Abstract
Purpose
This study sought to determine the efficacy of a therapeutic iodine-restricted diet during a 12 -month interval in cats with moderately to severely increased total thyroxine (TT4) concentrations.
Patients and methods
Eight hyperthyroid cats with serum creatinine <2.0 mg/dL and TT4 >6.0 µg/dL (reference interval 1.5–4.0 µg/dL) were enrolled. Each cat underwent an initial physical examination, complete blood count, serum chemistry panel, thyroid profile, thyroid scintigraphy, and echocardiogram. Physical examination and all blood samplings were repeated at 1, 2, 3, 6, and 12 months after enrollment. Clients were instructed to feed only the therapeutic iodine-restricted diet throughout the entire study.
Results
Median TT4 was 8.4 (range 6.2–24.0) µg/dL at study onset. Thyroid scintigraphy was abnormal in all cats, confirming hyperthyroidism. Six out of eight cats had normal serum TT4 after 4 weeks of feeding the therapeutic diet. The two cats that did not achieve normalization of TT4 at 4 weeks had the highest initial TT4 concentrations. Three cats were withdrawn from the study due to emergence of chronic kidney disease.
Conclusion
An iodine-restricted diet was able to control moderate-to-severe hyperthyroidism in most (six out of eight) of the cats by 4 weeks. Cats with highest baseline TT4 took substantially longer before TT4 concentrations normalized, if at all.
Introduction
Hyperthyroidism resulting from functional adenomas/adenomatous hyperplasia of the thyroid gland is the most common endocrine disease of the cat.Citation1,Citation2 It is most frequently diagnosed in cats >10 years of age.Citation2 Awareness of the disease has steadily increased over the past 35 years and early diagnosis is becoming increasingly common.
Treatment for hyperthyroidism includes medical and surgical modalities. Medical treatment with methimazole, a drug that inhibits thyroperoxidase and thereby reduces thyroid hormone synthesis, is widely employed.Citation3 Although effective in most hyperthyroid cats, methimazole has to be administered daily, may cause side effects, and does not definitively address the underlying cause of the disease. There also is concern that the prevalence of adenocarcinoma may increase with time from diagnosis.Citation4 Surgery to remove the affected thyroid lobe(s) can be performed, but includes risks of anesthesia, iatrogenic hypoparathyroidism and hypothyroidism, and recurrent laryngeal nerve damage. Definitive treatment with radioactive iodine (I131) is often considered the gold-standard treatment because of its efficacy and safety; however, cost of the treatment, required quarantine period, and availability of licensed radioiodine treatment facilities for cats limit this option.
Recently, a therapeutic iodine-restricted diet has become available for cats with hyperthyroidism. Studies by the manufacturer and independent investigators have established efficacy of iodine-restricted diets in the treatment of feline hyperthyroidism.Citation5–Citation9 However, there are also drawbacks to dietary management. Cats must be fed only the therapeutic diet to be maximally effective. Dietary treatment, similarly to methimazole, does not address continued growth of the abnormal thyroid tissue. Previous studies have assessed various dietary iodine concentrations, did not focus on cats with moderate-to-severe elevations in serum thyroid hormone with definitive clinical signs, or often were limited to ≤6 months.Citation5–Citation9 The objective of this study was to determine the efficacy of the commercially available iodine-restricted diet in cats with moderately to severely increased total thyroxine (TT4) concentrations (>6.0 µg/dL) for a longer interval (12 months).
Patients and methods
Eligible cats were recruited by an email announcing the trial to local primary care veterinary practices and the Cornell University Hospital for Animals. The study was conducted under the best practices criteria and guidelines of our American Animal Hospital Association accredited hospital, and was approved by the Institutional Animal Care and Use Committee of Cornell University (20120084). Eight cats diagnosed with moderate-to-severe hyperthyroidism having serum creatinine concentrations <2.0 mg/dL (reference range 0.6–2.0 mg/dL) were enrolled in the study. Moderate-to-severe hyperthyroidism was diagnosed by TT4 >6.0 µg/dL (reference interval 1.5–4.0 µg/dL) in cats with appropriate clinical signs and physical examination findings (eg, weight loss, polyphagia, hyperactivity, or palpable goiter). Enrolled cats were domestic shorthair (n=6) or domestic longhair (n=2) with six spayed females and two castrated males, and the median age was 14.5 (range 10–17) years (). All cats were indoor only, able to be fed an exclusive diet, and had no history of poor appetite or food refusal. All clients signed informed consent forms. Clients completed surveys regarding their cat’s clinical signs and perceived efficacy of the diet after feeding for 12 months.
Table 1 Signalment, initial body weight, and initial TT4 concentration in eight cats with moderate-to-severe hyperthyroidism
Complete physical examinations including body weight, body condition scoring (BCS) (1–9 scale)Citation10 and muscle condition scoring (MCS) (0–3 scale)Citation11 were performed by two of the authors (JPL or JJW). Complete blood count, serum chemistry panel, and thyroid profile (TT4, free T4 [fT4], and thyroid stimulating hormone [TSH]) were performed by an accredited veterinary laboratory (Animal Health Diagnostic Center, Ithaca, NY, USA). Briefly, TT4 was measured by radioimmunoassay, fT4 by radioimmunoassay after equilibrium dialysis, and TSH by chemiluminescent immunoassay.Citation12 Thyroid scintigraphy was performed on seven out of eight cats after intravenous administration of pertechnetate with interpretation by a Diplomate of the American College of Veterinary Radiology. A region of interest (ROI) was manually applied to densitometry plot areas corresponding to thyroid activity, salivary gland uptake, and representative background regions. Ratios of thyroid gland ROI pixel density to salivary gland ROI pixel density (T:S) were calculated. With two thyroid ROI, the pixel counts were added to calculate the ratio; this resulted in a higher RCitation2 value than choosing the higher single thyroid ROI as assessed via Pearson’s tests. Echocardiograms were interpreted by a Diplomate of the American College of Veterinary Internal Medicine Specialty of Cardiology.
Data are presented as individual values when possible and for group representation, medians and interquartile ranges are presented. Due to missing data points, the Kruskal– Wallis test was performed for comparison of body weight, BCS, and MCS. Linear regression analysis was performed to compare thyroid scintigraphy ratios to TT4 concentrations. Statistical analyses were performed with commercial software (GraphPad Prism version 7; GraphPad Software, La Jolla, CA, USA).
Results
Once the initial database was collected, enrolled cats were transitioned to the iodine-restricted veterinary therapeutic diet (Hill’s Prescription Diet™ y/d™ Feline; Hill’s Pet Nutrition, Inc., Topeka, KS, USA). Clients were instructed to transition slowly to the new diet over a 3–5 day period and feeding practices (ad libitum vs meal feeding) could be maintained as long as this diet was exclusively fed. All cats were on municipal water, which contains no known iodine.
At study onset, median TT4 was 8.4 (range 6.2–24.0) µg/dL and five out of eight cats had fT4 >10 ng/dL (reference interval 1.2–4.0 ng/dL), which exceeded the limit of the assay (). Concentrations of TSH were undetectable at baseline and throughout the study with the exception of a single normal value in one cat at 6 months. This lack of TSH in all cats may be a reflection of marginal control of thyroid hormone since many cats’ free T4 concentrations were still slightly higher than the high end of the reference range during dietary therapy. One cat (#4) was managed off site and neither thyroid scintigraphy nor echocardiography was performed. No clinically relevant cardiac changes were detected during echocardiography in the remaining seven cats, despite the association between hyperthyroidism and cardiac abnormalities in cats.Citation13 This is likely a reflection of the small sample size. On initial thyroid scintigraphy, cats had increased T:S (≥3.4, reference interval 0.48–1.66),Citation14 confirming hyperthyroidism and correlating positively with TT4 concentrations when assessed via Pearson’s testing (RCitation2=0.68, P=0.02).
Figure 1 Serum TT4 and fT4 concentrations decrease in cats with moderate-to-severe hyperthyroidism fed an iodine-restricted diet.
Abbreviations: fT4, free thyroxine; TT4, total thyroxine.
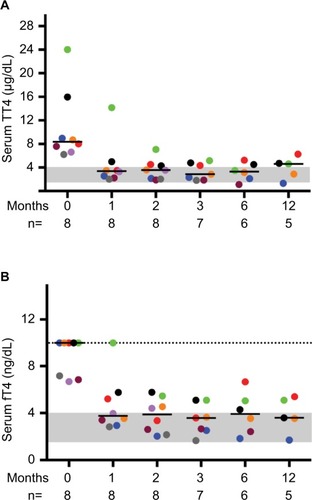
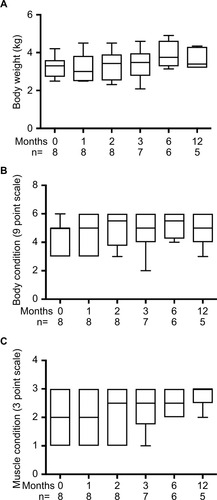
After 1-month consumption of the diet, the median TT4 concentration was 3.39 µg/dL, with six out of eight cats having TT4 concentrations within the reference interval (). In most cats, body weight, BCS and MCS stabilized or improved with the iodine-restricted diet (). Although improvement in MCS was the clearest trend over time, the change did not reach statistical significance. Overall, client survey responses noted improvement or no difference in clinical signs during consumption of the therapeutic diet with most notably mild improvements in hair coat, body condition, stool quality, and overall health (Table S1). Our findings concurred with the owners’ perceptions on physical examination.
Figure 2 Body composition in cats with moderate-to-severe hyperthyroidism during 1-year consumption of an iodine-restricted diet.
Abnormalities detected in the hemograms () included mild erythrocytosis, eosinopenia, and lymphopenia, which is consistent with changes documented in cats with hyperthyroidism.Citation15 Hyperthyroid cats may show increased red blood cell mean corpuscular volume,Citation16 but surprisingly, red blood cell microcytosis was noted in at least 25% of the cats throughout this study. An explanation for this finding is unclear. Common serum biochemistry changes detected at baseline were increased liver enzyme activities and hypokalemia () consistent with findings in hyperthyroid cats.Citation15,Citation16 Three cats developed azotemia and their creatinine concentrations obtained from chemistry panels performed as part of the study are summarized (). Increased creatinine concentrations were evident in one out of eight and one out of six cats at 3 and 6 months, respectively. A third cat (#7), began to refuse the diet after the 3-month time point and was removed from the study. He was later reported to have developed renal azotemia. It was presumed, considering that all cats had creatinine within the normal reference range at the initiation of the study, that kidney disease was absent or masked due to renal hypertension induced by hyperthyroidism and that cats could have been classified as International Renal Interest Society (IRIS) Stage one chronic kidney disease (CKD) at the time of enrollment. One cat would have been categorized as IRIS Stage 2 CKD at baseline; however, after 1 month, this cat’s creatinine concentration decreased to a value consistent with IRIS Stage 1 CKD (). Based on our inclusion/exclusion criterion of a normal creatinine value, cats with IRIS Stages 1 or 2 CKD could have been included and presumably these three cats all fell into this category. Ultimately, all three cats (#4, #7, and #8) that were withdrawn from the study developed CKD.
Table 2 Hemogram abnormalities in eight cats with moderate-to-severe hyperthyroidism at indicated month time points during 1-year consumption of an iodine-restricted diet
Table 3 Serum chemistry abnormalities in eight cats with moderate-to-severe hyperthyroidism at indicated month time points during 1-year consumption of an iodine-restricted diet
Table 4 Serum creatinine concentrations in cats that developed azotemia and were subsequently excluded from the study
Two out of the three cats withdrawn from the study because of increasing creatinine concentrations had clinical signs of hyporexia and vomiting; one cat was euthanized within 1 month of withdrawal, but the other two cats were lost to follow-up. Of the remaining five cats completing the 12-month study, one cat (#1) was subsequently treated with methimazole and another (#5) with I131 therapy due to suboptimal responses to dietary iodine restriction. The cat treated with methimazole was well-managed medically at the time of study completion, whereas the cat treated with I131 was euthanized 6 months after study completion for metastatic basal cell carcinoma. Cat #6 was euthanized 18 months after conclusion of the study due to progressive CKD, which was diagnosed 6 months after the trial was complete. Cats #2 and # 3 continued to eat the iodine-restricted diet at last communication with the owner (approximately 18 months after initiating diet).
Discussion
In this study, we reported on eight cats with moderate-to-severe hyperthyroidism treated with an iodine-restricted diet for 1 year. Overall, the response to the diet was acceptable with six out of eight cats achieving normalization of TT4 within 4 weeks. However, at all study time points (1, 2, 3, 6, and 12 months), at least two to three cats had TT4 and fT4 concentrations above the reference intervals. Persistently undetectable TSH concentrations in most cats throughout the study also might suggest incomplete control of hyperthyroidism. Nevertheless, TT4 and fT4 concentrations did decline in all cats. The six cats with initial TT4 concentrations between 6.2 and 9.0 µg/dL achieved normal values within 4 weeks. However, the cat with the baseline TT4 concentration of 24.0 µg/dL took 6 months before its TT4 normalized and by the next time point 6 months later, the TT4 had increased above the reference interval again. The cat with the baseline TT4 of 16.0 µg/dL never normalized TT4 during the 12-month study. Overall, clients were pleased with the clinical response to the diet based on surveys completed at 6 months. In some cases, clinical improvement without normalizing TT4 or fT4 may be appropriate where an iodine-restricted diet is the only viable option.
Dietary iodine may play a role in the development of hyperthyroidism. Although currently unknown, low or fluctuating iodine levels in commercial cat foods could predispose to thyroid hyperplasia and hyperthyroidism.Citation17 There is wide variation in the levels of iodine in commercial cat food as well as controversy regarding the minimum dietary requirement for iodine in cats. The National Research Council recommends adequate iodine concentrations to be 1.4 mg/kg of food on a dry matter (DM) basis.Citation18 This contrasts with the American Association of Feed Control Officers (AAFCO) guideline, which was 0.35 mg/kg iodine (DM basis) until two years ago when it increased to 0.6 mg/kg iodine (DM basis). The feline iodine requirement recently determined by break-point analysis of thyroid scintigraphy in cohorts of cats fed various iodine concentrations in their diet for 1 year was 0.46 mg/kg iodine (DM basis).Citation19 Therefore, the earlier AAFCO guideline may have resulted in commercial diets containing suboptimal amounts of dietary iodine. This could theoretically lead to insufficient iodine levels for thyroid hormone synthesis with ensuing TSH responses that could promote thyroid hyperplasia. The risks of thyroid hyperplasia has been studied in normal cat populations using the identical diet for long-term use and use of this diet did not show any risk of thyroid tissue hyperplasia.Citation9 In fact, it proved to be effective at maintaining serum thyroid hormone concentrations for 2 years, further clarifying the safety of feeding a diet of this nature to a multi-cat household if needed for a single cat in that household.
The diet assessed in this study provides 0.2 mg/kg iodine (DM basis) as reported by the manufacturer. This concentration is lower than current recommended allowances or estimated requirements. Restriction of dietary iodine limits iodine available for thyroid hormone synthesis, thus moderating thyroid hormone concentrations in cats with hyperthyroidism. Efficacy of dietary iodine restriction in hyperthyroid cats was previously tested by the manufacturer and the diet used in this study was evaluated in several prospective studies.Citation5–Citation8,Citation20 These studies concluded efficacy overall, but generally monitored cats for ≤6 months. Similar to the case series reported herein, median TT4 concentrations in these earlier studies normalized within the first month of treatment, but a proportion of cats never achieved euthyroidism. More interestingly, in our population, three out of the eight cats dropped out of our small prospective trial due to kidney insufficiency, whereas in the recent study by Pateau-Robinson et al, serum creatine in a population of 49 cats did not show any increases.Citation20 Yet, in this previous study 20% of the cats were removed from the study at 180 days for inappetence. This may have been due to emergence of other comorbidities such as kidney insufficiency, lack of thyroid hormone normalization, or problems with administering the diet. In addition, although we did not see a significant increase in body weight or BCS, there was a trend for increases in both BCS and MCS, although cardiac rate was unchanged. Again, this is different from the prior larger retrospective study,Citation20 which showed no changes in BCS in the cats that continued the diet beyond 180 days. but was similar to our study as there were no changes in the cardiac rate of the cats during treatment. Our population is very small and our findings must be interpreted cautiously; however, kidney insufficiency monitoring after any hyperthyroidism intervention is of the utmost importance.
With consumption of the iodine-restricted diet, cats demonstrated a reduction in TT4 from baseline following a relatively predictable pattern. This may guide clinicians in early identification of patients that might respond sub-optimally to dietary management. Based on the reduction in TT4 over time in this cohort, cats with TT4 concentrations ≥12 µg/dL may require more than 1 month to achieve euthyroidism. These data may aid in the establishment of reasonable client expectations by assuming a reduction of ~50% in TT4 monthly at the initiation of treatment for most cats. However, caution should be exercised for widespread application of this guideline given the low numbers of cats in this dataset.
The iodine-restricted diet provides 8.2 g/100 kcal protein as reported by the manufacturer. This is less than the proposed 12 g/100 kcal of dietary protein considered optimal for cats with hyperthyroidismCitation21 but would be considered sufficient based on National Research Council standardsCitation18 and would not be considered egregiously high for cats in early CKD. However, cats in this study trended toward improvement in MCS with reductions in TT4. Although overall significance was not achieved for changes in body weight, BCS, or MCS, no cat that normalized TT4 without a comorbidity had diminished BCS or MCS. The moderate dietary protein provided in the iodine-restricted food appeared to be sufficient for cats when adequate control of hyperthyroidism was achieved during this 1-year study. Studies involving larger number of cats for longer periods of time are warranted to support this finding.
The major limitation of this study was the small sample size. Unfortunately, recruitment of cases fell below that anticipated. Poor compliance with the diet was identified in one cat (#1) to be a contributing factor for persistently increased TT4 toward the end of the study. It is possible that dietary compliance was an issue with other cats but was not reported to investigators. During the 12-month study interval, CKD emerged in three cats. Likely, these cats had IRIS Stage 1 or 2 CKD at the time of admission to the study. Evaluation of urine specific gravity may have aided in identification of these cats; however, hyperthyroidism can also decrease urine specific gravity in cats without concurrent renal pathology.Citation22 There is also considerable overlap in urine specific gravity values between hyperthyroid cats that do and do not develop azotemia following treatment.Citation22 The comorbidity of renal disease may have affected body condition results overall, as these were the only cats that lost weight during the study.
Conclusion
An iodine-restricted veterinary therapeutic diet can be considered an effective alternative to more established therapies for feline hyperthyroidism. The TT4 concentrations prior to institution of dietary therapy may be useful in predicting potential time to response.
Acknowledgments
We thank the members of the Imaging and Cardiology Services at the Cornell University Hospital for Animals, particularly Drs Marc Kraus and Ned Dykes, for their participation. This study was funded with the support of Hill’s Pet Nutrition, Inc.
Supplementary material
Table S1 Summary of client questionnaire responses for hyperthyroid cats fed an iodine-restricted diet for 6 months
Disclosure
The authors report no conflicts of interest in this work.
References
- McLeanJLLobettiRGSchoemanJPWorldwide prevalence and risk factors for feline hyperthyroidism: a reviewJ S Afr Vet Assoc2014851109725685895
- PetersonMHyperthyroidism in cats: what’s causing this epidemic of thyroid disease and can we prevent it?J Feline Med Surg2012141180481823087006
- TrepanierLAPharmacologic management of feline hyperthyroidismVet Clin North Am Small Anim Pract200737477578817619011
- PetersonMEBroomeMRRishniwMPrevalence and degree of thyroid pathology in hyperthyroid cats increases with disease duration: a cross-sectional analysis of 2096 cats referred for radioiodine therapyJ Feline Med Surg20161829210325673019
- YuSWedekindKBurrisPForresterDLocniskarMControlled level of dietary iodine normalizes serum total thyroxine in cats with naturally occurring hyperthyroidismJ Vet Intern Med201125683684
- MelendezLMDYamkaR ABurrisPTitration of dietary iodine for maintaining normal serum thyroxine concentrations in hyperthyroid catsJ Vet Intern Med201125683
- MelendezLMYamkaRMForresterSDTitration of dietary iodine for reducing serum thyroxine concentrations in newly diagnosed hyperthyroid catsJ Vet Intern Med201125683
- van der KooijMBečvářováIMeyerHPTeskeEKooistraHSEffects of an iodine-restricted food on client-owned cats with hyperthyroidismJ Feline Med Surg201416649149824232246
- HuiTYBruyetteDSMooreGEScott-MoncrieffJCEffect of feeding an iodine-restricted diet in cats with spontaneous hyperthyroidismJ Vet Intern Med20152941063106826081922
- LaflammeDPDevelopment and validation of a body condition score system for cats: a clinical toolFeline Pr1997251318
- MichelKEAndersonWCuppCLaflammeDPCorrelation of a feline muscle mass score with body composition determined by dual-energy X-ray absorptiometryBr J Nutr2011106S1S57S5922005437
- PetersonMEGuterlJNNicholsRRishniwMEvaluation of serum thyroid-stimulating hormone concentration as a diagnostic test for hyperthyroidism in catsJ Vet Intern Med20152951327133426192742
- SangsterJKPancieraDLAbbottJAZimmermanKCLantisACCardiac biomarkers in hyperthyroid catsJ Vet Intern Med201428246547224350989
- HenriksonTDArmbrustLJHoskinsonJJThyroid to salivary ratios determined by technetium-99m pertechnetate imaging in thirty-two euthyroid catsVet Radiol Ultrasound200546652152316396272
- ThodayKLMooneyCTHistorical, clinical and laboratory features of 126 hyperthyroid catsVet Rec1992131122572641413411
- PetersonMEKintzerPPCavanaghPGFeline hyperthyroidism: pretreatment clinical and laboratory evaluation of 131 casesJ Am Vet Med Assoc198318311031106874510
- van HoekIHestaMBiourgeVA critical review of food-associated factors proposed in the etiology of feline hyperthyroidismJ Feline Med Surg2015171083784725366172
- National Research CouncilNutrient Requirements of Dogs and Cats. Rev volWashington, DCNational Academies Press2006
- WedekindKJBlumerMEHuntingtonCESpateVMorrisJSThe feline iodine requirement is lower than the 2006 NRC recommended allowanceJ Anim Physiol Anim Nutr (Berl)201094452753919906136
- Paetau-RobinsonIMelendezLDForresterSDArmbrustLJRefsalKRBurrisPAComparison of health parameters in normal cats fed a limited iodine prescription food vs a conventional dietJ Feline Med Surg201820214214828379113
- PetersonMEEirmannLDietary management of feline endocrine diseaseVet Clin North Am Small Anim Pract201444477578824951346
- WilliamsTLPeakKJBrodbeltDElliottJSymeHMSurvival and the development of azotemia after treatment of hyperthyroid catsJ Vet Intern Med201024486386920649748
