Abstract
Purpose
Rodents live in proximity to humans and domestic animals. These creatures can serve as reservoir hosts for many zoonotic parasites; therefore, they increase the risk of human infections. The aim of this study was to investigate Toxoplasma gondii and Neospora caninum in rodents caught in Meshgin-Shahr District, Iran.
Patients and methods
In a cross-sectional study, brain samples were collected from 70 rodents caught in Meshgin-Shahr District during March and December 2015. The specimens were examined for exposure to T. gondii and N. caninum with molecular methods.
Results
Seventy rodents were caught, including 50 Meriones persicus, 11 Mus musculus and 9 Cricetulus migratorius. Thirty rodents were female and 40 were males. Using PCR (B1 gene), T. gondii was detected in 7.1% (5/70) of the rodents while N. caninum was not detected. The prevalence of Toxoplasma infection was higher in female rodents (4.28%) compared to male rodents (2.86%), but the difference was not significant.
Conclusion
The results showed a low risk of T. gondii and N. caninum among rodents. Finally, further research is needed to understand the role of these rodent species in the transmission of the above protozoan pathogens to humans and livestock in this area.
Introduction
Rodents are the largest group of mammals with around 2700 species worldwide.Citation1 They are important intermediate hosts for several parasites affecting cats, dogs and wild carnivores such as Toxoplasma, Neospora, Hammondia, Sarcocystis, Echinococcus and Toxocara.Citation2 Rodents are also important zoonotic carriers of agents to humans, because they rapidly adapt to human habitats and ecologic and environmental changes.Citation3 Toxoplasma gondii and Neospora caninum are intracellular protozoan parasites belonging to the phylum Apicomplexa. Cats and dogs are the definitive hosts of T. gondii and N. caninum, respectively. Both parasites can use a wide range of domestic and wild animals as intermediate hosts.Citation4,Citation5 Infection can be acquired vertically by transplacental transmission of tachyzoites or horizontally following ingestion of tissue cysts from uncooked or undercooked meat (bradyzoites), or ingestion of oocysts excreted in definitive hosts feces.Citation4,Citation5
Previous investigations have reported N. caninum infection in different species of rodents.Citation6,Citation7 Infection in rodents has been associated with the spread of N. caninum infection and its effects on reproduction in dairy cattle.Citation8 Toxoplasma is one of the most common zoonotic parasites infecting one-third of the world’s population.Citation9,Citation10 Neospora, however, unlike Toxoplasma, is not considered a zoonotic protozoan.Citation11,Citation12 N. caninum has been reported in different hosts such as hooded crows (Corvus cornix),Citation13 chickens (Gallus domesticus)Citation14 and stray dogsCitation15 in Iran, but there is no reported survey in rodents. There are scant reports of the prevalence of T. gondii infection in rodents in Iran. Serological surveys carried out in rodents have shown a prevalence of 0–31%.Citation16 A recent epidemiological study carried out by Seifollahi et alCitation17 revealed a prevalence of 5.7% in rodents. Therefore, considering the fact that both T. gondii and N. caninum have a global distribution and our knowledge of these species in Iran is limited, the aim of this study was to evaluate the molecular prevalence of N. caninum and T. gondii infections in rodents living in Meshgin-Shahr District, north-western Iran.
Patients And Methods
Study Site And Sampling
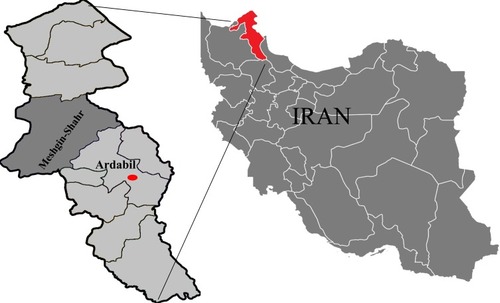
The location of sampling was Meshgin-Shahr District in north-western Iran (38°23′56″N and 47°40′55″E) (). It is located near the Sabalan Mountains and has a cold mountainous climate. During the period of this cross-sectional study (March to December 2015), 70 rodents were trapped with live traps baited with fresh cucumber and walnut. Identification of rodents was based on their skull and tooth structures as presented by Etemad.Citation18
Figure 1 Map of Iran showing the geographical location of Ardabil Province and the study area, Meshgin-Shahr.
Brain samples were collected, preserved in ethanol 40%, and transferred to the protozoology laboratory of the Public Health School of Tehran University of Medical Sciences for molecular evaluation. To avoid cross-contamination, a sterile scalpel was used to collect each sample. All procedures were approved by the Animal Ethics Committee of Kermanshah University of Medical Sciences, Iran (No. code: 94065) and performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
DNA Extraction And PCR Amplification
Genomic DNA from brain tissues of the rodents was extracted using the Qiagen DNA Minikit (Qiagen, Venlo, The Netherlands) according to the manufacturer’s recommendations and stored at −20°C until further use. Polymerase chain reaction (PCR) was performed to detect the T. gondii and N. caninum DNA using the specific primers targeted B1 and Nc5, respectively.Citation19,Citation20 DNA extracted from RH strain tachyzoites, NC-1 strain tachyzoites and bidistilled water was used as the positive control for T. gondii and N. caninum and the negative control for PCR reactions, respectively. PCR reaction was performed in 15 µL volume including 1.5 mM MgCl2, 200 µM of each dNTP, 0.5 µM of each primer, 1X PCR buffer, 1 U Taq DNA polymerase and 100 ng of T. gondii or N. caninum DNA. Amplification was conducted in a thermal cycler (Eppendorf, Germany) with an initial 5 mins denaturation at 95°C, followed by 35 amplification cycles, denaturation at 94°C for 45 s, 45 s annealing at specific annealing temperature for each primer and elongation at 72°C for 1 min, followed by a final extension step at 72°C for 5 mins. Annealing temperature was 61°C for gene B1 and 67°C for Nc5. PCR products were electrophoresed in a 1.5% agarose gel stained with Simply Safe (Eurx, Cat. No. E4600-01).
For data analysis, SPSS software (ver. 16.0) was used. The chi-square test was used to examine the relationships between rodent sex and the presence of T. gondii DNA. The P < 0.05 was considered as statistically significant.
Results
Seventy small rodents, including the three species of Meriones persicus, Mus musculus and Cricetulus migratorius, were collected. The most abundant species in the present study was Meriones persicus (71.43%). Thirty rodents were female and 40 were male. Totally, 5/70 (7.14%) brain samples of rodents were found positive for the T. gondii B1 gene (). The prevalence of Toxoplasma infection was higher in female rodents (4.28%) compared to male rodents (2.86%). There was no significant difference between rodent sex and the presence of T. gondii DNA. One of the positive T. gondii amplicons was sequenced and had 100% nucleotide similarity with T. gondii isolates. The nucleotide sequence was deposited in GenBank under the accession number: MK184480. None of the rodents tested positive for N. caninum by PCR. Frequency of infection of N. caninum and T. gondii in the brain tissue of rodents is summarized in
Figure 2 PCR amplification of the B1 gene.
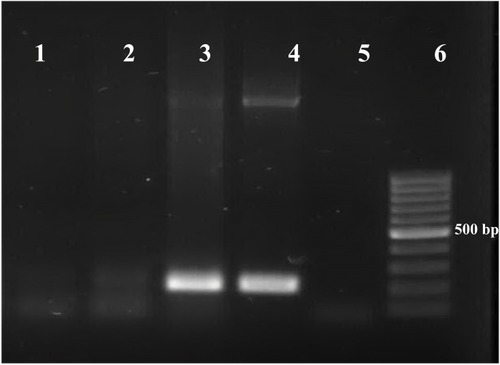
Table 1 Frequency Of Infection Of Neospora caninum And Toxoplasma gondii In The Brain Tissue Of Rodents In Meshgin-Shahr District, Iran
Discussion
Our increased knowledge of the parasitological diseases of rodents in different parts of the world has a fundamental role in the evaluation of environmental health conditions and the assessment of health plans aimed at preventing certain prevalent infectious diseases in human and animal populations.
In the current investigation, T. gondii infection was detected in 7.1% of the rodents by molecular analysis, which is comparable to the results of Saki and KhademvatanCitation21 in rodents of Ahvaz District in southwestern Iran. Thomasson et alCitation22 reported a prevalence of up to 40.78% for T. gondii in Apodemus sylvaticus in England. In a study by Fuehrer et al,Citation23 the DNA of T. gondii was detected in 0.7% of common voles and 4.7% of water voles in the western part of Australia.
Molecular surveys conducted in Mus musculus on the Czech-German border showed N. caninum in 13 out of 360 animals (3.6%).Citation6 A study in Mexico showed a N. caninum prevalence of 77% in Mus musculus, 71% in Spermophilus variegatus, and 50% in Rattus norvegicus.Citation7 Dellarupe et alCitation24 did not detect the DNA of N. caninum in any of the sampled rodents.
In the current study, none of 70 mouse brains was positive for N. caninum DNA in molecular surveys. Several factors may have contributed to the failure to identify the N. caninum DNA in rodents in this study. For example, Barratt et alCitation25 suggested that screening wild rodents for the presence of cysts or N. caninum DNA in the brain alone would result in the under-reporting of the infection. According to Ferroglio et al,Citation26 the brain tissue of only 4 of 25 rodents was positive for DNA of N. caninum, whereas 19 muscle samples and 6 kidney samples showed evidence of exposure to infection. However in this study, it was not possible to detect N. caninum DNA from the tissue samples due to our financial limitations.
The district of Meshgin-Shahr is considered the coldest province in Iran with a very cold climate in the winter and a mild climate in the summer; therefore, decreased oocyst survival and sporulation under cool conditions could explain the low infection rates seen in the current study. Some studies suggest that cohabitation of rodent with the definitive hosts of N. caninum is a putative risk factor for seropositivity due to high level of exposure to oocysts.Citation7,Citation27 Therefore, low prevalence rates in rodents can be associated with different distribution patterns of host species such as cats, dogs and livestock, which were found to be largely infected by N. caninum.
In this study, only one of the positive T. gondii amplicons was sequenced and had 100% nucleotide similarity with T. gondii isolates. Sequencing of the other four PCR T. gondii-positive samples was not successful, which could be explained by the small amount of T. gondii DNA in the samples. It can be concluded that, although the results of the current study indicated a low risk of T. gondii and N. caninum infection among rodents of this district, the infection could be much more widespread in animals; the prevalence might be underestimated due to low DNA concentrations in samples. In addition, only a few cases of Toxoplasma infection might develop into clinical lesions. Moreover, we must be cautious about the interpretation of our results, as different studies have used different genetic markers to detection T. gondii and N. caninum infection and these markers have different power to detection parasite.
Rodent plays an important role in the life cycle of T. gondii and N. caninum because they are generally preyed by domestic and feral cats and dogs hence can spread the parasite to humans and other animals. Besides, hunting and eating of rodents by man as an ancient practice in some nations increased the risk of acquiring zoonotic diseases such as toxoplasmosis and neosporiasis.Citation28 However, to the best of the authors’ knowledge, this research is the first study of Toxoplasma and Neospora prevalence in rodents in the Meshgin-Shahr District. Further research is needed to understand the role of these rodent species in the epidemiology of such protozoan pathogens to humans and livestock in this area. In addition, we recommend evaluating other molecular markers for the detection of T. gondii and N. caninum infection in rodent.
Acknowledgment
This study received financial support from Kermanshah University of Medical Sciences, Kermanshah, Iran (Grant No. 94065).
Disclosure
The authors report no conflicts of interest in this work.
References
- Wilson DE, Reeder DM. Mammal Species of the World: A Taxonomic and Geographic Reference. 3nd ed. Baltimore, Maryland: Johns Hopkins University Press; 2005.
- Krücken J, Blümke J, Maaz D, et al. Small rodents as paratenic or intermediate hosts of carnivore parasites in Berlin, Germany. PLoS One. 2017;12(3):e0172829. doi:10.1371/journal.pone.017282928278269
- Chaisiri K, Chaeychomsri W, Siruntawineti J, Ribas A, Herbreteau V, Morand S. Diversity of gastrointestinal helminths among murid rodents from northern and northeastern Thailand. Southeast Asian J Trop Med Public Health. 2012;43(1):21–28.23082550
- de Barros LD, Miura AC, Minutti AF, Vidotto O, Garcia JL. Neospora caninum in birds: a review. Parasitol Int. 2018;67:397–402. doi:10.1016/j.parint.2018.03.00929614327
- Torrey EF, Yolken RH. Toxoplasma oocysts as a public health problem. Trends Parasitol. 2013;29(8):380–384. doi:10.1016/j.pt.2013.06.00123849140
- Hůrková-Hofmannová L, Qablan M, Jurankova J, Modrý D, Pialek J. A survey of Toxoplasma gondii and Neospora caninum infecting house mice from a hybrid zone. J Parasitol. 2014;100(1):139–141. doi:10.1645/13-255.123927367
- Medina-Esparza L, Macías L, Ramos-Parra M, Morales-Salinas E, Quezada T, Cruz-Vázquez C. Frequency of infection by Neospora caninum in wild rodents associated with dairy farms in Aguascalientes, Mexico. Vet Parasitol. 2013;191(1–2):11–14. doi:10.1016/j.vetpar.2012.08.00722989953
- Dubey J, Schares G, Ortega-Mora L. Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev. 2007;20(2):323–367. doi:10.1128/CMR.00031-0617428888
- Flegr J, Prandota J, Sovičková M, Israili ZH. Toxoplasmosis – a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS One. 2014;9(3):e90203.24662942
- Nazari N, Bozorgomid A, Janbakhsh A, Bashiri F. Toxoplasma gondii and human immunodeficiency virus co-infection in western Iran: a cross sectional study. Asian Pac J Trop Med. 2017;10(10):58–62.
- Oshiro LM, Motta-Castro ARC, Freitas SZ, et al. Neospora caninum and Toxoplasma gondii serodiagnosis in human immunodeficiency virus carriers. Rev Soc Bras Med Trop. 2015;48(5):568–572. doi:10.1590/0037-8682-0151-201526516966
- Calero-Bernal R, Horcajo P, Hernández M, Ortega-Mora LM, Fuentes I. Absence of Neospora caninum DNA in Human Clinical Samples. Spain Emerg Infect Dis. 2019;25(6):1226–1227. doi:10.3201/eid2506.18143131107232
- Abdoli A, Arbabi M, Pirestani M, et al. Molecular assessment of Neospora caninum and Toxoplasma gondii in hooded crows (Corvus cornix) in Tehran, Iran. Comp Immunol Microbiol Infect Dis. 2018;57:69–73. doi:10.1016/j.cimid.2018.06.00830017081
- Sayari M, Namavari M, Mojaver S. Seroprevalence of Neospora caninum infection in free ranging chickens (Gallus domesticus). J Parasit Dis. 2016;40(3):845–847. doi:10.1007/s12639-014-0590-827605795
- Yakhchali M, Bahrami M, Asri-Rezaei S, Bokaie S. The enzymes and electrolytes profiles in sera of Iranian stray dogs naturally infected with Neospora caninum. Annals Ann Parasitol. 2017;63(1):63–68.
- Khademvatan S, Foroutan M, Hazrati-Tappeh K, et al. Toxoplasmosis in rodents: a systematic review and meta-analysis in Iran. J Infect Public Health. 2017;10(5):487–493. doi:10.1016/j.jiph.2017.01.02128237696
- Seifollahi Z, Sarkari B, Motazedian MH, Asgari Q, Ranjbar MJ, Abdolahi Khabisi S. Protozoan parasites of rodents and their zoonotic significance in Boyer-Ahmad District, Southwestern Iran. Vet Med Int. 2016;2016:5.
- Etemad E. Rodents and Identification Key of Them. Vol. I Tehran: National Association of Natural Source Protection and Human Environment; 1979.
- Burg JL, Grover CM, Pouletty P, Boothroyd J. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J Clin Microbiol. 1989;27(8):1787–1792.2768467
- Müller N, Zimmermann V, Hentrich B, Gottstein B. Diagnosis of Neospora caninum and Toxoplasma gondii infection by PCR and DNA hybridization immunoassay. J Clin Microbiol. 1996;34(11):2850–2852.8897199
- Saki J, Khademvatan S. Detection of Toxoplasma gondii by PCR and mouse bioassay in rodents of Ahvaz District, Southwestern Iran. Biomed Res Int. 2014;2014:1–5. doi:10.1155/2014/383859
- Thomasson D, Wright E, Hughes J, et al. Prevalence and co-infection of Toxoplasma gondii and Neospora caninum in Apodemus sylvaticus in an area relatively free of cats. Parasitology. 2011;138(9):1117–1123. doi:10.1017/S003118201100090421756421
- Fuehrer H-P, Blöschl I, Siehs C, Hassl A. Detection of Toxoplasma gondii, Neospora caninum, and Encephalitozoon cuniculi in the brains of common voles (Microtus arvalis) and water voles (Arvicola terrestris) by gene amplification techniques in western Austria (Vorarlberg). Parasitol Res. 2010;107(2):469–473. doi:10.1007/s00436-010-1905-z20480373
- Dellarupe A, Fitte B, Pardini L, et al. Toxoplasma gondii and Neospora caninum infections in synanthropic rodents from Argentina. Rev Bras Parasitol Vet. 2019;28(1):113–118. doi:10.1590/S198430916257
- Barratt J, Al Qassab S, Reichel MP, Ellis JT. The development and evaluation of a nested PCR assay for detection of Neospora caninum and Hammondia heydorni in feral mouse tissues. Mol Cell Probes. 2008;22(4):228–233. doi:10.1016/j.mcp.2008.03.00118420378
- Ferroglio E, Pasino M, Romano A, Grande D, Pregel P, Trisciuoglio A. Evidence of Neospora caninum DNA in wild rodents. Vet Parasitol. 2007;148(3–4):346–349. doi:10.1016/j.vetpar.2007.06.03117651897
- Zanet S, Sposimo P, Trisciuoglio A, Giannini F, Strumia F, Ferroglio E. Epidemiology of Leishmania infantum, Toxoplasma gondii, and Neospora caninum in Rattus rattus in absence of domestic reservoir and definitive hosts. Vet Parasitol. 2014;199(3–4):247–249. doi:10.1016/j.vetpar.2013.10.02324295953
- Suwannarong K, Chapman RS, Lantican C, Michaelides T, Zimicki S. Hunting, food preparation, and consumption of rodents in Lao PDR. PLoS One. 2015;10(7):e0133150. doi:10.1371/journal.pone.013315026196134
