Abstract
Purpose
Parrot bornavirus is the etiological agent of Parrot bornavirus syndrome, also referred to and comprising proventricular dilatation disease or PDD, macaw wasting disease, enteric ganglioneuritis and encephalitis, and avian ganglioneuritis. It has been suggested that nonsteroidal anti-inflammatory drugs may be able to ameliorate this disease. Therefore, this study investigated the effects of two commonly used nonsteroidal anti-inflammatory drugs, celecoxib and meloxicam, on cockatiels experimentally inoculated with Parrot bornavirus-2 (PaBV-2).
Materials and methods
Twenty-seven cockatiels were randomized into 3 groups of 9 birds, matched with respect to historical PaBV shedding, weight, and sex. The cockatiels were inoculated with cell culture-derived PaBV-2 by the intranasal and intramuscular routes. Beginning at 23 days post-inoculation, birds in each group received oral treatment once daily with placebo, meloxicam (1.0 mg/kg), or celecoxib (10.0 mg/kg).
Results
Within 33–79 days post-inoculation, 2 birds died and 6 birds were euthanized based on neurological or gastrointestinal signs consistent with Parrot bornavirus syndrome: 2 birds were euthanized in the placebo group, 1 bird died and 1 bird was euthanized in the meloxicam-treated group, and 1 bird died and 3 birds were euthanized in the celecoxib-treated group. Of these 8 birds, black intestinal contents were found upon necropsy in 2 birds of the meloxicam-treated group and 2 birds of the celecoxib-treated group. At day 173 (±2) post-inoculation, the remaining 19 birds were euthanized. Necropsy and histopathology showed lesions characteristic of Parrot bornavirus syndrome in 23 cockatiels. Histopathologic lesions were present in birds of all 3 groups. There was no statistical difference between the groups nor was there a statistical difference among the 3 treatment groups in the detection of PaBV RNA and PaBV nucleoprotein using RT-PCR and immunohistochemistry, respectively.
Conclusion
Meloxicam and celecoxib treatments do not appear to alter the clinical presentation, viral shedding, gross lesions, histopathology, or viral distribution. Treatment with NSAIDs may cause gastrointestinal toxicity in cockatiels experimentally inoculated with PaBV-2.
Introduction
Parrot bornaviruses 1–8 (PaBV- 1-8) are the causal agents of a progressive fatal avian neurologic syndrome referred to as Parrot bornavirus syndrome, a complex of clinical problems that can include proventricular dilatation disease or PDD, macaw wasting disease, enteric ganglioneuritis and encephalitis, avian ganglioneuritis, or neurological deficits.Citation1–Citation5 Parrot bornavirus syndrome primarily affects captive birds of the Psittacidae and Cacatuidae family, such as cockatoos, cockatiels, lovebirds, conures, parakeets (other than Budgerigars), and especially macaws, but has been diagnosed in over 80 bird species.Citation6–Citation8 Tissue distribution of the virus following infection is extensive. Parrot Bornavirus and viral RNA are detected in the brain, eye, retinal nerve, spinal cord, heart, adrenal glands, kidneys, and intestines.Citation9,Citation10 The disease is characterized by infection of the central and peripheral nervous system and other organs with PaBV, leading to lymphoplasmacytic infiltration of those tissues, and culminating in nervous system disorders and gastrointestinal malfunctions.Citation8–Citation14
No effective treatment for PaBV infection or Parrot bornavirus syndrome currently exists. Experimentally, IFN-α inhibits virus infection and reduces viral load in quail cell cultureCitation15 and ribavirin inhibits transcription and reduces the viral load in cultured duck embryo fibroblasts.Citation16,Citation17 Symptomatic treatment and management are the only currently recommended therapies for Parrot bornavirus syndrome.Citation7,Citation18 Based on clinical and pathological signs of PaBV infection, disease may result from inflammatory reactions to the virus within the brain, nerves, and other tissues.Citation10–Citation12 Thus, nonsteroidal anti-inflammatory drugs (NSAIDs) and immunosuppressive drugs could inhibit or reduce the inflammation caused by PaBV infection and lessen the severity of the clinical disease.Citation7,Citation18
The use of NSAIDs is reported to reduce the severity of clinical signs in birds affected with PDD.Citation7 Celecoxib and meloxicam are NSAIDs commonly used for the symptomatic treatment of birds diagnosed with Parrot bornavirus syndrome.Citation18–Citation21 However, meloxicam did not reduce the clinical signs in cockatiels experimentally infected with PaBV-4 and in fact may have exacerbated the disease progression.Citation22
Due the discrepancies in reported outcomes and the limited controlled studies on the effectiveness of NSAIDs and dearth of research comparing celecoxib and meloxicam in treating PaBV infected birds, the objective of this study was therefore to evaluate the effects of celecoxib and meloxicam treatment on clinical signs, viral shedding, and pathology of cockatiels infected with PaBV-2. We hypothesized that NSAID administration will reduce the severity of disease in infected cockatiels.
Materials And Methods
Parrot Bornavirus
PaBV-2 was isolated from the brain of experimentally infected cockatiels (Nymphicus hollandicus).Citation14 Virus for inoculation was grown as previously described.Citation23 Briefly, duck embryo fibroblast cultures were inoculated with stock virus and maintained in Dulbecco’s modified eagle medium (Gibco®, Life Technologies Co., Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum (Gibco®, Life Technologies Co) at 37°C in an atmosphere of 5% CO2. After 3 days of incubation, cells were harvested, divided into 1.0 mL aliquots, and stored at −80°C. Virus was confirmed to be PaBV-2 by RNA extraction and RT-PCR analysis followed by sequence analysis of the PCR product, as described below. Birds were inoculated using a combined intranasal and intramuscular administration of infected cells containing 8 × 104 focus forming units of the virus.
Nonsteroidal Anti-Inflammatory Drugs
A suspension containing 1.0 mg/mL meloxicam was made by crushing three meloxicam 15.0 mg tablets (Lupin, Pharmaceuticals, Inc. Baltimore, MD, USA) using a mortar and pestle, and dissolving the powder in 1 mL deionized water. Ten millilitre of Ora-Plus (Perrigro® Co., Dublin, Ireland) suspending vehicle was added, and then Ora-Sweet (Perrigro® Co.) was added to the suspension to obtain a final volume of 45.0 mL. A suspension containing 10.0 mg/mL celecoxib was made by adding the contents of nine celecoxib 50.0 mg capsules (Pfizer Inc., Mission, KS, USA) to 1.0 mL deionized water. Ten millilitre of Ora-Plus suspending vehicle was added, and then Ora-Sweet added to the suspension to obtain a final volume of 45.0 mL. All solutions were stored at 4ºC and prepared fresh every 30 days. Prior to administration, all solutions were warmed to room temperature.
Animals
Twenty-seven cockatiels (Nymphicus hollandicus), ranging from 79 to 145 g (mean 101 g), were used. The birds were assessed as healthy by physical examination and medical history. The cockatiels were quarantined for 60 days, during which time each bird was tested three times over 4 weeks for psittacid herpesvirus (genotype 1-4), Chlamydia spp. and Macrorhabdus ornithogaster (avian gastric yeast). Birds needed to have all tests negative to be included in the study. The cockatiels were housed 14 or 13 birds per cage, with a light-dark cycle of 12 hrs and a room temperature of 23.3 (±5.0)°C, at the Schubot Exotic Bird Health Center aviary, Texas A&M University. Birds were fed a 1/6 cup per bird of premium daily FruitBlend with natural fruit flavors (ZuPreem®, Shawnee, KS, USA), and had access to tap water ad libitum. An animal use protocol detailing the experimental protocol was reviewed and approved by the Texas A&M University Office of Research Compliance, complying with guidelines included in the National Research Council of the National Academies’ publication Guide for the Care and Use of Laboratory Animals, 8th edition.
Experimental Protocol
Birds were matched with respect to historical shedding of PaBV, weight, and sex, and randomly assigned into three groups of nine birds each: Group 1 birds (placebo) were inoculated with PaBV-2 and beginning 23 days post-inoculation were treated orally, once daily with only the delivery solution (water, Ora-plus, and Ora-Sweet). Group 2 birds (meloxicam treated) were inoculated with PaBV-2 and beginning 23 days post-inoculation were treated orally, once daily with 1.0 mg/kg meloxicam. Group 3 birds (celecoxib treated) were inoculated with PaBV-2 and beginning 23 days post-inoculation were treated orally, once daily with 10.0 mg/kg celecoxib. All treatments were administered prior to morning feeding. The dosage of drug administered was recalculated after each weekly weighing. The drug treatment administered to each group was unknown to the drug administrator/evaluator, assay technician, and pathologist until completion of the study.
Birds were observed daily for clinical signs and deviations from normal behavior: these included feather fluffing, bowed head, overly quiet, almond-shaped eyes, over-eating, lethargy, and reluctance to fly. Prior to inoculation, cloacal swabs were collected twice; weights and body condition scores (BCS) were assessed once. After viral inoculation, birds were weighed and BCS was assessed weekly; cloacal swabs were collected every third week and stored at −80ºC until assayed by RT-PCR. Body condition scores were determined by assessing the region of the keel and pectoral muscle. Body condition scores ranged from 1 to 5, thin to obese, respectively.Citation24 A bird was withdrawn from the study and euthanized if it met the following pre-established criteria: weight loss >20% of the initial weight; BCS of 1; or as recommended by the attending veterinarian.
On day 173 (±2) following experimental inoculation [day 150 (±2) of NSAID treatment or placebo treatment], surviving birds were anesthetized with 5% isoflurane in 100% oxygen; weight, BCS, cloacal swab, and urine were collected. Urine was collected as previously described by Heatley et al.Citation25 Immediately after sample collection while the bird was still in deep anesthesia, the bird was humanely killed by chamber exposure to 100% CO2. A complete necropsy was performed immediately and gross lesions were recorded. Paired samples of heart, liver, feather follicle, spleen, crop, proventriculus, ventriculus, intestine, gonad, pancreas, adrenal gland, kidney, lung, spinal cord, brain, eye, aqueous humor, optic nerve, brachial plexus, and sciatic nerve were collected and stored at −80ºC for later analyses by RT-PCR. The remainder of each organ was placed in 10% neutral-buffered formalin for histologic examination and immunohistochemistry (IHC) testing.
RT-PCR
Tissue, urine, and cloacal swabs were tested for the presence of viral RNA by RT-PCR as previously described.Citation23 All samples were tested in duplicate for both the matrix protein and phosphoprotein. Samples were considered negative for cycle threshold (CT) ≥37.0. If a sample was positive for only one of the two proteins, the sample was retested. Results were analyzed using the Sequence Detection System, Version 2.4.1 (SDS 2.4) software (Life Technologies, Thermo Fisher, Carlsbad, CA, USA).
Histopathology & Immunohistochemistry
Tissue samples were fixed in 10% neutral-buffered formalin, processed overnight, embedded in paraffin, sectioned at 4 µm, stained with haematoxylin and eosin (H & E), and examined by light microscopy according to standard procedure. Immunohistochemistry, to demonstrate the presence of PaBV nucleoprotein, was performed on tissues obtained at necropsy according to previously described methods.Citation26 The reviewing pathologist assigned a semiquantitative viral score to the tissue sample using the following ordinal scale: none detected (-), small quantity detected (+), moderate quantity detected (++), large quantities detected (+++).
Statistical Analysis
The Gehan–Breslow method was used to analyze differences in survival between the treatment groups. Two-way repeated measures Analysis of Variance (ANOVA) was used to compare weight changes between the treatment groups and day over the experimental time course. The Kruskal–Wallis One Way ANOVA on ranks was used to ascertain differences in histological findings and IHC results. A P ≤ 0.05 was considered statistically significant. Sigma Plot version 10.0.1 was used for performing all statistical analyses (Systat Software, Inc., San Jose, CA, USA).
Results
Clinical Observations
Survival did not differ significantly between the 3 study groups (). In the placebo group (group 1), 2 of 9 birds were euthanized prior to the end of the study on 54 and 61 days post-inoculation. In the meloxicam-treated group (group 2), 2 of 9 birds were euthanized or found dead on 33 and 79 days post-inoculation. In the celecoxib-treated group (group 3), 4 of 9 birds were euthanized or found dead on 37, 43, 45, and 74 days post-inoculation. Of the 8 birds that died prior to study completion, 2 were found dead, one each in the meloxicam and the celecoxib-treated groups, while the remaining 6 showed neurological or gastrointestinal signs characteristic of PaBV infection ().
Figure 1 Survival analysis of cockatiels (Nymphicus hollandicus) inoculated with PaBV-2 and treated with either placebo, meloxicam (1.0 mg/kg), or celecoxib (10.0 mg/kg).
Notes: The Gehan–Breslow statistic for survival curve was used to generate the survival analysis. Birds were experimentally inoculated with PaBV-2 on day 0. On day 23 post-inoculation (dashed arrow), birds started once daily, oral administration with the following: placebo control group (solid black line), meloxicam (dotted grey line) and celecoxib (dashed grey line).
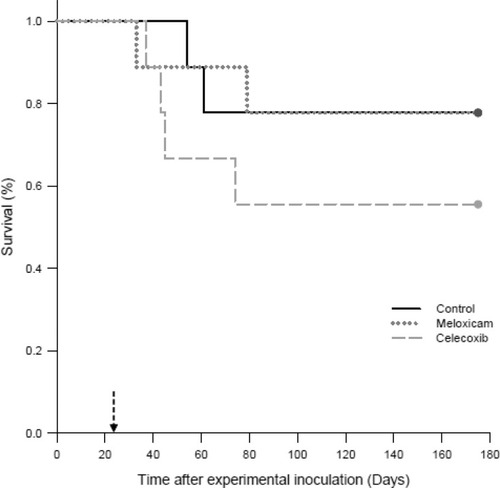
Table 1 Cockatiels Euthanized Or Found Dead Prior To Study’s End
Body weights did not differ significantly between the study groups over the study period. Within each treatment group, significant daily differences in weight were noted. All groups had a reduction in weight on day 7 after treatment, with or without NSAID, was administered. Between 7 and 47 days, the body weights of the NSAID-treated groups were significantly lower than the weights prior to inoculation or the commencement of the NSAID treatment; however, from 54 days after treatment until study’s end, there was no significant difference between their weight prior to inoculation or treatment. Body condition scores between or within the groups did not differ significantly during the study period.
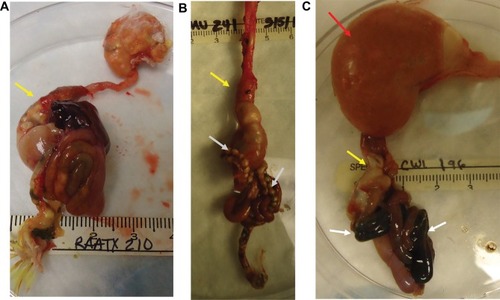
Most birds that died or were euthanized early tended to have lost weight and BCS over the course of the study. A single bird from the meloxicam group was an exception. At the time of euthanasia, this bird had a greatly increased weight compared to other birds, but had a low BCS. Necropsy findings of a profoundly dilated crop and proventriculus that were full of feed were most likely the cause of the increased weight ().
Figure 2 Gross necropsy findings in cockatiels (Nymphicus hollandicus) inoculated with PaBV-2 and treated with either placebo, meloxicam (1.0 mg/kg), or celecoxib (10.0 mg/kg). (A) Bird 15, meloxicam-treated group: euthanized on day 173 post-inoculation. Moderate dilation of proventriculus (yellow arrow). (B) Bird 21, celecoxib-treated group: euthanized on day 45 post-inoculation. Mild dilation of proventriculus (yellow arrow) with undigested seed present in the intestines (grey arrows). (C) Bird 22, celecoxib-treated group: euthanized on day 74 post-inoculation. Severe dilation of crop (red arrow), mild dilation of proventriculus (yellow arrow), and blacken intestinal content (grey arrows).
Detection Of PaBV RNA
The results of viral RNA detection in cloacal swab samples are summarized in . Prior to experimental inoculation, cloacal swab testing for viral RNA was negative for all birds, with the exception of one cockatiel that had a positive cloacal swab on day −17 of inoculation, but was negative on days −31, 13, and 20 post-inoculation. PaBV shedding was first detected 42 days post-inoculation in groups 2 and 3. At study end, all birds that had not died or been euthanized early for humane concerns had positive cloacal swabs, with the exception of one bird in group 2, which was negative throughout the study period. The cumulative number of birds with positive cloacal swabs by the end of the study was 8/9, 6/9, and 7/9 in groups 1, 2, and 3, respectively.
Table 2 Detection Of Viral RNA In Cloaca Samples From Cockatiels (Nymphicus hollandicus) Inoculated With PaBV-2 And Treated With Placebo, Meloxicam (1.0 mg/kg), Or Celecoxib (10.0 mg/kg)
At the study’s end, viral RNA was detected in the midbrain, hindbrain, cerebellum, forebrain, kidney, and urine of all surviving birds, except for one. There was no significant difference in the amount of detectable viral RNA between the treatment groups.
Necropsy And Histopathology
The predominant gross abnormalities seen at necropsy were dilation of the crop and proventriculus and an enlarged, dilated heart with thin walls (–). However, these abnormalities were not consistently present nor of consistent severity. Birds that were euthanized or died early in the study had more pronounced crop and proventricular dilatation than birds that survived until day 173 (±2) post-inoculation. Black intestinal contents were present in 2 birds in group 2 and 2 birds in group 3; these 4 birds had been euthanized prior to the study’s endpoint ( and ). Other abnormalities such as liver mottling, enlarged spleen, mild intestinal distension, and pale pancreas were occasionally noted, with no predilection for any group. Histopathological changes occurred in many tissues, but no significant differences in the severity of the lesions occurred based on group (). The lesions observed included: lymphoplasmacytic myenteric ganglioneuritis in the crop, proventriculus, ventriculus and intestines; multifocal dilation of tubules/interstitial inflammation, fibrosis and/or mineralization, and scattered lymphoid nodule formation in the kidney; lymphoplasmacytic infiltration within the epicardial, myocardial, and/or Purkinje cells of the heart; and, lymphoplasmacytic perivascular cuffing in the central nervous system (). Though infrequent, histopathological changes were also seen in the liver, pancreas, lung, spleen, optic nerves, and adrenal glands.
Figure 3 Histologic findings in cockatiels (Nymphicus hollandicus) inoculated with PaBV-2 and treated with either placebo, meloxicam (1.0 mg/kg), or celecoxib (10.0 mg/kg). (A) Lymphoplasmacytic infiltration of serosal ganglia of the crop. Bird 3, placebo, euthanized 173 (±2) days post-inoculation. (B) Moderate lymphoplasmacytic infiltration of subserosal ganglia in the proventriculus. Bird 9, placebo, euthanized 173 (±2) days post-inoculation. (C) Moderate lymphoplasmacytic infiltration of subserosal ganglia in the ventriculus. Bird 17, meloxicam treated, euthanized 173 (±2) post-inoculation. (D) Lymphocytic infiltration in the interstitium of kidney with formation of a lymphoid nodule. Bird 3, placebo, euthanized 173 (±2) days post-inoculation. (E) Lymphoplasmacytic infiltration in the epicardial ganglia of the heart. Bird 25, celecoxib treated, euthanized 173 (±2) days post-inoculation.
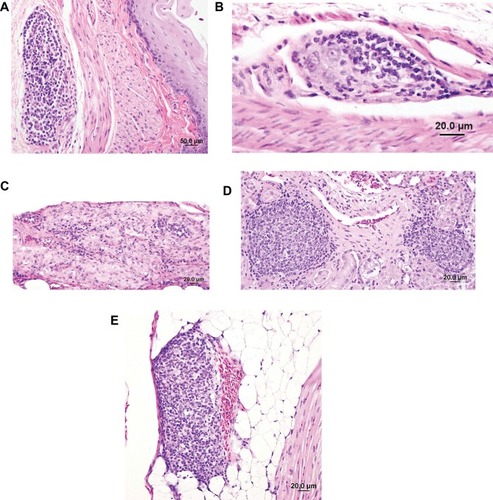
Table 3 Distribution Of Histological Lesions In Cockatiels (Nymphicus hollandicus) Inoculated With PaBV-2 And Treated With Placebo, Meloxicam (1.0 mg/kg), Or Celecoxib (10.0 mg/kg)
Immunohistochemistry
Distribution or amount of viral nucleoprotein did not differ statistically between the three groups (). Virus (PABV-2) was predominantly detected in the brain, heart, gastrointestinal tract and kidneys but also detected in liver, pancreas, lung, spleen, adrenal optic nerve, uropygial gland, cloaca, gonads, and skin/feather follicles. Skeletal muscle was the only tissue consistently negative for the virus. No virus was detected in any tissues of four birds: one in group 1, two in group 2, and one in group 3.
Table 4 Tissue Distribution And Relative Amount Of PaBV Nucleoprotein (N-protein) As Detected By Immunohistochemistry
Discussion
Oral administration of meloxicam, 1.0 mg/kg, or celecoxib, 10.0 mg/kg, once daily for 150 days failed to show differences in the clinical presentation, viral shedding, gross lesions, viral distribution, nor histopathology in cockatiels experimentally inoculated with PaBV-2 when compared to untreated birds. These results agree with previously published experimental study on meloxicam usage,Citation22 but conflict with reports on the treatment of clinically affected birds.Citation19–Citation21 Bird species differences, viral genotype differences, and evaluation criteria may account for the lack of agreement between our studies and previous reports on clinical cases.
In this study, 4 cockatiels treated with NSAIDs had black intestinal material that may have been autolyzed blood, and all 4 were euthanized after 10 to 56 days of NSAID treatment. In a prior study that treated PaBV inoculated cockatiels with meloxicam, 1 bird had blood-filled, blackened intestine, while another bird had black material that may have been autolyzed blood in its intestines.Citation22 In mammals, gastrointestinal abnormalities, such as ulcers and bleeding, and renal necrosis due to ischemia are major features of NSAID toxicity; however, these have not been reported as major side effects of NSAID use in birds.Citation27–Citation35 The combined effect of PaBV induced pathological changes and NSAID treatment may exacerbate gastrointestinal irritation and toxicity of the NSAID and may increase the risk for gastrointestinal bleeding.
The administration of NSAIDs to birds infected with PaBV did not affect their weight nor BCS. Birds that displayed severe clinical signs and either were removed early or died, had decreased weight and BCS. This is consistent with the gastrointestinal dysfunction that leads to starvation in the birds.Citation7,Citation14,Citation22,Citation36 It is interesting to note that while most birds infected with PaBV decreased in weight, one bird in the meloxicam group had gained weight at the time of its removal from the study. Upon necropsy, the crop and proventriculus were distended with feed. Presumably, the weight gain was due to the proventricular contents. This reinforces that bird body weight alone should not be used to assess disease progression or severity.
Survival and early removal of the birds from the study were not statistically different between the groups; however, the number of birds with clinical conditions warranting early removal was twice as high in the celecoxib-treated group as compared to the meloxicam-treated or placebo group (). In addition to the NSAIDs having no apparent effect on overall mortality, the time at which cockatiels started to show clinical signs and had to be removed early (33–79 days post-PaBV inoculation) was not affected by NSAID treatment and was consistent with the time period seen in other studies of PaBV-2 experimentally inoculated birds.Citation3,Citation9,Citation14,Citation22,Citation36,Citation37
Gross pathology showed no significant difference between the three treatment groups and all groups had clinical presentations indicative of Parrot bornavirus syndrome, with a dilated proventriculus being most represented, as described in the literature.Citation3,Citation9,Citation14,Citation22,Citation36,Citation37 NSAID treatment in cockatiels inoculated with PaBV-2 was expected to decrease lesion distribution and tissue inflammatory response; however, in this study treatment lacked any significant impact.
Shedding of PaBV-2 was not affected by the administration of NSAIDs. In fact, treated birds tended to shed earlier than the placebo group, though there was no significant difference. Shedding was first detected on day 42 post-inoculation and became more consistent by day 63 post-inoculation, a time period that corresponded with previous work in our lab. At the end of the study, only one bird had not shed virus. Previous research has shown that there is not a 100% correlation between histopathology and virus detection between and among tissues and fluids.Citation25,Citation38
One bird in the meloxicam-treated group was negative for the presence of PaBV by RT-PCR and IHC throughout the study. However, at necropsy, its proventriculus was dilated and contained undigested seed. Histopathology showed perivascular cuffing with increased glial cells in the brain and lymph nodules in the mucosa, ganglia, and serosa of the ventriculus. Tissues may be negative for viral antigens by IHC but the animal can still have a subclinical or asymptomatic infection.Citation38 Cryptosporidium was identified in the proventriculus of this bird which may have been a factor in causing some of these lesions.Citation39
Conclusion
The administration of meloxicam or celecoxib failed to alter the progression or severity of clinical signs, gross or histopathological changes, viral shedding, or distribution of viral RNA in cockatiels infected with PaBV-2. Caution should be stressed when prescribing NSAIDs in birds with gastrointestinal dysfunction, such as may occur with PaBV infection, due to the potential for gastrointestinal irritation, bleeding, or other unwanted side effects of NSAIDs.
Acknowledgments
The authors thank Debra Turner and Dr. Jordan Gentry for technical assistance. Supported in part by the Schubot Exotic Bird Health Center. The open access publishing fees for this article have been covered by the Texas A&M University Open Access to Knowledge Fund (OAKFund), supported by the University Libraries and the Office of the Vice President for Research.
Disclosure
All authors declare no conflicts of interest relevant to the research performed.
References
- Honkavuori KS, Shivaprasad HL, Williams BL, et al. Novel bornavirus in psittacine birds with proventricular dilatation disease. Emerg Infec Dis. 2008;14(12):1883–1886. doi:10.3201/eid1412.08098419046511
- Kistler AL, Gancz A, Clubb S, et al. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent. Virol J. 2008;5:88–102. doi:10.1186/1743-422X-5-8818671869
- Gray P, Hoppes S, Suchodolski P, et al. Use of avian bornavirus isolated to induce proventricular dilatation disease in conures. Emerg Infec Dis. 2010;16(3):473–479. doi:10.3201/eid1603.09125720202423
- Kuhn JH, Dürrwald R, Bào Y, et al. Taxonomic reorganization of the family Bornaviridae. Arch Virol. 2015;160(2):621–632. doi:10.1007/s00705-014-2276-z25449305
- Philadelpho NA, Rubbenstroth D, Guimaraes MB, et al. Survey of bornaviruses in pet psittacines in Brazil reveals a novel parrot bornavirus. Vet Micro. 2014;174(3–4):584–590. doi:10.1016/j.vetmic.2014.10.020
- Doneley RJT, Miller RI, Fanning TE. Proventricular dilatation disease: an emerging exotic disease of parrots in Australia. Aust Vet J. 2007;85(3):119–123. doi:10.1111/j.1751-0813.2007.00109.x17359314
- Gancz AY, Clubb S, Shivaprasad HL, et al. Advanced diagnostic approaches and current management of proventricular dilatation disease. Vet Clin North Am Exot Anim Pract. 2010;13(3):471–494. doi:10.1016/j.cvex.2010.05.00420682431
- Hoppes S, Gray PL, Payne S, et al. The isolation, pathogenesis, diagnosis, transmission, and control of avian bornavirus and proventricular dilatation disease. Vet Clin North Am Exot Anim Pract. 2010;13(3):495–508. doi:10.1016/j.cvex.2010.05.01420682432
- Piepenbring AK, Enderlein D, Herzog S, et al. Pathogenesis of avian bornavirus in experimentally infected cockatiels. Emerg Infect Dis. 2012;18(2):234–241. doi:10.3201/eid1802.11152522304809
- Leal de Araujo J, Rech RR, Heatley JJ, et al. From nerves to brain to gastrointestinal tract: A time-based study of parrot bornavirus 2 (PaBV-2) pathogenesis in cockatiels (Nymphicus hollandicus). PLoS One. 2017;12(11):e0187797. doi:10.1371/journal.pone.018779729121071
- Ouyang N, Storts R, Tian Y, et al. Histopathology and the detection of avian bornavirus in the nervous system of birds diagnosed with proventricular dilatation disease. Avian Pathol. 2009;38(5):393–401. doi:10.1080/0307945090319103619937526
- Shivaprasad HL, Barr BC, Woos LW, et al. Spectrum of lesions (Pathology) of proventricular dilatation syndrome. Proc Annu Conf Assoc Avian Vet. 1995;505–506.
- Berthane Y, Smith DA, Newman S, et al. Peripheral neuritis in psittacine birds with proventricular dilatation disease. Avian Pathol. 2001;30(5):563–570. doi:10.1080/0307945012007877019184947
- Mirhosseini N, Gray PL, Hoppes S, et al. Proventricular dilatation disease in cockatiels (Nymphicus hollandicus) after infection with a genotype 2 avian bornavirus. J Avian Med Surg. 2011;25(3):199–204. doi:10.1647/2010-030.122216720
- Reuter A, Ackermann A, Kothlow S, et al. Avian bornaviruses escape recognition by the innate immune system. Viruses. 2010;2(4):927–938. doi:10.3390/v204092721994661
- Escandon P, Heatley JJ, Kranz JB, et al. Effects of ribavirin on avian bornavirus in duck embryonic fibroblast cell culture. Proc ICARE Paris. 2015.
- Musser JMB, Heatley JJ, Koinis AV, et al. Ribavirin inhibits parrot bornavirus 4 replication in cell culture. PLoS One. 2015;10(7):e0134080. doi:10.1371/journal.pone.013408026222794
- Hoppes S, Tizard I, Shivaprasad HL. Avian Bornavirus and proventricular dilatation disease. diagnostics, pathology, prevalence, and control. Vet Clin Exot Anim. 2013;16(2):339–355. doi:10.1016/j.cvex.2013.01.004
- Dalhausen B, Aldred S, Colaizzi E, et al. Resolution of the clinical proventricular dilatation disease by cyclooxygenase 2 inhibition. Proc Annu Conf Assoc Avian Vet. 2002;9–12.
- Clubb SL. Clinical management of psittacine birds affected with proventricular dilatation disease. Proc Annu Conf Assoc Avian Vet. 2006;85–90.
- Keller DL, Honkavuori KS, Briese T, et al. Proventricular dilatation disease associated with avian bornavirus in a scarlet macaw (Ara Macao). J Vet Diagn Invest. 2010;22(6):961–965. doi:10.1177/10406387100220061921088184
- Hoppes S, Heatley JJ, Guo JH, et al. Meloxicam treatment in cockatiels (Nymphicus hollandicus) infected with avian bornavirus. J Exot Pet Med. 2013;22(3):275–279. doi:10.1053/j.jepm.2013.08.014
- Guo JH, Payne S, Zhang S, et al. Avian bornaviruses: diagnosis, isolation, and genotyping. Curr Protoc Microbiol. 2014;supplement 34:15i.1–15i.1.33. doi:10.1002/9780471729259.mc15i01s34.
- Tully TN, Dorrestein GM, Jones AK. Handbook of Avian Medicine. 2nd ed. Philadelphia: Saunders/Elsevier; 2009.
- Heatley JJ, Villalobos AR. Avian bornavirus in the urine of infected birds. Vet Med Res Rep. 2012;3:19–23. doi:10.2147/VMRR.s31336
- Weissenböck H, Sekulin K, Bakonyi T, et al. Novel avian bornavirus in a nonpsittacine species (Canary; Serinus canaria) with enteric ganglioneuritis and encephalitis. J Virol. 2009;83(21):11367–11371. doi:10.1128/JVI.01343-0919706702
- Swarup D, Patra RC, Prakash V, et al. Safety of meloxicam to critically endangered Gyps vultures and other scavenging birds in India. Anim Conserv. 2007;10(2):192–198. doi:10.1111/j.1469-1795.2006.00086.x
- Pereira ME, Werther K. Evaluation of the renal effects of flunixin meglumine, ketoprofen and meloxicam in budgerigars (Melopsittacus undulatus). Vet Res. 2007;160(24):844–846. doi:10.1136/vr.160.24.844
- Desmarchelier M, Troncy E, Fitzgerald G, et al. Analgesic effects of meloxicam administration on postoperative orthopedic pain in domestic pigeons (Columba livia). Am J Vet Res. 2012;73(3):361–367. doi:10.2460/ajvr.73.3.36122369527
- Musser JMB, Heatley JJ, Phalen DN. Pharmacokinetics after intravenous administration of flunixin meglumine in budgerigars (Melopsittacus undulates) and Patagonian conures (Cyanoliseus patagonus). J Am Vet Med Assoc. 2013;242(2):2005–2208. doi:10.2460/javma.242.2.205
- Molter CM, Court MH, Cole GA, et al. Pharmacokinetics of meloxicam after intravenous, intramuscular, and oral administration of a single dose to Hispaniolan Amazon parrots (Amazona ventralis). Am J Vet Res. 2013;74(3):375–380. doi:10.2460/ajvr.74.3.37523438111
- Montesinos A, Ardiaca M, Juan-Sallés C, et al. Effects of meloxicam on hematologic and plasma biochemical analyte values and results of histologic examination of kidney biopsy specimens of African grey parrots (Psittacus erithacus). J Avian Med Surg. 2015;29(1):1–8. doi:10.1647/2013-05625867660
- Montesinos A, Ardiaca M, Gilabert JA, et al. Pharmacokinetics of meloxicam after intravenous, intramuscular and oral administration of a signal dose of African grey parrots (Psittacus erithacus). J Vet Pharmacol Ther. 2017;40(3):279–284. doi:10.1111/jvp.1235027597532
- Dhondt L, Devreese M, Croubels S, et al. Comparative population pharmacokinetics and absolute oral bioavailability of COX-2 selective inhibitors celecoxib, mavacoxib and meloxicam in cockatiels (Nymphicus hollandicus). Sci Rep. 2017;7(1):12043. doi:10.1038/s41598-017-12159-z28947805
- Dijkstra B, Guzman DSM, Gustavsen K, et al. Renal, gastrointestinal, and hemostatic effects of oral administration of meloxicam to Hispaniolan Amazon parrots (Amazona ventralis). Am J Vet Res. 2015;76(4):308–317. doi:10.2460/ajvr.76.4.30825815572
- Payne S, Shivaprasad HL, Mirhosseini N, et al. Unusual and severe lesions of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) acting as healthy carriers of avian bornavirus (ABV) and subsequently infected with a virulent strain of ABV. Avian Pathol. 2011;40(1):15–22. doi:10.1080/03079457.2010.53697821331944
- Gancz AY, Kistler AL, Greninger AL, et al. Experimental induction of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) inoculated with brain homogenates containing avian bornavirus 4. Virol J. 2009;6:100. doi:10.1186/1743-422X-6-10019589169
- Raghav R, Taylor M, DeLay J, et al. Avian bornavirus is present in any tissues of psittacine birds with histopathologic evidence of proventricular dilatation disease. J Vet Diagn Invest. 2010;22(4):495–508. doi:10.1177/10406387100220040220622218
- Ravich ML, Reavill DR, Hess L, et al. Gastrointestinal cryptosporidiosis in captive psittacine birds in the United States: a case review. J Avian Med Surg. 2014;28(4):297–303. doi:10.1647/1082-6742-28.4.29725843467
