronic EIPH worsens with repeated exercise and consequently increased age. An important question is how much exercise and EIPH-induced damage is required to induce these changes. Certainly, it appears to be >2 weeks of intensive exercise training at least for the expected alterations in mRNA and protein expression which may be necessary precursors.Citation6,Citation94
Mechanical stresses of respiration and locomotion
Lung tissue is damaged by thoracic impact, and Schroter et al have suggested that the locomotory impact of the forelimbs produces a localized impulsive load on the chest wall sending pressure waves through the lung parenchyma.Citation52,Citation53,Citation113 These waves increase in magnitude, and therefore destructive potential, as the mass of affected tissue decreases toward the dorsocaudal lobe – the primary site of capillary rupture. For a 500 kg horse at the gallop, it is estimated that impulsive forces in excess of 100 kPa of 10 ms duration would be applied to the horses’ chest by each scapula which is expected to produce edema and hemorrhage. This elegant theory helps explain why horses that jump (steeplechase, eventing) generally experience greater EIPH than flat racers.Citation2
Prevention and treatment of EIPH
Given the complex etiology of EIPH, it is not surprising that this condition has not been eradicated or that a unified treatment strategy has not been identified. Therapeutic countermeasures at best reduce but do not prevent EIPH and are aimed at either constraining the severity of EIPH or reducing the pernicious sequelae including inflammation and infection as well as possibly fibrosis and venous remodeling. Moreover, the issue is complicated by fact that the weight loss induced by the diuretic furosemide is a substantial performance enhancer as absolute is unaltered and the ~20 kg weight loss therefore increases mass-specific
.Citation114–Citation117 The failure of investigations to quantify EIPH by BAL has also led to confusion, and in some instances, misdirection (eg, using endoscopy).Citation75 Given the pressing and continued demand for effective prophylaxis and/or treatment to reduce EIPH, an extensive variety of pharmacotherapeutic and management interventions have been developed. However, under scientific scrutiny, few of these have demonstrated efficacy in treating EIPH.Citation1–Citation3,Citation6,Citation118–Citation120 Treatments proposed for EIPH are generally directed at or more than 1 of the putative causal mechanisms for EIPH considered earlier and includeCitation2,Citation6 furosemide and other diuretics, dehydration, antihypertensive agents or pulmonary vasodilators that dilate the pulmonary vasculature (eg, nitric oxide [NO] inhalation, endothelin inhibition), drugs to decrease blood viscosity (eg, pentoxifylline), inhibition of platelet aggregation, transhexamic acid to inhibit fibrinolysis, nasal dilator strips to reduce the resistance and maintain full patency of the nasal passages, bronchodilators, surgical correction of laryngeal hemiplegia to decrease upper airway resistance, anti-inflammatory drugs/strategies to reduce lower airway inflammation, hesperidin-citrus bioflavinoids to reduce capillary fragility, aminocaproic acid and herbal remedies.Citation2,Citation6,Citation68,Citation118–Citation120 Given that EIPH risk does not relate to any discernible combination of horse’s age, race distance/speed, air quality or track hardness,Citation53 the following discourse focuses on interventions and treatments mentioned that either offer significant potential to treat EIPH and/or for which novel information is available.
Furosemide (Lasix, Salix, Frusemide)
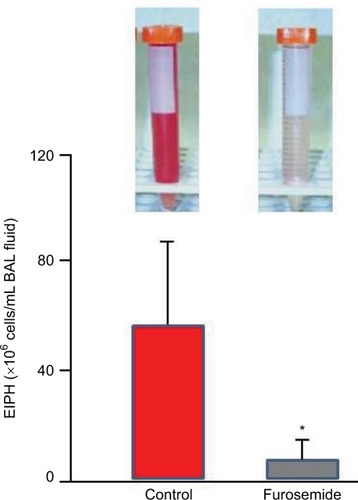
Where permitted in North America, South America, Saudi Arabia and the Philippines,Citation2,Citation119 furosemide is the most commonly used race-day EIPH treatment.Citation121–Citation127 Typically administered 4 hours prior to racing, furosemide induces a vigorous diuresis and reduces EIPH (as assessed by BAL; )Citation68,Citation69,Citation127,Citation128 by constraining the exercise-induced elevation of right atrial, pulmonary arterial, pulmonary wedge and pulmonary capillary pressures.Citation42,Citation68,Citation69,Citation129–Citation132 There may also be a redistribution of pulmonary blood flow such that flow decreases to the “at-risk” dorsal lung regions.Citation133 Specifically, furosemide administered intravenously decreased EIPH by 80–90% in Thoroughbred horses run to ~95% (1 mg/kg furosemide) and 120% (0.5 mg/kg furosemide) of their V˙O2max on the high-speed treadmill.Citation68,Citation69 Moreover, a systematic review and meta-analysis of 11 studies documented furosemide’s efficacy for reducing EIPH in over 5,000 Thoroughbred and Standardbred racehorses.Citation24 There has been much consideration of furosemide’s vasodilatory effect on the smooth muscle of the pulmonary vasculature and bronchi. However, its pharmacokinetics is such that mean plasma levels will be in the very low ng/mL range 4 hours after administration of the regulation dose,Citation134 and as such, these direct effects are highly unlikely.
Figure 8 The diuretic furosemide significantly (*) reduced exercise-induced pulmonary hemorrhage during high-intensity exercise as measured by bronchoalveolar lavage.
Abbreviations: EIPH, exercise-induced pulmonary hemorrhage; BAL, bronchoalveolar lavage.
Nasal dilators
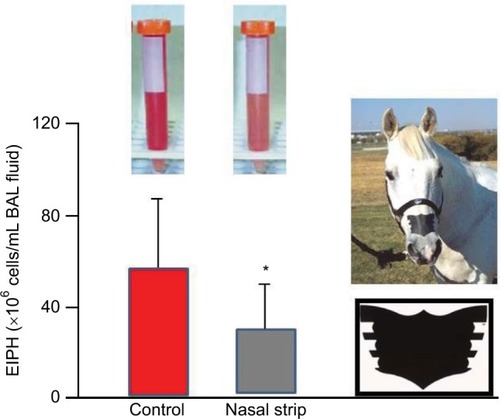
Although external nasal dilator strips are of questionable efficacy for humans during exercise, at least where oral breathing is unobstructed, during exercise, horses are obligate nasal breathers. Accordingly, nasal resistance, and changes thereof, is of substantially greater importance to inspiratory mechanics and airflow in horses than humans. During forceful inhalations when galloping, partial collapse of the unsupported nasal passages occurs, and this increases airways resistance. Use of the FLAIR™ nasal strip to stabilize the nasal wall and prevent the partial airway collapse reduces intrapleural and alveolar pressure swings that contribute to capillary transmural pressures,Citation100,Citation103,Citation135 thereby reducing EIPH by up to 50% as assessed by BAL ().Citation45,Citation68,Citation69,Citation71 Moreover, those studies demonstrated that EIPH was reduced the most in those horses that were the heaviest bleeders on the control (no nasal strip) runs. Interestingly, during submaximal exercise, the nasal strip decreased , consistent with the horse performing less respiratory muscle work. As humans can redistribute their cardiac output among locomotory and respiratory muscles during maximal exercise,Citation136 this raises the intriguing possibility that the nasal strip may enhance exercise performance if the blood flow (and O2) “spared” by the respiratory muscles was redistributed to the locomotory muscles. McDonough et alCitation71 demonstrated that, in the presence of a substantial EIPH reduction, performance was enhanced by wearing the nasal strip.
Figure 9 The nasal strip pictured at right significantly (*P<0.05) reduced exercise-induced pulmonary hemorrhage during high-intensity exercise as measured by bronchoalveolar lavage.
Abbreviations: EIPH, exercise-induced pulmonary hemorrhage; BAL, bronchoalveolar lavage.
Support for nasal strips benefitting racehorses on the track has emerged from studies of 400 horses at the Calder Race Course in Florida across the 1999–2000 Thoroughbred season (Scollay M and Hernandez J, unpublished findings, 2000). Horses wearing the nasal strip achieved a 3.4% higher win percentage than their non-strip-wearing counterparts. Moreover, the nasal strip reduced the between-race interval from 29 to 23 days (p<0.05). Subsequently, 30 Thoroughbred racehorses at the Golden Gate Fields Racetrack in California, who were identified as having a history of severe EIPH, achieved a 65% decrease in BAL-assessed EIPH.Citation72 These studies emphasize the importance of using BAL rather than endoscopy to quantify BAL.Citation75
The FLAIR nasal strip has been broadly approved for use in flat- and harness-racing within North America; most notably, it was approved for California Chrome, the Triple Crown contender, at Belmont Park in 2014. The FLAIR strip has also been approved for use by all key non-racing regulatory bodies, including the Federation Equestrian International, the American Horse Shows Association, the National Reining Association, the United States Equestrian Team, the United States Polo Association, the National Barrel Horse Association, and the American Quarter Horse Association. In the international market, the FLAIR strip has been approved for racing in the following countries: Mexico, Brazil, Trinidad, Barbados, Jamaica, the Netherlands, United Arab Emirates, Qatar, India, Australia (Harness), New Zealand, Korea and Singapore.
Whereas insufficient investigations have been performed to date on the nasal strip to conduct a meta-analysis, as for furosemide,Citation24 the reduction in EIPH measured by BAL is substantial and robust enough to demonstrate statistical significance even with relatively small numbers of horses in each instance.Citation45,Citation68,Citation69,Citation71,Citation72
Nitric oxide
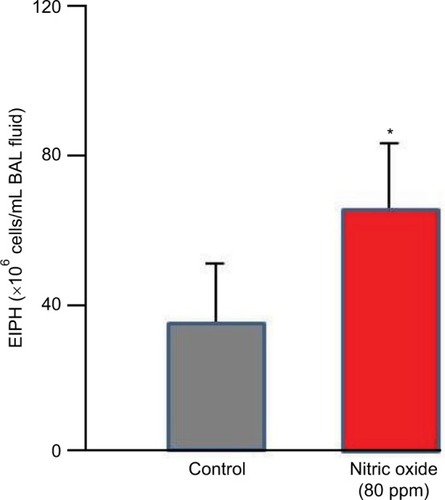
NO is a vascular smooth muscle-relaxing factor that is produced endogenously via the action of NO synthase (NOS) on l-arginine within vascular endothelial cells, muscles (preferentially Type II, fast twitch) and nervous tissues.Citation137,Citation138 In addition, dietary nitrate can be reduced to nitrite by facultative bacteria in the mouth; upon resorption, circulating nitrate is further reduced, especially by deoxygenated hemoglobin to NO.Citation139 Inhibition of NOS using the l-arginine analog L-NAME increases pulmonary artery pressure and EIPH in maximally exercising horsesCitation140–Citation142 suggesting a key role for NO in mediating endothelial function during exercise. Whereas nitroglycerin forms NO and is a potent vasodilator, neither intravenous nor oral dosing reduces exercise-induced hypertension in the exercising horse.Citation143,Citation144 The same is true for the phosphodiesterase inhibitor sildenafil.Citation145 In contrast, whereas inhalation of 80 ppm NO reduces pulmonary artery pressure in horses during heavy-/severe-intensity exercise, it simultaneously increases EIPH as assessed by BAL ().Citation146–Citation148 This dissociation of pulmonary artery pressure and EIPH by NO inhalation has revealed that pulmonary hypertension may, in part, be the consequence of downstream arteriolar vasoconstriction that actually protects the fragile dorsocaudal capillaries from high blood flows and associated pressures. Although NO lowers pulmonary artery pressure, it prevents this protective effect, and EIPH is actually worsened.Citation148 This conclusion, regarding the effect of high pulmonary artery pressures being protective in certain instances, is also supported by the elevated EIPH seen concomitant with reduced pulmonary artery pressures during inclined running.Citation66
Figure 10 Inhaled nitric oxide (80 ppm) lowered pulmonary vascular pressures substantially but significantly increased exercise-induced pulmonary hemorrhage as measured by bronchoalveolar lavage.
Abbreviations: EIPH, exercise-induced pulmonary hemorrhage; BAL, bronchoalveolar lavage.
Concentrated equine serum
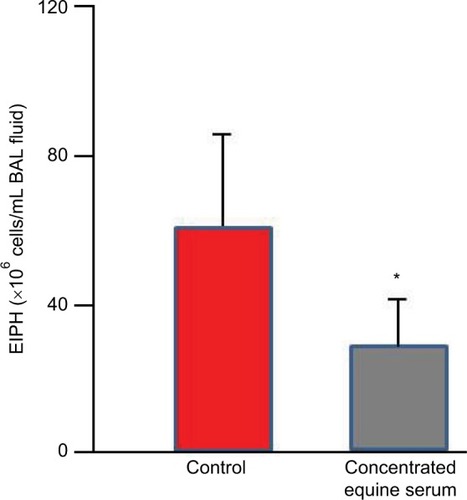
Because EIPH may be potentiated by inflammatory airway diseaseCitation60,Citation107,Citation149 and EIPH severity is associated with increased IL-6 expression,Citation150 strategies to reduce post-EIPH parenchymal tissue damage and subsequent scarring may be valuable. The concentrated equine serum (CES) Seramune® contains high levels of equine IgG and other immunoglobulins collected from draft horse donors. Following field studies that supported its potential therapeutic benefit to treat chronic EIPH sufferers and safety assessment, a dosage of 20 mL intratracheally and 10 mL intravenously was administered for 5 consecutive days with a booster dose weekly and 24–48 hours before heavy treadmill running. This treatment regimen was completed at different racetracks over a 5-year period and demonstrated a reduction in EIPH and mucus. This CES treatment reduced EIPH by 53% and white blood cells by 32% as assessed by BAL ().Citation120,Citation151,Citation152
Figure 11 Concentrated equine serum decreased exercise-induced pulmonary hemorrhage during high-intensity exercise as measured by bronchoalveolar lavage.
Abbreviations: EIPH, exercise-induced pulmonary hemorrhage; BAL, bronchoalveolar lavage.
Omega-3 fatty acids
Equine supplements often contain omega-3 fatty acids, and their capacity to impede the arachadonic acid cascade and thus airway inflammation has been hypothesized to decrease EIPH. Moreover, diets rich in docasahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) may also constrain the reduction in erythrocyte membrane fluidity during exercise and improve pulmonary hemodynamics reducing EIPH.Citation153 This hypothesis was supported at Kansas State University where 10 Thoroughbred horses fed a DHA- and EPA-enriched diet for 83 and 145 days evidenced a decreased EIPH.Citation67
Herbal formulations
A variety of herbal formulations that claim to “clear heat and edema from the lung, cool and nourish the blood, and move stagnated blood out of the airways”Citation154,Citation155 have been used to treat EIPH in horses. Some herbal formulations have also been designed to treat impaired coagulation and modify platelet function, both of which have been considered to contribute to EIPH. Within our laboratory, we find no evidence that Yunnan Paiyao and Single Immortal have efficacy for reducing EIPH in Thoroughbred racehorses.Citation73
Conjugated estrogens and antifibrinolytics
Despite the absence of scientific evidence for increased fibrinolysis or impaired coagulation in horses with EIPH,Citation156–Citation158 use of conjugated estrogens and antifibrinolytics is widespread in the racing industry. Aminocaproic acid at 30 and 100 mg/kg (intravenous) doses in resting horses has an antifibrinolytic effect.Citation159 Whereas both of these treatments, conjugated estrogens and aminocaproic acid, tended to decrease EIPH after severe-intensity treadmill, there was evidence for compromised running performance after aminocaproic acid.Citation67,Citation160,Citation161 The potential of conjugated estrogens to decrease EIPH in some horses along with enhancing performance indicates the need for broader mechanistic investigation.
Conclusion
EIPH occurs in essentially all performance horses evaluated by BAL where, although rarely fatal, greater severity of bleeding has been associated with poor performanceCitation6 and may curtail racing longevity. EIPH becomes worse with advancing age most likely because older horses have more racing history and lifetime starts and therefore more structural and functional (mal)adaptations of the lungs. EIPH results from catastrophic mechanically induced rupture of the exquisitely thin blood–gas barrier that interposes between the pulmonary capillary blood and alveolar gas with the dorsocaudal regions of the lung most heavily impacted (). Whereas the mechanistic bases for EIPH are complex, they summate as the effective capillary transmural pressure and the strength or integrity of the intervening blood–gas barrier (). As such, anything that increases the positive capillary intravascular pressures or the negative alveolar pressures will exacerbate EIPH as will scarification or weakness of the barrier itself. Because the performance horse must achieve magnificently high cardiac outputs during maximal exercise, intravascular pressures reach levels normally associated with the systemic circulation (ie, 100 mmHg).Citation45,Citation46 Just 0.5 μm away, the alveolar pressure must be extremely negative to draw air into the lungs at peak flow rates of ≥120 L/s against an airways resistance that is accentuated by long extrathoracic airways, partial collapse of the nasal passages and possibly inflammatory compromise of small airway patency. In horses, pulmonary capillary (and presumably capillary transmural) pressures >~90 mmHg in vitroCitation48 and in vivoCitation50 rupture the blood–gas barrier which heals rapidly after the pulmonary capillary hypertension is relieved.Citation162 However, parenchymal damage and associated inflammatory processes combined with RBCs remaining in the lung interstitium drive structural and mechanical changes that not only compromise barrier strength and integrity but also elevate pulmonary vascular pressures during subsequent exercise bouts (ie, veno-occlusive disease).Citation19,Citation93,Citation94,Citation97–Citation99,Citation112
Recently, it has become evident that high pulmonary artery pressures may reflect a selective vasoconstriction within the pulmonary arterioles that actually serve to protect capillaries within the dorsocaudal lung regions that would otherwise rupture. Thus, conditions such as inclined running and 80 ppm NO inhalation that lowers pulmonary artery pressures exacerbate EIPH severity.Citation2,Citation66,Citation148 In this regard, it would be extremely valuable to define the role played by different segments of the pulmonary vasculature in EIPH, especially as this relates to their structure and vasomotor control, and potentially, changes thereof with repeated EIPH episodes within and across racing seasons. In this regard, the studies of Stack et al particularly on the pulmonary venules and veins may prove to be extremely insightful.Citation93,Citation94,Citation96–Citation99
In terms of therapeutic countermeasures that have scientifically proven efficacy in reducing but not abolishing EIPH, furosemide is by far the most widely used (). Given the likelihood that furosemide will be banned in North America as a race-day drug in the near future, there is an urgent need for an effective alternative. At present, perhaps, the best candidate for race-day alleviation of EIPH is the nasal strip (), use of which is gaining in popularity. Resistance to use of the nasal strip as an alternative to furosemide in the racing industry has likely resulted from the overarching fact that, without the ~20 kg weight loss from furosemide-induced diuresis, nasal strips (or other countermeasures) do not increase performance sufficiently, or at least not to the extent that furosemide does. At present, there is a pressing need for large-scale field trials of the nasal strip ideally using BAL to measure reductions in EIPH severity. In addition, given the hopeful (albeit small scale) results of immunomodulation therapy and conjugated estrogens these putative therapeutic avenues are deserving of closer scientific scrutiny (). Resolution of therapeutic efficacy is, to a great extent, contingent on an accurate and sensitive method to quantify EIPH. At present, the more invasive but sensitive BAL is far better than endoscopy but more challenging to build into field studies. However, in the absence of a better method, effective progress in EIPH reduction/prevention may be contingent on wider use of BAL in the field. For EIPH mitigation, strategies incorporating longer race–race intervals, investigating dehydration strategies (to mimic the plasma volume/weight loss and lower pulmonary vascular pressures of furosemide), and in the longer term, selective breeding programs based upon deselecting for EIPHCitation26 are worthy of serious consideration and investigation.
Disclosure
The authors report no conflicts of interest in this work.
References
- EricksonHHA review of exercise-induced pulmonary hemorrhage and new concepts for preventionProc Ann Conv Am Assoc Equine Pract200046193196
- EricksonHHPooleDCExercise-induced pulmonary hemorrhage: current conceptsLekeuxPEquine Respiratory DiseasesIthaca, NYInternational Veterinary Information Services2007
- PooleDCEppTSEricksonHHScience Overview. Exercise-induced pulmonary haemorrhage (EIPH): mechanistic bases and therapeutic interventionsEquine Vet J200739429229317722718
- MoranGFolchHExercise-induced pulmonary hemorrhage in horses – reviewActa Vet Brno2013823309316
- WestJBFragility of pulmonary capillariesJ Appl Physiol2013115111523640584
- SullivanSHinchcliffKUpdate on exercise-induced pulmonary hemorrhageVet Clin North Am Equine Pract201531118719825770069
- HinchcliffKWCouetilLLKnightPKExercise induced pulmonary hemorrhage in horses: American College of Veterinary Internal Medicine consensus statementJ Vet Intern Med201529374375825996660
- EppTSSzladovitsBBuchannanAThe presence and severity of exercise-induced pulmonary hemorrhage in racing GreyhoundsComp Exerc Physiol200852132
- HopkinsSRSchoeneRBHendersonWRSpraggRGMartinTRWestJBIntense exercise impairs the integrity of the pulmonary blood-gas barrier in elite athletesAm J Respir Crit Care Med19971553109010949116992
- KoYCDaiMPOuCCPlaying saxophone induced diffuse alveolar hemorrhage: a case reportIr J Med Sci2010179113713919565318
- LéguilletteRSteinmannMBondSLStantonBTracheobron-choscopic assessment of exercise-induced pulmonary hemorrhage and airway inflammation in barrel racing horsesJ Vet Intern Med20163041327133227278854
- EppTSMcDonoughPPadillaDJExercise-induced pulmonary haemorrhage during submaximal exerciseEquine Vet J Suppl20063650250717402474
- LyleCHUzalFAMcGorumBCSudden death in racing Thoroughbred horses: an international multicentre study of post mortem findingsEquine Vet J201143332433121492210
- GunsonDESweeneyCRSomaLRSudden death attributable to exercise-induced pulmonary hemorrhage in racehorses: nine cases (1981–1983)J Am Vet Med Assoc198819311021063417520
- HinchcliffKWJacksonMAMorleyPSAssociation between exercise-induced pulmonary hemorrhage and performance in Thoroughbred racehorsesJ Am Vet Med Assoc2005227576877416178399
- EricksonHHMcAvoyJLWestfallJAExercise-induced changes in the lung of Shetland ponies: ultrastructure and morphometryJ Submicrosc Cytol Pathol199729165729066143
- PascoeJRExercise-induced pulmonary hemorrhage: a unifying conceptProc Annu Conv Am Assoc Equine Pract199642220226
- RobertsCAEricksonHHExercise-induced pulmonary haemorrhage workshopEquine Vet J Suppl19993064264410659338
- RobinsonNEWilliamsKJStackAJacksonWFDerksenFJExercise-induced pulmonary haemorrhage: a progressive disease affecting performance?Equine Vet J201547333934025712624
- MarkhamGMarkham’s Masterpiece Revived: Containing all Knowledge Belonging to the SmithLondonJohn Richardson for Tho. Passinger1688
- YouattWThe HorseLondonLongmans, Green, and Co.1874
- RobertsonJBBiological searchlight on racehorse breeding: VI. The heredity of blood-vessel breaking in the ThoroughbredBloodstock Breeders Rev19132265281
- SullivanSLAndersonGAMorleyPSHinchcliffKWProspective study of the association between exercise induced pulmonary hemorrhage and long term performance in Thoroughbred racehorsesEquine Vet J201547335035724617564
- SullivanSLWhittemTMorleyPSHinchcliffKWA systematic review and meta-analysis of the efficacy of furosemide for exercise-induced pulmonary haemorrhage in Thoroughbred and Standardbred racehorsesEquine Vet J201547334134925291214
- MorleyPSBromberekJLSaulezMNHinchcliffKWGuthrieAJExercise-induced pulmonary haemorrhage impairs racing performance in Thoroughbred racehorsesEquine Vet J201547335836525290262
- VelieBDRaadsmaHWWadeCMKnightPAHamiltonNAHeritability of epistaxis in the Australian Thoroughbred racehorse populationVet J2014202227427825011713
- PrestonSARiggsCMSingletonMDTroedssonMHDescriptive analysis of longitudinal endoscopy for exercise-induced pulmonary haemorrhage in Thoroughbred racehorses training and racing at the Hong Kong Jockey ClubEquine Vet J201547336637125055713
- HarveyWExercitatio Anatomica de Motu Cordis et Sanguinis in Anamalibus [An Anatomical Disquisition on the Motion of the Heart and Blood in Animals]FrankfurtGuilielmi Fitzeri1628 Latin
- MalpighiMDe Pulmonibus [Lungs]LondonPhilosophical Transactions of the Royal Society1661 Latin
- WestJBMarcello Malpighi and the discovery of the pulmonary capillaries and alveoliAm J Physiol Lung Cell Mol Physiol20133046L383L39023377345
- LowFNElectron microscopy of the rat lungAnat Rec1952113443744414943972
- LowFNThe pulmonary alveolar epithelium of laboratory mammals and manAnat Rec1953117224126313104990
- WoodPAn appreciation of mitral stenosis. I. Clinical featuresBr Med J1954148701051106313149899
- WoodPAn appreciation of mitral stenosis. II. Investigations and resultsBr Med J1954148711113112413149911
- CaulfieldJBBorgTKThe collagen network of the heartLab Invest1979403364372423529
- BorgTKCaulfieldJBMorphology of connective tissue in skeletal muscleTissue Cell19801211972077361300
- WagnerPDGaleGEMoonRETorre-BuenoJRStolpBWSaltzmanHAPulmonary gas exchange in humans exercising at sea level and simulated altitudeJ Appl Physiol19866112602703090012
- PascoeJRFerraroGLCannonJHArthurRMWheatJDExercise-induced pulmonary hemorrhage in racing Thoroughbreds: a preliminary studyAm J Vet Res19814257037077258791
- WhitwellKEGreetTRCCollection and evaluation of tracheobronchial washes in the horseEquine Vet J19841664995086519041
- PooleDCEricksonHHHighly athletic terrestrial mammals: horses and dogsCompr Physiol20111113723737162
- EricksonBKEricksonHHCoffmanJRPulmonary artery, aortic and oesophageal pressure changes during high-intensity treadmill exercise in the horse: a possible relation to exercise induced pulmonary haemorrhageEquine Vet J Suppl199094752
- EricksonBKEricksonHHCoffmanJRPulmonary artery, aortic and oesophageal pressure changes during high-intensity treadmill exercise in the horse; effect of frusemide and phentolamineEquine Vet J19922432152191606935
- JonesJHSmithBLTylerWSWhy do horses bleed? Source and cause of exercise-induced pulmonary hemorrhage in horsesClarkAEProceedings of the International Exercise Induced Pulmonary Hemorrhage ConferenceGuelphEquine Research Center19932324
- WestJBMathieu-CostelloOJonesJHStress failure of pulmonary capillaries in racehorses with exercise-induced pulmonary hemorrhageJ Appl Physiol1993753109711098226517
- PooleDCKindigCAFentonGFergusonLRushBREricksonHHEffects of external nasal support on pulmonary gas exchange and EIPH in the horseJ Equine Vet Sci2000209578585
- DucharmeNGHackettRPGleedRDAinsworthDMErbHNMitchellLMSoderholmLVPulmonary capillary pressure in horses undergoing alteration of pleural pressure by imposition of various upper airway resistive loadsEquine Vet J Suppl199930273310659217
- CookWREpistaxis in the racehorseEquine Vet J19746245584833210
- CookWRWilliamsRMKirker-HeadCAVerbridgeDJUpper airway obstruction (partial asphyxia) as the possible cause of exercise-induced pulmonary hemorrhage in the horse: an hypothesisEquine Vet Sci1988811126
- PascoeJRRaphelCFPulmonary hemorrhage in exercising horsesComp Cont Educ Pract Vet Suppl19824S411S416
- TakahashiTHiragaAOhmuraHKaiMJonesJHFrequency of and risk factors for epistaxis associated with exercise-induced pulmonary hemorrhage in horses: 251 609 race starts (1992-1997)J Am Vet Med Assoc200121891462146411345311
- SchroterRCMarlinDJDennyEExercise-induced pulmonary haemorrhage (EIPH) in horses results from locomotory impact induced trauma - a novel, unifying conceptEquine Vet J19983031861929622318
- SchroterRCLeemingADennyEBharathAMarlinDJModelling impact-initiated wave transmission through lung parenchyma in relation to the aetiology of exercise-induced pulmonary haemorrhageEquine Vet J Suppl1999303438
- HinchcliffKWMorleyPSJacksonMARisk factors for exercise-induced pulmonary haemorrhage in Thoroughbred racehorsesEquine Vet J Suppl20103822823421059011
- CrispeEJLesterGDRobertsonIDSecombeCJBar shoes and ambient temperature are risk factors for exercise-induced pulmonary haemorrhage in Thoroughbred racehorsesEquine Vet J201648443844125962341
- FogartyUEvaluation of a bronchoalveolar lavage techniqueEquine Vet J19902231741762361503
- MooreBRCoxJHDiagnostic use of bronchoalveolar lavage in horsesEquine Pract1996185715
- RobinsonNEDerksenFJSlocombeRFBronchoalveolar lavageHuntingdonPProceedings of the AEVA (Bain-Fallon Memorial Lectures)Proceedings of the American Association of Equine PractitionersAEEP, Lexington, KY1986612
- SweeneyCRRossierYZiemerELEffect of lung site and fluid volume on results of bronchoalveolar lavage fluid analysis in horsesAm J Vet Res1992538137613791510313
- FogartyUA bronchoalveolar lavage technique for routine diagnostic purposesEquine Vet Educ199022102104
- McKaneSACanfieldPJRoseRJEquine bronchoalveolar lavage cytology: survey of Thoroughbred racehorses in trainingAust Vet J199370114014048280021
- MeyerTSFeddeMRGaughanEMLangsetmoIEricksonHHQuantification of exercise-induced pulmonary haemorrhage with bronchoalveolar lavageEquine Vet J19983042842889705109
- DepeckerMRichardEAPitelPHFortierGLeleuCCouroucé-MalblancABronchoalveolar lavage fluid in Standardbred racehorses: influence of unilateral/bilateral profiles and cut-off values on lower airway disease diagnosisVet J2014199115015624225534
- FogartyUBuckleyTBronchoalveolar lavage findings in horses with exercise intoleranceEquine Vet J19912364344371778160
- LangsetmoIFeddeMRMeyerTSEricksonHHRelationship of pulmonary arterial pressure to pulmonary haemorrhage in exercising horsesEquine Vet J200032537938411037258
- KindigCARamselCMcDonoughPPooleDCEricksonHHInclined running increases pulmonary haemorrhage in the Thoroughbred horseEquine Vet J200335658158514515958
- EricksonHHEppTSPooleDCA review of alternative therapies for EIPHProc Am Assoc Equine Pract2007536871
- KindigCAMcDonoughPFentonGPooleDCEricksonHHEfficacy of nasal strip and furosemide in mitigating exercise-induced pulmonary hemorrhage in Thoroughbred horsesJ Appl Physiol20019131396140011509541
- GeorRJOmmundsonLFentonGPaganJDEffects of an external nasal strip and frusemide on pulmonary haemorrhage in Thoroughbreds following high-intensity exerciseEquine Vet J200133657758411720029
- EricksonHHKindigCAPooleDCExercise-induced pulmonary hemorrhage: a new concept for preventionJ Equine Vet Sci2000203164167
- McDonoughPKindigCAHildrethTSEffect of furosemide and the equine nasal strip on exercise-induced pulmonary hemorrhage and time-to-fatigue in maximally exercising horsesEquine Comp Exerc Physiol200413177184
- ValdezSCNietoJESpierSJOwensSDBeldomenicoPSnyderJREffect of an external nasal strip on cytological characteristics of bronchoalveolar lavage fluid in Thoroughbred racehorsesJ Am Vet Med Assoc2004224455856114989550
- EppTSMcDonoughPPadillaDJCoxJHPooleDCEricksonHHThe effect of herbal supplementation on the severity of exercise-induced pulmonary haemorrhageEquine Comp Exer Physiol2005211725
- GoetzTEManoharMHassanASBakerGJNasal strips do not affect pulmonary gas exchange, anaerobic metabolism, or EIPH in exercising ThoroughbredsJ Appl Physiol20019062378238511356805
- MacNamaraBBauerSIafeJEndoscopic evaluation of exercise-induced pulmonary hemorrhage and chronic obstructive pulmonary disease in association with poor performance in racing StandardbredsJ Am Vet Med Assoc199019634434452298674
- LapointeJMVrinsAMcCarvillEA survey of exercise-induced pulmonary haemorrhage in Quebec Standardbred racehorsesEquine Vet J19942664824857889923
- HillidgeCJLaneTJJohnsonELPreliminary investigations of exercise-induced pulmonary hemorrhage in racing quarter horsesJ Equine Vet Sci1984412123
- RaphelCFSomaLRExercise-induced pulmonary hemorrhage in Thoroughbreds after racing and breezingAm J Vet Res1982437112311277103190
- MasonDKCollinsEAWatkinsKLRespiratory systemSnowDHPerssonSGBRoseRJEquine Exercise PhysiologyCambridgeGranta Editions19835763
- BurrellMHEndoscopic and virologic observations on respiratory disease in a group of young Thoroughbred horses in trainingEquine Vet J1985172991032985380
- VoynickBTSweeneyCRExercise-induced pulmonary hemorrhage in polo and racing horsesJ Am Vet Med Assoc198618833013023949607
- MairTSStokesCRBourneFJCellular content of secretions obtained by lavage from different levels of the equine respiratory tractEquine Vet J19871954584623678190
- DerksenFJBrownCMSoneaIDarienBJRobinson NE Comparison of transtracheal aspirate and bronchoalveolar lavage cytology in 50 horses with chronic lung diseaseEquine Vet J198921123262920696
- MarlinDJExercise-induced pulmonary haemorrhage: a global review of recent findingsProceedings of the World Equine Airways Symposium and Veterinary and Comparative Respiratory Society, Edinburgh, Scotland, 19–23 July 2001CanadaWorld Equine Airways Society2001
- O’CallaghanMWGouldenBERadiographic changes in the lungs of horses with exercise-induced epistaxisN Z Vet J198230811711816030891
- O’CallaghanMWPascoeJRO’BrienTRHornofWJMasonDKExercise-induced pulmonary haemorrhage in the horse: results of a detailed clinical, post mortem and imaging study: VI. Radiological/pathological correlationsEquine Vet J19871954194223678184
- LekeuxPArtTThe respiratory system: anatomy, physiology, and adaptations to exercise and trainingHodgsonDJRoseRJThe Athletic Horse: Principles and Practice of Equine Sports MedicinePhiladelphia, PAWB Saunders Co199478127
- RantanenNWByarsTDHauserMLGainesRDSpontaneous contrast and mass lesions in the hearts of race horses: ultrasound diagnosis - preliminary dataJ Equine Vet Sci198445220223
- MahoneyCRantanenNWDeMichaelJAKincaidBSpontaneous echocardiographic contrast in the Thoroughbred: high prevalence in racehorses and a characteristic abnormality in bleedersEquine Vet J19922421291331582391
- MarrCCardiology of the HorseLondonWB Saunders Co1999
- VotionDMRobertsCAMarlinDJLekeuxPMFeasibility of scintigraphy in exercise-induced pulmonary haemorrhage detection and quantification: preliminary studiesEquine Vet J Suppl19993013714210659238
- DerksenFWilliamsKStackAExercise-induced pulmonary hemorrhage in horses: the role of pulmonary veinsCompend Contin Educ Vet2011334E6
- StackADerksenFJSordilloLMEffects of exercise on markers of venous remodeling in lungs of horsesAm J Vet Res20137491231123823977896
- BernardSLGlennyRWEricksonHHMinimal redistribution of pulmonary blood flow with exercise in racehorsesJ Appl Physiol (1985)1996813106210708889735
- PelletierNRobinsonNEKaiserLDerksenFJRegional differences in pulmonary artery endothelial function: a role in the site of EIPH?Equine Vet J Suppl199527S1399103
- StackADerksenFJWilliamsKJRobinsonNEJacksonWFRegional heterogeneity in the reactivity of equine small pulmonary blood vesselsJ Appl Physiol (1985)2016120659960726769957
- StackADerksenFJWilliamsKJRobinsonNEJacksonWFLung region and racing affect mechanical properties of equine pulmonary microvasculatureJ Appl Physiol (1985)2014117437037624925981
- WilliamsKJRobinsonNEDefeijter-RuppHDistribution of venous remodeling in exercise-induced pulmonary hemorrhage of horses follows reported blood flow distribution in the equine lungJ Appl Physiol (1985)2013114786987823372148
- HolcombeSJBerneyCCornelisseCJDerksenFJRobinsonNEEffect of commercially available nasal strips on airway resistance in exercising horsesAm J Vet Res20026381101110512171161
- HolcombeSJCornelisseCJDerksenFJThe effect of FLAIR™ nasal strips on upper airway mechanics in exercising horses. Proc World Equine Airways Symp and Veterinary and Comparative Respiratory Society. Edinburgh, Scotland, 19–23 July 2001Canada: World Equine Airways Society2001221
- ArtTSerteynDLekeuxPEffect of exercise on the partitioning of equine respiratory resistanceEquine Vet J19882042682733168986
- Di SommaEMWestSNWheatleyJRAmisTCNasal dilator strips increase maximum inspiratory flow via nasal wall stabilizationLaryngoscope1999109578078410334230
- ManoharMRight heart pressures and blood-gas tensions in ponies during exercise and laryngeal hemiplegiaAm J Physiol19862511 Pt 2H121H1263728688
- ManoharMBlood flow in respiratory muscles during maximal exertion in ponies with laryngeal hemiplegiaJ Appl Physiol (1985)19876212292373104287
- Van Erck-WestergrenEFranklinSHBaylyWMRespiratory diseases and their effects on respiratory function and exercise capacityEquine Vet J201345337638723368813
- NewtonJRWoodJLEvidence of an association between inflammatory airway disease and EIPH in young Thoroughbreds during trainingEquine Vet J Suppl20023441742412405727
- MichelottoPVJrMuehlmannLAZanattaALPulmonary inflammation due to exercise-induced pulmonary haemorrhage in Thoroughbred colts during race trainingVet J20111902e3e622108190
- McKaneSASlocombeRFExperimental mild pulmonary inflammation promotes the development of exercise-induced pulmonary haemorrhageEquine Vet J Suppl201038235239
- DerksenFJWilliamsKJUhalBDPulmonary response to airway instillation of autologous blood in horsesEquine Vet J200739433433917722725
- WilliamsKJDerksenFJDefeijter-RuppHLRobinsonNERepeated blood instillation into the airway of the horse does not cause pulmonary fibrosisEquine Vet J201143335435821492214
- DerksenFJWilliamsKJPannirselvamRRde Feijter-RuppHSteelCMRobinsonNERegional distribution of collagen and haemosiderin in the lungs of horses with exercise-induced pulmonary haemorrhageEquine Vet J200941658659119803055
- NewtonJRRogersKMarlinDJWoodJLWilliamsRBRisk factors for epistaxis on British racecourses: evidence for locomotory impact-induced trauma contributing to the aetiology of exercise-induced pulmonary haemorrhageEquine Vet J200537540241116163941
- GrossDKMorleyPSHinchcliffKWWittumTEEffect of furosemide on performance of Thoroughbreds racing in the United States and CanadaJ Am Vet Med Assoc1999215567067510476714
- HinchcliffKWEffects of furosemide on athletic performance and exercise-induced pulmonary hemorrhage in horsesJ Am Vet Med Assoc1999215563063510476706
- BaylyWMSlocombeRFSchottHC2ndHodgsonDREffect of intravenous administration of furosemide on mass-specific maximal oxygen consumption and breathing mechanics in exercising horsesAm J Vet Res199960111415142210566819
- ZawadzkasXASidesRHBaylyWMIs improved high speed performance following frusemide administration due to diuresis-induced weight loss or reduced severity of exercise-induced pulmonary haemorrhage?Equine Vet J Suppl20063629129317402434
- EricksonHHHildrethTSPooleDCManagement of exercise-induced pulmonary hemorrhage in non-racing performance horsesComp Cont Educ Pract Vet20012310901093
- HinchcliffKWManagement and pharmacotherapy of exercise-induced pulmonary hemorrhage in horsesProc World Equine Airways Symp20012110
- EricksonHHHildrethTSNovel and emerging therapies for EIPHProc Am Coll Vet Intern Med200422734736
- HinchcliffKWMcKeeverKHFrusemideEquine Vet J Suppl199518256258
- HinchcliffKWHubbellJAEGrosenboughDHemodynamic effects of furosemide are dependent on diuresisProc Annu Conv Am Assoc Equine Pract199642229230
- PascoeJRMcCabeAEFrantiCEArthurRMEfficacy of furosemide in the treatment of exercise-induced pulmonary hemorrhage in Thoroughbred racehorsesAm J Vet Res1985469200020034051304
- SweeneyCRSomaLRExercise-induced pulmonary hemorrhage in Thoroughbred horses: response to furosemide or hesperidin-citrus bioflavinoidsJ Am Vet Med Assoc198418521951976746389
- SweeneyCRSomaLRBucanCARaySGExercise-induced pulmonary hemorrhage in exercising Thoroughbreds: preliminary results with preexercise medicationCornell Vet19847432632686234149
- SweeneyCRSomaLRMaxsonADThompsonJEHolcombeSJSpencerPAEffects of furosemide on the racing times of ThoroughbredsAm J Vet Res19905157727782337276
- LesterGClarkCRiceBSteible-HartlessCVetro-WidenhouseTEffect of timing and route of administration of furosemide on pulmonary hemorrhage and pulmonary arterial pressure in exercising Thoroughbred racehorsesAm J Vet Res199960122289918144
- HopperMKPieschlRLJrPelletierNGEricksonHHCardiopulmonary effects of acute blood volume alteration prior to exercisePerssonSGBLindholmAJeffcottLBEquine Exercise Physiology 3Davis, CAICEEP Publications1991916
- OlsenSCCoyneCPLoweBSPelletierNRaubEMEricksonHHInfluence of furosemide on hemodynamic responses during exercise in horsesAm J Vet Res19925357427471524299
- ManoharMHutchensEConeyEPulmonary hemodynamics in the exercising horse and their relationship to exercise-induced pulmonary hemorrhageBr Vet J199314954194288298955
- ManoharMHutchensEConeyEFrusemide attenuates the exercise induced rise in pulmonary capillary blood pressure in horsesEquine Vet J199426151548143666
- GleedRDDucharmeNGHackettRPEffects of frusemide on pulmonary capillary pressure in horses exercising on a treadmillEquine Vet J Suppl19993010210610659232
- EricksonHHBernardSLGlennyRWEffect of furosemide on pulmonary blood flow distribution in resting and exercising horsesJ Appl Physiol19998662034204310368371
- ChaySWoodsWERowseKNugentTEBlakeJWTobinTThe pharmacology of furosemide in the horse. V. Pharmacokinetics and blood levels of furosemide after intravenous administrationDrug Metab Dispos19831132262316135581
- AmisTCKirknessJPdi SommaEWheatleyJRNasal vestibule wall elasticity: interactions with a nasal dilator stripJ Appl Physiol (1985)19998651638164310233129
- HarmsCAWetterTJMcClaranSREffects of respiratory muscle work on cardiac output and its distribution during maximal exerciseJ Appl Physiol (1985)19988526096189688739
- CoppSWHoldsworthCTFergusonSKHiraiDMPooleDCMuschTIMuscle fibre-type dependence of neuronal nitric oxide synthase-mediated vascular control in the rat during high speed treadmill runningJ Physiol2013591112885289623507879
- MónicaFZBianKMuradFThe endothelium-dependent nitric oxide-cGMP pathwayAdv Pharmacol20167712727451093
- FergusonSKHiraiDMCoppSWImpact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in ratsJ Physiol2013591254755723070702
- KindigCAGallatinLLEricksonHHFeddeMRPooleDCCardiorespiratory impact of the nitric oxide synthase inhibitor L-NAME in the exercising horseRespir Physiol20001202161176
- MillsPCMarlinDJScottCMPulmonary artery pressure during exercise in the horse after inhibition of nitric oxide synthaseBr Vet J19961521191228680833
- ManoharMGoetzTEL-NAME does not affect exercise-induced pulmonary hypertension in thoroughbred horsesJ Appl Physiol (1985)1998846190219089609783
- ManoharMGoetzTEPulmonary vascular pressures of strenuously exercising Thoroughbreds during intravenous infusion of nitroglycerinAm J Vet Res199960111436144010566823
- HackettRPDucharmeNGGleedRDErbHNMitchellLMSoderholmLVOral nitroglycerin paste did not lower pulmonary capillary pressure during treadmill exerciseEquine Vet J Suppl199930153158
- ColahanPTJacksonCARiceBSzaboNJonesJHThe effect of sildenafil citrate administration on selected physiological parameters of exercising Thoroughbred horsesEquine Vet J Suppl20103860661221059068
- MillsPCMarlinDJScottCMSmithNCNitric oxide during exercise and pulmonary disease in the horsePferdeheikunde199612551556
- MillsPCMarlinDJDemoncheauxENitric oxide and exercise in the horseJ Physiol1996495Pt 38638748887788
- KindigCAMcDonoughPFinleyMRNO inhalation reduces pulmonary arterial pressure but not hemorrhage in maximally exercising horsesJ Appl Physiol (1985)20019162674267811717233
- RaglandWLMcCulloughRWilkeyWCPassive immunotherapy of equine respiratory disease: treatment of acutely ill horses with equine immunoglobulinEquine Athlete1995861621
- SaulezMNGodfroidJBosmanACytokine mRNA expressions after racing at a high altitude and at sea level in horses with exercise-induced pulmonary hemorrhageAm J Vet Res201071444745320367053
- EppTSMcDonoughPBehnkeBJThe effects of concentrated equine serum on exercise-induced pulmonary hemorrhage in Thoroughbred horsesEquine Comp Exerc Physiol200521725
- EppTSMcDonoughPMyersDEThe effectiveness of immunotherapy in treating exercise-induced pulmonary haemorrhageJ Equine Vet Sci2009296527532
- PortierKde MoffartsBFellmanNThe effects of dietary N-3 and antioxidant supplementation on erythrocyte membrane fatty acid composition and fluidity in exercising horsesEquine Vet J Suppl20063627928417402432
- MaciociaGThe Practice of Chinese Medicine: The Treatment of Diseases with Acupuncture and Chinese HerbsEdinburghChurchill and Livingstone1998
- XieHHerbal formulas for respiratory diseases in equine practice. A Course in Traditional Chinese Herbal Medicine: International Veterinary Acupuncture SocietyModule200122527
- BaylyWMMeyersKMKeckMTHustonLJGrantBDEffects of furosemide on exercise-induced alterations in haemostasis in Thoroughbred horses exhibiting post-exercise epistaxisSnowDHPerssonSGBRoseRJEquine Exercise PhysiologyCambridgeGranta Editions19836470
- JohnstoneIBVielLCraneSWhitingTHemostatic studies in racing Standardbred horses with exercise-induced pulmonary hemorrhage. Hemostatic parameters at rest and after moderate exerciseCan J Vet Res19915521011061909208
- GiordanoAMeazzaCSalvadoriMPaltrinieriSThromboelastometric profiles of horses affected by exercise-induced pulmonary hemorrhagesVet Med Int2010201094578920953331
- HeidmannPTornquistSJQuACebraCKLaboratory measures of hemostasis and fibrinolysis after intravenous administration of epsilonaminocaproic acid in clinically normal horses and poniesAm J Vet Res200566231331815757133
- EppTSEdwardsKLPooleDCEricksonHHEffects of conjugated estrogens and aminocaproic acid upon exercise-induced pulmonary hemorrhageComp Exerc Physiol20085295103
- BuchholzBMMurdockABaylyWMSidesRHEffects of intravenous aminocaproic acid on exercise-induced pulmonary haemorrhage (EIPH)Equine Vet J Suppl20103825626021059015
- ElliottARFuZTsukimotoKPredilettoRMathieu-CostelloOWestJBShort-term reversibility of ultrastructural changes in pulmonary capillaries caused by stress failureJ Appl Physiol (1985)1992733115011581400030