Abstract
In this retrospective study all suspect bovine intoxications submitted to the California Animal Health and Food Safety Laboratory between January 1, 2000 and December 31, 2011 were reviewed. A total of 1199 cases were submitted, but a diagnosis of intoxication was only established in 13.5% of cases. In these cases, overexposures to minerals, metals, and poisonous plants were determined as the most commonly diagnosed poisonings in cattle in California. Nitrate/nitrite poisoning was the most commonly diagnosed plant-associated intoxication, followed by gossypol and oleander. This study details the diagnostic challenges and treatment options for the most commonly diagnosed intoxications. To ensure proper treatment and prevention of new cases, accurate diagnosis is necessary, and therefore this review provides an essential tool for the food animal practitioner. Available toxicological analyses are offered at select laboratories, which can be time consuming and expensive, yet the potential for residues in consumed animal products and implications for human health necessitate testing and consultation. Any potential exposure to a toxicant in cattle should be reviewed to determine whether a residue hazard exists. Therapy focuses on immediate removal of the toxicant from the environment and from the gastrointestinal tract. With few antidotes available, most are cost prohibitive to treat numerous affected cattle. In addition, most antidotes will require extra-label drug use and establishment of meat and milk withdrawal times.
Keywords:
Introduction
Although infrequent in practice, food animal intoxications require extensive diagnostic and rapid therapeutic measures. The diagnostic approach is key for adequate treatment and prevention of further cases. Unfortunately, no single procedure will test for all toxicants, and these cases require a multifaceted approach to assemble and solve a diagnostic puzzle. A complete case history, clinical and clinicopathological data, postmortem findings, chemical analyses, and occasionally bioassay findings all provide pieces of this puzzle.Citation1
Toxic residues in food animals may pose a public health risk in edible products. In addition and have to diagnosis and treatment, practitioners often face publicity and medico-legal issues consult closely with regulatory agencies on food safety and public health. Crucial in complex cases, referral veterinary toxicology laboratories can help the bovine practitioner establish an accurate diagnosis and provide advice regarding food safety. In this report, we review and organize existing data from cattle poisonings in California, identify the most common toxicants, and aim to provide a tool for bovine practitioners to enable early recognition, rapid diagnosis and confident treatment.
The purpose of this study was to characterize the most commonly diagnosed intoxications of cattle in California, and to provide details on diagnostic work-up and treatment in view of public health risks.
Materials and methods
Source of case material and categorization
The cases were collected from submissions to the California Animal Health and Food Safety Laboratory System (CAHFS) between January 1, 2000 and December 31, 2011. The terms “poison,” “poisoning,” “toxic,” or “toxicosis” were used to search the laboratory information database. Data obtained from the case reports included production class, location, clinical history, pathological findings, toxicological results, other diagnostic testing, and diagnosis. Cattle were subdivided into two production classes: beef and dairy. Cases were categorized as “intoxication” when toxicological testing was confirmatory and consistent with clinical history, or when pathological findings combined with exposure history, clinical and clinicopathological information were consistent with “intoxication.” For example, a positive finding of oleandrin in rumen content after an unexpected death of a cow was considered diagnostic for oleander intoxication, without detailed pathological or histopathological evaluation. In contrast, hepatic lesions suggestive of amanitin poisoning would be categorized as “hepatotoxicity” rather than amanitin intoxication, unless collected samples tested positive for amanitin.
Data analysis
This is retrospective study and data are presented descriptively.
Results
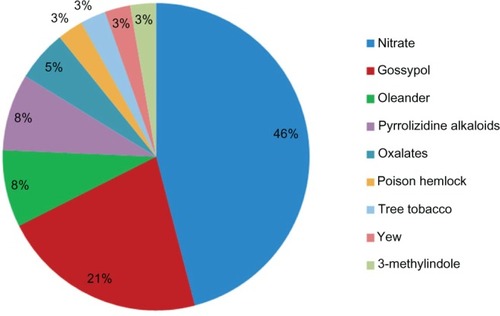
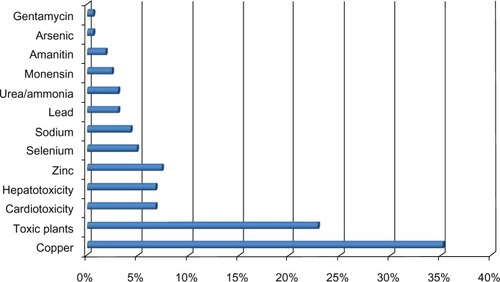
A total of 1199 bovine cases matched the search terms. Ninety percent of these cases occurred in California; 55% in dairy cattle, 41% in beef cattle, and the remainder was unspecified as to production class. A diagnosis of intoxication was established in 13.5% (162) cases (). Ingestion of toxic plants, plant-associated toxins, and copper overexposure comprised the majority of diagnosed cases (). Nitrate/nitrite poisoning, followed by gossypol, oleander, and pyrrolizidine alkaloids (), comprised the majority of plant-associated intoxications in this dataset.
Figure 1 Estimated incidents of poisonings and identified causes diagnosed in cattle by the California Animal Health and Food Safety Laboratory System.
Discussion
While presented data reflects overall trends in California, the annual incidence of cattle intoxications remains uncertain due to challenges and cost for both the practitioner and producer to pursue in-depth analytical toxicology testing of suspect poisoning cases. Depending upon the state, regulatory agencies may assist in the work-up of suspect intoxications of food-producing animals in order to guarantee a safe food supply. The California Department of Food and Agriculture (CDFA) requires reporting of a suspect intoxication within 24 hours.Citation2 While the CAHFS toxicology laboratory provides extensive testing, specific analytical tests have been developed for many but not all toxicants. Our data enhances the understanding of frequency and etiology of cattle poisoning in California. But intoxications are likely under-reported because of lack of submission to the laboratory and because of lack of identification of all toxicants. One limitation of this retrospective study is that regional differences regarding toxic poisonous plants can occur, and therefore the presented data may not reflect the same incidence of cattle in other geographical areas of the country. A larger retrospective evaluation of cattle in multiple or even all states would give more information about the common intoxications countrywide and their geographical distribution. California is also one of the leading states in the dairy industry. This could have possibly influenced the number of submissions from dairy versus beef cattle operations. However, no central poisoning reporting center for cattle exists, and the gathering of the information is difficult and problematic if attempted in multiple states. The authors therefore concentrated on the common cattle poisoning in the state of California.
Diagnostic approach
Accurate and rapid diagnosis of intoxication is challenging, as no single test detects all possible toxicants. Nonspecific clinical signs (eg, diarrhea or weakness) along with absent postmortem lesions often confound the diagnosis. A systematic approach to collecting all evidence, proper sampling techniques, and good communication between clinician, technicians, client, and laboratory are critical for successful toxicology investigations (). A consultation with a veterinary toxicologist can facilitate and enhance the work-up of a poisoning case and ensure proper sample collection. Prior to ante-mortem examination and/or necropsy, the legal status of the case should be determined to ensure proper documentation (chain-of-custody) and sampling.
Table 1 Specimens for analytical toxicology testing: preferred amount, container, storage condition, and extensive list of possible analytes
Along with crucial history, information on age, sex, reproductive status, morbidity and mortality, and progression of clinical signs, additional questions regarding the following must be asked: recent changes in feed or water, movements of animals, administration of medications or supplements, changes in weather conditions, nearby pesticide applications or industries, changes in personnel, and potential for deliberate poisoning.Citation3,Citation4
Toxicosis should be considered when there is a sudden onset of clinical signs in a number of cattle. Individual animals must be assessed in addition to the herd. A complete physical examination may suggest a specific toxicant, yet many poisonings have nonspecific clinical signs and rate of onset and progression of signs may prove useful to an astute diagnostician. Response to treatment can also provide important information about the possible toxicant.
Suitable samples for toxicology testing from affected, live animals include gastrointestinal (GI) contents (rumen lavage fluid, fecal material), urine, whole blood, serum, and milk. For most analyses performed on serum, blood, urine, or milk, a 1 mL volume of sample is sufficient. If excessive testing is expected, volumes of 5 mL are desired (). Tissue biopsies, such as liver collection for copper analysis, or fat collection for persistent organic pollutant analysis, can be useful for certain investigations.Citation5,Citation6 Samples should be stored in separate containers and frozen.Citation3 Whole blood can be refrigerated at 4°C. Serum must be separated from the clot as soon as possible before freezing. Some analytes, such as potassium, magnesium, phosphorus, and zinc, may be altered by time spent in contact with the clot, because red cells continue to metabolize them or release additional material.Citation7 Special care has to be taken to avoid contact with rubber products that can be a source of zinc.Citation8 The best choice for trace metal, including zinc testing, is a royal-blue top tube, or equivalent, designed for trace element analysis to avoid an artificial increase.Citation9
Often a complete postmortem examination of an animal is necessary to obtain information regarding the cause of an unexpected death or a suspected toxicosis. While practitioners can perform postmortem examinations, a referral veterinary diagnostic laboratory should be considered for thorough diagnostic workup. Necropsy samples can be used for histological, toxicological, and microbiological examination. Samples of major organs should be placed in 10% buffered formalin for histopathological examination. The formalin to tissue sample ratio should be 9:1, and the thickness of the collected specimen should not exceed 0.5 cm. Fresh frozen samples, not formalin fixed samples, must be collected for toxicology testing.Citation10 To avoid any dilution effect, samples should not be pooled and should be packed separately in whirlpak bags. Thorough assessment and sampling of rumen and intestinal contents is critical for sudden deaths, because unusual material (chemicals) or toxic plant parts may be identified. Generally, the most useful postmortem specimens for toxicology testing include GI contents, liver, kidney, urine, brain, and ocular fluid ().Citation4 For most tissue analysis, 5 g of specimen is sufficient for toxicology testing. With regards to samples from the GI tract, it is of great use to collect contents from the rumen, small intestine, and large intestine. With regards to brain, special care must be considered when analysis for cholinesterase activity is desired. Reference ranges are typically based on the analysis of half-brain (sagittal section). It is best to collect as many samples as possible for toxicology testing at the time of necropsy. Samples can be held frozen (tissues, serum, urine, and milk) or refrigerated (blood) until results of other tests (eg, histopathology, bacteriology, and virology) are completed before proceeding to specific toxicological analyses.
Etiologies
The incidents identified in this study allowed us to focus on the most commonly reported intoxications. In addition, we included tetracycline and sulfur as feed additive and metal toxicants. Although our search criteria did not reveal tetracycline or sulfur intoxications, such intoxications can occur in cattle and might be underdiagnosed or a regional problem. Analysis of feeds and water for total sulfur is routinely recommended in cases of polioencephalomalacia (PEM) diagnosis. A common clinical or postmortem diagnosis in cattle, the etiology of PEM is rarely identified, and pathological lesions and/or response to thiamine treatment often provide sufficient diagnostic criteria. Documentation of tetracycline overexposures in our records does not exist because of lack of routine assays for these antimicrobials in biological specimens.
Toxic plants
Poisonous plants cause significant losses of cattle each year. However, cattle usually only eat poisonous plants when forced by environmental circumstances such as drought or when toxic plants are distributed in the ration or embedded in pellets or cubes. Poisonings can be prevented by providing ample forage and rations free of poisonous plants. Areas infested with poisonous plants should be avoided when trailing, holding, or unloading animals. Supplemental feed may help protect animals if poisonous plants are unavoidable; however, changes in palatability (possibly due to herbicides or drying) or increased toxicity of some plants (due to pesticide residues or high toxin concentrations during certain growth stages) can complicate this approach.
During the investigation of suspect plant intoxications, mixed feed from bunks or stalls as fed, unusual pasture plants, all feed ingredients going into a ration, feed supplements, tags, and labels should be collected. In addition, information on recent feed changes (with dates), feed quality (visual), preparation of mixed feed, feeding practices, feed supplements (recent changes, lot numbers, use level), lot numbers, storage facilities and conditions, pasture changes, and weather changes must be obtained. Diagnosing a plant poisoning can be difficult. In many cases, clinical signs are nonspecific (such as diarrhea), and postmortem lesions are not characteristic. Specialized veterinary toxicology laboratories may provide testing for plant toxins, but the assays do not cover the wide variety of plant toxins. In many cases, the best way to support a diagnosis of a plant poisoning is to confirm the presence of a toxic plant in the animal’s environment (this will require positive identification of the suspect plant), to confirm that the plant has been ingested (noting that the candidate plants have been chewed and/or finding plant fragments in vomitus or GI tract samples), and to correlate clinical findings, where possible, with those known to be associated with the suspect plant. If diagnostic tests are available, the diagnosis can be confirmed. This is especially important in insurance or legal investigations.
Plant intoxications may result from a single ingestion of a large amount of a poisonous plant, but small amounts of acutely toxic plants may also result in severe disease or death. In our retrospective study, acute plant-related intoxications were caused, in descending order, by nitrate-containing plants, gossypol, oleander (Nerium oleander), oxalates, poison hemlock (Conium maculatum), tree tobacco (Nicotiana spp.), Yew (Taxus spp.), and 3-methylindole. Chronic poisoning typically results in clinical signs long after exposure to the toxic plant material, and treatment may no longer be possible. Plants that resulted in chronic intoxications of cattle in our study included pyrrolizidine alkaloid-containing plants such as common groundsel (Senecio vulgaris), tansy ragwort (Senecio jacobaea), and fiddleneck (Amsinckia intermedia). In this article, we highlight the most commonly identified toxic plants.
Nitrate containing plants
Nitrate accumulates in vegetative tissue, particularly in the lowest 15 cm of stems.Citation11 The most common nitrate accumulating weeds, pigweed (Amaranthus retroflexus) and lamb’s quarters (Chenopodium spp.), regularly contaminate alfalfa hay, but crop plants, especially oat hay, corn, ryegrass, and sorghum (Sorghum spp.) have been incriminated in nitrate toxicosis.Citation12,Citation13 Fertilization, herbicide treatment, drought, cloudy weather, and decreased temperatures all may increase the nitrate concentrations in plants. Water contaminated with nitrate from manure or fertilizer runoff can also result in acute intoxications.
Nitrate is reduced to nitrite by rumen microbes. In healthy ruminants, nitrite is further reduced and converted to ammonia by propionate-producing bacteria.Citation14 Sudden ingestion of toxic amounts of nitrate results in rapid absorption of nitrite. Nitrite oxidizes Fe2+ to Fe3+, converting hemoglobin to methemoglobin and resulting in tissue anoxia.Citation15 Clinical signs begin between 30 minutes and 2 hours after exposure, with death possible within 6–24 hours. Acute nitrate poisoning results in depression, respiratory distress, tremors, ataxia, tachycardia, and terminal convulsions. While chocolate-brown discoloration of tissues and blood has been described as a specific diagnostic marker for nitrate/nitrite intoxication, it is not routinely observed. Postmortem, analysis of aqueous humor for nitrate and nitrite concentration proves reliable if eyeballs are collected immediately or several hours after death and refrigerated.Citation16 A rapid, yet presumptive field diagnosis can be made using a standard nitrate dipstick to test ocular fluid. Testing of suspect source material (forage, ration, water) for confirmation and quantitation must be done if ocular fluid is consistent with intoxication, to prevent additional exposures. The established laboratory method for nitrate and nitrite quantatition is ion chromatography with a conductivity detector.Citation17
An emergency situation, acute nitrate/nitrite poisoning must be treated as soon as possible after exposure, with minimal possible stress to cattle at risk of tissue hypoxia and acute death. While methylene blue provides the antidote to methemoglobinemia (8.8 mg/kg of a 1% solution intravenous),Citation18 it has a 180-day withdrawal time in milk and meat. Although not approved for use in food animals, the United States Food and Drug Administration (FDA) exercises enforcement discretion in cases where treatment is needed, to prevent animal suffering.Citation19 Chronic exposure to elevated nitrate may lead to fetal anoxia, abortions and stillbirths, and progesterone alterations.Citation20 In addition, long-term exposure to elevated nitrate concentrations in feed or water can lead to decreased production and vitamin deficiencies.
With acute poisoning likely if the feed nitrate concentrations exceed 1% (dry weight), forage management techniques should reduce nitrate concentrations to below 0.3% (dry weight). Careful use of nitrogen fertilizers, harvest under appropriate conditions, supplementation of ration with corn, ensiling, and testing hay and forage for nitrate content will help minimize intoxication risk. Hay with nitrate accumulating plants should be tested prior to feeding. If unavoidable, slow introduction of such feed is recommended to allow the rumen microbial environment to acclimatize to higher nitrate concentrations. A bacterial feed additive (propionibacterium acidipropionicic strain P5) may be supplemented when exposure to high concentrations of nitrate in feed or water cannot be avoided. Water should be tested if nitrate contamination is suspected due to manure or fertilizer runoff. Water nitrate concentrations should be below 440 mg/L, but acute toxicosis is unlikely to occur until water nitrate concentrations exceed 1300 mg/L.
Oleander
A drought-tolerant, ornamental evergreen shrub found year round in the United States, especially California, Arizona, and Texas, oleander (Nerium oleander) grows along roads, pastures, and fence lines and can invade pastures and contaminate hay. California Animal Health and Food Safety (University of California, Davis, CA) diagnoses 50–100 cases annually in numerous species including cattle, usually from ingestion of plant clippings, contaminated hay, or silage (). Its cardiac glycosides (predominantly oleandrin) inhibit Na+-K+-ATPase,Citation21 resulting in increased intracellular Na+, subsequent increased intracellular Ca2+, and positive inotropic effects. Cardiac glycosides also may increase vagal tone and lead to direct atrioventricular (AV) nodal depression and bradycardia.Citation22
Figure 3 Oat hay contaminated with oleander (Nerium oleander). Oleander leaves (▲). Oleander stems (+).

Between five and ten leaves of oleander can be lethal to an adult bovine. The entire plant, including seeds, fruit, and root, fresh or dried is toxic. Clinical signs include diarrhea, salivation, cardiac arrhythmias, and death within 2–8 hours. Cardiac abnormalities include bradycardia, AV blocks, ectopic beats, and gallop rhythms.Citation23 Usually rapidly progressing and with many cattle found dead, rare cases present with clinical signs delayed by 12 hours or more. Postmortem findings depend on the time course and may be minimal in cases that rapidly progress to death. Reddening of the GI mucosa, pulmonary congestion, pale myocardium, or subepicardial and subendocardial hemorrhages may be noticed.Citation24 Histopathological cardiac lesions may include myocardial necrosis, interstitial edema, hemorrhage, and an inflammatory response.
One of the authors has observed transfer of oleandrin into milk of dairy cattle and into edible tissues of poisoned cows. Therefore, specific toxicology work-up of suspect oleander poisonings must address the public health risk. With zero tolerance for oleandrin in edible tissue, any detection is considered unsafe and the product adulterated. Oleandrin has been detected in milk samples for several days after exposure, while muscle tissues may remain positive for up to 10 days, albeit at low ng/g concentrations. Consultation with a toxicologist and confirmatory testing are imperative to protect the food supply. Highly specific and sensitive liquid chromatography/mass spectrometry methods detect oleandrin in suspect plant material and specimens from animals, including GI contents, liver, serum, urine, milk, and muscle.Citation25 In live animals, serum is the sample of choice for oleandrin detection because it contains higher concentrations than urine. Postmortem, rumen contents are of greatest diagnostic use in acute deaths, while colon contents, liver, and heart are useful specimens.
There is no antidote for cardiac glycosides approved for use in cattle, and due to the acute nature, treatment may not be beneficial. Symptomatic and supportive treatment should include administration of intravenous fluids and antiarrhythmics. Activated charcoal should be administered multiple times over several days to prevent further absorption through enterohepatic circulation of the toxins.Citation26 Cardiac evaluation may warrant the use of atropine and propanolol. Digoxin-specific Fab antibody fragments cross-react with cardiac glycosides in oleander,Citation27 but dosages are empirical and potentially cost-prohibitive.
Pyrrolizidine alkaloid containing plants
Pyrrolizidine alkaloids (PAs) naturally occur in many plant species, including Senecio vulgaris (common groundsel), S. jacobea (tansy ragwort), and Amsinckia sp. (fiddleneck). Unpalatable in pastures, these plants present a major concern if incorporated into hay. Common groundsel is commonly found in spring-cut alfalfa hay in California. Flowering, growing PA plants are most toxic, while PA concentrations remain stable in hay and decrease by 90% with ensiling.Citation28 Silage still poses a risk for PA residues.
Chronic PA exposure for 2–6 months results in clinical signs of weight loss, depression, icterus, and anorexia.Citation29 Hepatogenous photosensitization can develop. In contrast to very susceptible cattle and horses, small ruminants and herbivores remain unaffected. Cows ingesting more than 5% of their bodyweight of fresh S. jacobaea over 1–3 months can develop severe poisoning and death. Alkaloid concentrations vary considerably in plants; thus, no comparative toxicity data exist for individual PAs or related plants. Other liver-damaging agents (copper, aflatoxins, endotoxins, or viruses) can act synergistically to increase PA susceptibility.Citation30–Citation32
Bioactivated to highly reactive pyrroles in the liver, PAs result in crosslinking of DNA, RNA, and proteins and subsequent cytotoxicity, antimitotic, and megalocytic activity.Citation33 Histopathologically, hepatomegalocytes, bile duct hyperplasia, and fibrosis can be seen. Liver enzymes (aspartate aminotransferase, sorbitol dehydrogenase, alkaline phosphatase, and gamma-glutamyltransferase) only present alteration in early stages of disease. Quickly eliminated from blood, PA analysis is only available at select diagnostic laboratories and only meaningful in very recent exposures. With development of signs months after plant exposure, testing of feed for PAs becomes difficult or impossible because the animals are often on different, non-PA-contaminated feed. However, a thorough feed inspection and testing for PAs remains the best approach to reaching a diagnosis.Citation34 Along with the nonspecific pathological lesions, PA poisoning in cattle is mostly a presumptive diagnosis.
No specific treatment exists, and affected cattle are not expected to recover. With dilution of minute concentrations in milk processing and extremely low concentrations present in meat, this route of exposure is not considered to be of great concern to humans, with greater risk through contaminated salad mixtures, honey, grains, or herbal preparations.Citation35
Gossypol
A polyphenolic aldehyde found in cottonseed pigment glands, the bark of plant roots, leaves, seed hulls, and flowers, gossypol concentration varies among the species of cotton plant. Its free toxic form presents great concern in cottonseed and cottonseed meal commonly used as protein supplement for cattle.Citation36 Especially toxic for monogastric animalsCitation37 and young calves,Citation38 adult ruminants are more resistant due to protein complex formation within the rumen.Citation39 Exceeding a dose of 24 g of free gossypol per day can lead to toxicosis because of inability of the animal to detoxify more than this amount.Citation40 Gossypol impairs spermatogenesis, increases the number of abnormal spermatozoa, and reduces the sperm motility in bulls fed with cottonseed products.Citation41
Gossypol intoxication can lead to acute death in calves. Clinical signs are dyspnea, abdominal pain, diarrhea, and congestive heart failure if illness is prolonged.Citation42 Chronic exposure to low concentrations of gossypol can result in poor growth and ill thrift in affected calves, enhanced by stress, such as change of feed, group housing, or other environmental factors. Gross necropsy of acute gossypol-poisoned cattle can be unremarkable. Chronic cases show evidence of pulmonary and subcutaneous edema, straw-colored effusions in body cavities, and pale streaking of myocardium indicative of cardiac necrosis.Citation43 Histopathology of the liver reveals periacinar necrosis, and clinical chemistry indicates hepatic failure in terminal cases.Citation43 Diagnosis results from history of exposure, clinical signs, necropsy findings, and analysis of feed samples. Bull semen evaluation with increased number of midpiece defects suggests gossypol overexposure.Citation44 Testing for gossypol can be done in serum or plasma of animals suspected to be exposed to excessive amounts, or in feed. Laboratories use high-performance liquid chromatography.Citation45
With no specific treatment available, it is important to remove the feed source and offer feed without cottonseed byproducts. Supportive therapy may help in individual cases. Bulls can return to normal spermatogenesis within 2–3 months after removal of the cottonseed products from the diet.
Feed-related toxicants
Monensin
By-products of Streptomyces spp, ionophores of veterinary clinical significance in beef and dairy cattle include monensin, salinomycin, and lasalocid. They all lead to similar clinical presentations when overdosed. Lipid soluble antimicrobials, commonly used as feed additives for ruminants, ionophores target the rumen microbial organism, alter the ruminal ecosystem, result in increased nitrogen and carbon retention by the animal, and lead to increased production efficiency of nutrients.Citation46
A carboxylic polyether ionophore widely used as a feed additive in beef cattle, monensin functions as described above in addition to its use for prevention and control of coccidiosis in cattle.Citation47 In 2004, the FDA approved use of monensin in total mixed rations for increased milk production efficiency in dairy cattle; the following year, ionophores were widely approved for use in dairy cattle.Citation48
Intoxications occur as a result of error in the amount of ionophore added to the diet,Citation49 inaccurate on-farm feeding,Citation50 administration to the wrong cohort, or exposure of cattle not acclimated to the ionophore-containing ration.Citation51 With no established LD50 for cattle, toxic monensin concentrations range between 21.9 mg/kg and 80 mg/kg of bodyweight.Citation52 Clinical signs of a monensin overdose include anorexia, rumen atony, lethargy, diarrhea, dehydration, muscle fasciculations, weakness, decreased milk production, and sudden death.Citation48,Citation53 Tachycardia, tachypnea, and cardiac arrhythmias can develop as signs of cardiac dysfunction. In general, monensin has been shown to have an enhanced toxicity when fed repeatedly at lower doses, compared with one single oral overdose.Citation51
Cardiac function of cattle with monensin intoxication is impaired due to myocardial necrosis. Echocardiography of cattle with an acute monensin overdose show left ventricular systolic dysfunction and reduced chamber size.Citation53 Echocardiographic interpretation requires expertise and special equipment, neither practical nor economic for the evaluation of a large number of cattle. A sensitive myocardial biomarker released from damaged myocardium, cardiac troponin I (cTnI) provides a noninvasive, and easy-to-perform ante-mortem diagnostic tool.Citation53 However, cTnI only confirms myocardial damage, not necessarily monensin intoxication.
Gross necropsy of cattle with monensin toxicosis shows cardiac dilatation, epicardial hemorrhages, and pale streaking of the myocardial muscle (indicating necrosis).Citation53,Citation54 In cases of prolonged ingestion of sublethal doses of monensin or survival of an acute monensin toxicosis, signs of congestive heart failure such as subcutaneous edema, hydrothorax, ascites, pulmonary edema, and liver congestion can be observed.Citation55 Histopathological evaluation reveals myocardial necrosis, vacuolar degeneration, swelling and eosinophilic staining of myocardial fibers, and vacuolation and swelling of mitochondria.Citation52,Citation53 Diagnosis relies upon history of exposure, quantitation of monensin concentration of the feed, and necropsy findings. Recently, detection of monensin in myocardial tissue in suspect cases has been used successfully to confirm exposure. However, analysis of feed for monensin concentration is of greatest diagnostic value and relies on liquid chromatography/mass spectrometry.Citation56
With no specific antidote, treatment focuses on supportive care and immediate removal of contaminated feed material. Administration of mineral oil in early cases may help sequester lipid soluble ionophore antibiotics still present in the GI system.Citation57 In general, the use of mineral oil in cases of intoxication is not advised.
Tetracyclines
The bacteriostatic tetracyclines, including chlortetracycline and oxytetracycline, which are equally effective against gram-positive and -negative bacteria, are used as feed additives in cattle for improved growth rate, increased feed efficiency, prevention and treatment of bacterial enteritis, and bovine respiratory disease complex. High oral dosages of dietary tetracyclines can lead to decreased activity of ruminal flora and ruminoreticular stasis.Citation58 Diagnosis is made by evaluation of the feed and exclusion of other toxicants leading to anorexia and rumen atony. Testing for tetracyclines is not routinely offered by veterinary diagnostic laboratories. Treatment focuses on restoration of normal rumen flora by offering good quality hay and correcting the tetracycline concentration in the diet.
Metals and minerals
Copper
An essential micronutrient, copper plays an important role in neutrophil function and triggers appropriate response of peripheral-blood lymphocytes. Below-normal serum copper concentrations significantly impact cytokine production in cattle.Citation59 Copper overexposure is hepatotoxic and nephrotoxic and can result in high mortality. Chronic copper poisoning can result from low dietary intake of molybdenum. With decreased intake of dietary molybdenum, GI uptake of copper increases and even normal copper feed concentrations can become toxic to cattle.
Oral or parenteral administration of an acutely toxic dose of copper frequently results from supplementation without nutritional consultation or assessment of herd copper status. Aside from the risk of acute intoxication, this type of supplementation is often not cost effective. Previously, injectable copper preparations, eg, Cu-EDTA, were commonly given to cattle raised on copper-deficient pasture or on molybdenum-rich soil. Slow release, orally administered copper boluses, can result in chronic copper intoxications in suckling calves, particularly if multiples sources of copper exist.Citation60 Occasionally, acute hepatotoxicosis due to soluble forms of injectable copper occurs even within the recommended dose; other mineral deficiencies (eg, selenium) may result in increased sensitivity.Citation61
Acute clinical signs include GI (eg, diarrhea), and central nervous system disturbances, such as circling, head pressing, and ataxia. Some cattle exhibit dyspnea and depression.Citation61 Others are just found dead. Death usually occurs 12–72 hours post-injection. Acute copper poisoning cases lack icterus or hemoglobinuria as seen in chronic cases with hemolytic anemia.Citation62
Gross necropsy shows peritoneal hemorrhage in some cases.Citation63 Acute copper toxicosis does not necessarily cause the triad of liver damage, hemolysis, and renal damage as seen in chronic toxicoses.Citation64 Severe acute generalized centrilobular to panlobular liver necrosis and vacuolar degeneration in the renal tubular epithelial cells can be observed.Citation61 Nonspecific hepatic degeneration and necrosis require additional testing for aflatoxins, PAs, or gossypol ingestion. Specific copper histochemical stains can confirm diagnosis.
Chronic copper poisoning occurs when the amount of copper absorbed from the diet exceeds the nutritional requirement and the animal’s capacity to excrete the excess. The abundant copper accumulates within the liver over a variable period of time.Citation65 Clinical signs manifest acutely when the liver’s copper storage is saturated, and the rupture of intracellular lysosomes results in hepatocellular necrosis and liberation of a large amount of copper into the bloodstream. This can lead to acute intravascular hemolysis (hemoglobinuria, icterus, and anemia).Citation65,Citation66 However the majority of cattle with chronic copper intoxication show unspecific signs, such as dull mentation and anorexia. Kidney failure in some cases of hemolytic crisis can occur due to hemoglobinuric and tubular nephrosis; death of the animal can occur within hours of first clinical signs. Chronic copper ingestion can also manifest in poor hair coat, weight loss, diarrhea, decreased appetite, milk production, and susceptibility to mastitis, metritis, and retained placenta.Citation67,Citation68
The unspecific clinical signs render diagnosis of chronic copper toxicosis challenging, and other differential diagnoses for liver failure should be considered. Postmortem observations in cattle with an acute hemolytic crisis include generalized icterus, dark swollen kidneys, and pigmenturia, while animals with silent chronic copper exposure usually show no prominent features on gross examination of the carcass. Serum/plasma copper concentrations correlate poorly with liver copper concentrations.Citation69 However, in acute hemolytic crisis, serum copper concentration increases from the massive release of copper stores from the hepatocytes. Hepatic enzyme activity also increases. The measurements are unlikely to be elevated prior to hemolytic crisis.Citation69 Thus, serum copper analysis can provide useful diagnostic data during the acute phase of the disease. Analysis is done routinely in laboratories using inductively coupled plasma emission spectrometry.Citation70 Both, liver and kidney copper concentrations must be evaluated in suspect intoxications. Analysis for copper is typically done by inductively coupled plasma emission spectrometry. Generally between 25 and 100 mg/kg wet weight, copper wet weight varies with age and production class. While liver copper concentrations of greater than 250 mg/kg wet weight are consistent with overexposure, pathological or additional toxicological results must also be considered.Citation71 In acute clinical cases, kidney copper concentrations typically exceed 10 mg/kg wet weight.Citation63
Lead
Lead poisoning results from cattle’s natural curiosity and their habit of licking and indiscriminate eating.Citation72 This presents a human food safety concern due to potential exposure to meat or milk products of affected animalsCitation73 and results in considerable economic loss in beef and dairy herds.Citation74 Asymptomatic animals may have substantial amounts of lead in tissues and milk; thus, a thorough investigation is required to adequately protect human health.Citation75,Citation76 Single ingestion of large quantities of lead-containing material, licking of lead-containing paint chips in old barns or discarded paint cans, consuming water from lead-containing water pipes, lubricants, and lead accumulator batteries result in acute intoxication.Citation74 Chronic exposure occurs from grazing on polluted pastures in the vicinity of lead mines or ingestion of forages harvested from such areas.Citation77
Only 2%–10% of the ingested lead is absorbed through the GI tract and rapidly distributed to kidney and liver.Citation74 Kidney tissue concentrations often reflect accurately the environmental level.Citation74 Harmful for unborn calves, lead can accumulate in the central nervous system of the neonate due to immaturity of the blood–brain barrier.Citation78 Secretion into milk and redistribution into bone (storage site) may also be significant.Citation74 Milk-based diets fed to young cattle enhance the absorption of lead from the GI tract. In addition, lead-contaminated milk from dams can result in exposure and risk.Citation79 More commonly, young cattle ingest a single material with high bioavailability (lead oxide, carbonate, or acetate).Citation72 Death usually occurs within 12–24 hours without previous clinical signs.Citation80
Neurological signs include cortical blindness, delayed menace, head pressing, delayed withdrawal reflex, reduced tongue tone, champing of the jaws with ptyalism and frothing at the mouth, rapid and difficult breathing, tachycardia, ataxia, and tremors.Citation81 Some animals may wander aimlessly, circle, or walk through objects such as fences or brush.Citation82 More common in adults, subacute lead poisoning can lead to depression, anorexia, and GI disturbances such as constipation followed by diarrhea, signs of colic, bruxism and rumen atony. Central nervous system signs also occur.Citation82 Hematological evaluation can include basophilic stippling of erythrocytes and increased nucleated red blood cells, but may not be present in acute cases.Citation83 In less common chronic exposures, cattle develop weakness, incoordination and muscle wasting, abortion, and sterility,Citation82 and develop normocytic, normochromic anemia, and basophilic stippling of red blood cells.Citation80
Gross necropsy can reveal metallic fragments in the reticulum, suggestive of lead poisoning.Citation84 However, many lead sources would not be noticeable during necropsy. While all forms of lead are toxic, organic lead is considered most bioavailable, followed by inorganic lead salts (eg, lead acetate, lead sulfide) and metallic lead. Although different lead species are absorbed to varying degrees, all are capable of causing adverse health effects. Histopathology of the brain will show evidence of polioencephalomalacia, such as cerebral laminar cortical necrosis, severe congestion of cerebrocortical tissue and meninges, edema, and endothelial swelling.Citation85,Citation86 Severe liver necrosis and lipid accumulation,Citation81 and renal tubular degeneration,Citation82 may occur. Ante-mortem diagnosis of acute intoxication relies on history of exposure, clinical signs, and blood lead concentration greater than 0.35 µg/mL.Citation85 It is critical to analyze a whole blood sample for lead. Serum and plasma samples are not appropriate for lead analysis because lead associated with red blood cells. Furthermore, clinically asymptomatic animals which could have been exposed should be tested for lead. Lead analyses are widely available through diagnostic laboratories, and most laboratories use atomic absorption analysis. Clinical signs correlate poorly with blood concentrations.Citation74 Postmortem diagnosis is made based on history and liver and kidney lead concentrations. It is best to determine the lead concentrations in both tissues, as concentrations vary based on the timeframe between exposure and death. Analysis of tissues for lead is typically done by inductively coupled plasma emission spectrometry.
Sulfur
Necessary for cellular function and growth of ruminal microorganisms, sulfur is an important component for rumen synthesis of the sulfur-containing amino acids, and for production of B-vitamins. A minimal 0.15% sulfur is recommended in beef cattle diets and maximum dietary sulfur concentration has been estimated at 0.4%.Citation87 Since sulfur content of feedstuffs relates directly to protein concentration and can vary greatly, sulfur-containing additives are often added but can lead to toxic levels.Citation88,Citation89 Water can be a significant source of sulfur, and increased water intake with rising ambient temperatures can influence total sulfur consumption. Furthermore, sulfur-containing plants can contribute to a large amount of sulfur.Citation90 Cattle exposed to manure gas with a high H2S content developed PEM, making inhalation of gas a potential source for sulfur intoxication.Citation91
In beef calves, experimental diets containing more than 4000 ppm sulfur produce neurologic disease due to PEM, and subclinical brain lesions have been observed in a few calves with consumption of sulfur lower than 4000 ppm. Dietary sulfates are reduced to sulfides within the rumen and form hydrogen sulfide gas, increasing risk of PEM.Citation92 A concentration of 2000 mg/L hydrogen sulfide typically precedes clinical cases of PEM in cattle.Citation93 Analysis for sulfur in feeds and tissues can be accomplished by inductively coupled plasma emission spectrometry, while analysis for hydrogen sulfide is done by a H2S-detector tube. Sulfate concentrations in water are determined by sulfate anion chromatography with conductivity detection.
Acute sulfur intoxication leads to neurologic manifestation, including cortical blindness, staggering, muscle fasciculations, recumbency, opisthotonus, uncontrolled paddling,Citation89 coma, or death in the absence of preceding clinical signs.Citation88 Exhaled breath may smell of hydrogen sulfide. Sulfates are less toxic than H2S, but can lead to an osmotic diarrhea due to poor absorption. Histopathology of the brain reveals signs of PEM, such as laminar and cortical spongiosis and neuronal necrosis within the cerebral cortex.Citation90
Differential diagnoses for neurologic disorders in cattle include lead poisoning, sodium poisoning, thiamine deficiency-induced PEM, hypovitaminosis A, and Histophilus somni meningoencephalitis. Sulfur-induced PEM does not cause a decrease in rumen or blood thiamine concentrations. Diagnosis of sulfur PEM is made by estimation of the total sulfur intake from feed and water. Impractical in the field, sampling of rumen gas for hydrogen sulfide concentration can help assess clinical cases. Treatment of cattle with sulfur-induced PEM consists of thiamine administration, removal of the sulfur containing diet or water, and administration of dexamethasone, if cerebral edema is suspected. Often nonresponsive to thiamine, this treatment may just hold a diagnostic and prognostic value.
Zinc
An essential trace element involved in protein synthesis, carbohydrate metabolism, and enzyme function,Citation94 zinc is relatively nontoxic to ruminants. Bioavailability of zinc complexes, amount of dietary fiber, amount and period of zinc feeding, along with age and concurrent disease, influence the susceptibility from environmental contamination or increased amounts in diet or water.Citation95 Acute clinical signs include anorexia, drastic decrease in milk production, light-green colored diarrhea, and weakness, while chronic exposure leads to diarrhea, followed by constipation, weight loss, or decreased weight gain in young animals. In live cows, serum and plasma samples are considered suitable for zinc determination. Special care must be taken to avoid contact with rubber products that can be a source of zinc (eg, rubber-topped tubes) and hemolysis, which may also increase zinc concentration. Necropsy of acute cases reveals pulmonary emphysema, pale myocardium, renal hemorrhages, and hepatic degeneration. Chronic exposure leads to pancreatic and renal damage. Histopathology reveals acinar cell degeneration and necrosis and renal cortical fibrosis in calves.Citation95 Diagnosis includes feed or water mineral analysis and measurement of tissue zinc concentrations. Liver zinc determination is of greatest diagnostic value in postmortem evaluations. Analysis for zinc is typically done by inductively coupled plasma emission spectrometry
Zinc muscle concentrations are similar to those in the liver, and, in clinical cases, a muscle sample is easy to obtain.Citation96 Treatment of zinc poisoning involves removal of feed material high in zinc and increased dietary roughage content.Citation97
Environmental toxicants and conditions
Microcystins
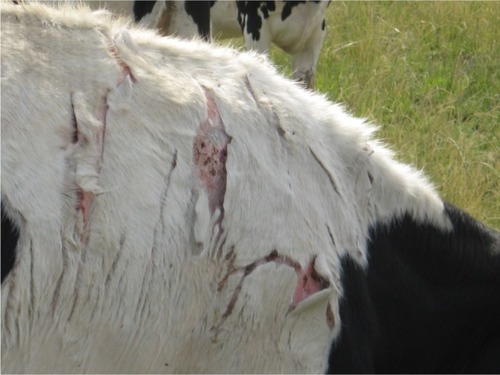
Increasing global water temperatures, nutrient and pollutant enrichment lead to eutrophication of fresh and coastal water bodies and can result in toxicogenic cyanobacterial (blue-green algae) blooms. Produced by multiple cyanobacteria, microcystins have been detected worldwide, resulting in numerous animal intoxications. These algal toxins present a constant threat to pastured animals because of their persistence in ponds and streams (). Cattle drink contaminated water readily, even consuming algal mats, and poisonings appear to be on the rise. The number of structural variants frequently present in surface water blooms makes estimating toxicity difficult. Cyclic heptapeptides causing acute liver damage through potent inhibition of protein phosphatases 1 and 2A,Citation98 microcystins result in diarrhea, weakness, pale mucous membranes, and shock within 30 minutes to several hours.Citation99,Citation100 Many die within hours to days, but individual cattle may survive and develop hepatogenous photosensitization (). The rapid onset of acute hepatotoxicosis renders therapeutic intervention quite difficult, and high mortality rates results. No specific therapy has proven effective in laboratory animals, including decontamination with activated charcoal and no data exists for adsorptive capacity in other animals.Citation101 Symptomatic and supportive care to treat hypovolemia and electrolyte imbalances, and antioxidants such as vitamin E and selenium should be included in the therapeutic regimen for cattle poisoned with microcystins. Cattle with photosensitization must be protected from sun exposure.
Figure 4 Pond with Microcystis bloom in California’s Central Valley.

Figure 5 Photosensitization resulting from microcystin exposure.
A diagnosis of microcystin poisoning is confirmed by liquid chromatography/mass spectrometry analysis of stomach contents, suspect water source, and algal material. As the toxicity of cyanobacteria is strain-specific, morphological identification of the algal genus alone cannot predict the hazard level of a given water source. Detection methods for microcystins in liver or other tissues remain to be developed. Acute hepatic damage results in an enlarged and friable liver resulting from intrahepatic hemorrhage and, histopathologically, in hepatocyte dissociation, degeneration, and necrosis. Pathological findings should quickly lead to an environmental assessment for possible microcystin contaminated water.
Consumption of milk, meat, or liver is unlikely to pose a significant health risk to humans.Citation102,Citation103 However, as a tumor promoter, microcystins may pose a cumulative health risk as they become more ubiquitous. Steps must be taken to reduce fertilizer runoff and applications in fields surrounding ponds used for drinking water. Treatment of water sources with algicides releases intracellular toxins, and prevention of blooms is key to protect animal and human health.
Amanitins
More frequently reported in humans, based on clinical presentation and mushroom identification, mushroom poisonings now can be diagnosed by testing of specimens of animals for specific toxins.Citation104 Amanitins are found in a number of mushroom genera including Amanita, Galerina, Lepiota, Cortinarius, and Conocybe spp. Amanita phalloides (death cap) and Amanita ocreata (Western North American destroying angel) are the most common amanitin-containing mushrooms associated with lethality in the United States.Citation105 A single mushroom can result in the death of a dog or human; while the exact toxicity to cattle is unknown, amanita intoxications may be more common than originally thought.Citation106
Amanitins decrease transcription and protein synthesis through inhibition of RNA polymerase II,Citation107 resulting in toxic effects on hepatocytes, crypt cells, and proximal convoluted tubules of the kidneys. Poisoning leads to severe GI duress (colic and diarrhea) approximately 8–12 hours after exposure. The animal may appear to recover for several hours before developing acute liver, renal, and possible multi-organ failure.Citation108 Elevated serum liver enzyme activities, hypoglycemia, and prolonged clotting times result. Cattle may just be found dead in pasture.
With treatment reliant on symptomatic and supportive care, prognosis is poor. Once acute hepatotoxicosis occurs, effective therapeutic intervention is no longer possible. If acute liver or multi-organ failure has not developed, treatment involves activated charcoal (two to three doses within 24 hours) and supportive care. Silibinin, milk thistle extract (Silybum marianum), has been successfully used in Europe to reduce the uptake of amanitins into hepatocytes in humans. No safety or efficacy data exist for use in cattle.
At necropsy, the liver is typically friable with an enhanced reticular pattern. Histopathological lesions indicate a diffuse, centrilobular to panlobular hepatic necrosis. Microcystins, copper, anemia, heart failure, or cocklebur (Xanthium spp.) present differential diagnoses, so further confirmation is needed. Analysis of serum, urine, gastric contents, liver, or kidney for amanitin can be performed at select veterinary toxicology laboratories. In live, symptomatic animals, urine is preferable for diagnosis. Postmortem, depending on time since exposure, kidney may contain higher concentrations than liver. Identification of mushroom pieces in the GI contents also may aid in diagnosis in addition to mushrooms collected from the environment. Accurate mushroom identification requires an experienced mycologist. The availability of diagnostic assays will help determine the frequency of mushroom poisonings in cattle.
Water deprivation/sodium ion intoxication
In cattle, sodium toxicosis or water deprivation can result in acute disease with high morbidity and mortality. Intoxications usually occur with ingestion of salt blocks or loose salts following limited exposure to salts for a prolonged period of time. Concurrent decreased water consumption increases clinical severity. A defective water trough system, no access to water,Citation109 or in cold weather due to freezing of the water source, can lead to hypertonic dehydration with hypernatremia. Hot weather, high milk production, or transportation contribute to toxicity through increased total body-water losses.Citation110 Nursing or diarrheic calves with limited or no access to water become intoxicated when offered milk replacer or electrolytes solution which is improperly formulated or has manufacturer errors.Citation111
Cattle become depressed and refuse to eat. Acute cases result in central nervous system disturbances, including excitability, blindness, incoordination, hyperesthesia, opisthotonus, nystagmus, muscle twitching, and convulsions, followed by death, due to osmotic differences and resulting water losses from the brain.Citation109,Citation112 Signs of dehydration, especially with prolonged water deprivation, include dry feces and mucus membranes, increased eye ball recession, and skin tent duration. Some animals experience GI disturbances, such as colic and diarrhea, and frequent urination. Although gross necropsy findings are nonspecific, histopathological evaluation of the central nervious system may reveal cerebral edema and lesions consistent with PEM.Citation113
Suspicion of diet-related intoxication is made based on the history of feed and water changes; however, definitive diagnosis of sodium poisoning is made by toxicological and pathological evidence. Toxic sodium concentrations can be measured in serum, aqueous humor, cerebrospinal fluid, and in rumen content and will be severely elevated in clinical cases. Ocular fluids present a useful and reliable specimen for measuring sodium concentrations. Sodium concentration within the aqueous or vitreous humor does not change significantly during postmortem autolysis and reflects the sodium concentration at the time of death. The ocular sodium concentration is approximately 95% of the serum sodium concentration. The sodium ion concentration within the brain can also be utilized.
Pesticides and baits
Although the database search did not confirm poisonings in cattle with pesticides or baits, this must be considered.Citation114,Citation115 The toxicants of greatest concern in this category are carbamate and organophosphorus insecticides.Citation115 In 1998, 167 lactating dairy cows died within a 24-hour period in the Central Valley of California after ingesting phorate (Puschner, personal communication). The organophosphorus insecticide was mistaken for a mineral supplement and added to the total mixed ration. Phorate poisoning was confirmed by the determination of depressed cholinesterase activity in brains, and phorate detection in liver, rumen contents, and feed. Other commonly used pesticides around cattle include strychnine, anticoagulant rodenticides, zinc phosphide, and pyrethrins/pyrethroids. While most of those toxicants have resulted in malicious or accidental intoxications in small animals, they present a risk to cattle as well.
General therapeutic approach to intoxications of cattle
Treatment begins with removal of the causative agent. If feed-mixing errors have occurred, the feed should be withheld and good quality of roughage should be provided to the animals. The ingestion of toxic materials should be immediately followed with decontamination (). Ideally, the toxic compound is removed from the rumen and GI tract. Rumen lavage may be attempted with a large diameter bore tube (eg, the Kingman tube), but the large rumen volume and fibrous contents capable of plugging the tube present a challenge. Aspiration pneumonia might be a risk. Surgical ruminal evacuation (rumenotomy) can be performed if the patient is stable and without cardiovascular compromise. All contaminated feed material can be removed, and further absorption of the toxic compound eliminated if rumenotomy is performed shortly after ingestion and before the substance is completely absorbed or transitioned into the intestine. Rumenotomy is recommended in individual cattle, with a high genetic, economic, or sentimental value. However, the procedure is time consuming and not practical or economic if numerous cattle are affected.
Table 2 The dos and don’ts when dealing with a poisoning case in cattle
For herd outbreaks, activated charcoal (AC) has been successfully used as an orally administered adsorbent of toxicants.Citation116–Citation118 In suspected intoxications AC should be administered as soon as possible, prior to absorption of toxicants from the GI tract. AC binds toxicants, preventing or reducing the absorption. The dosage for cattle is 1–3 g per kg of bodyweight, mixed with 5 mL water per gram AC.Citation119 The water-AC mix is administered per orogastric tube twice a day and can be given for multiple days without any concern for adverse effects. The Food Animal Residue Avoidance Database has listed a zero day meat and milk withdrawal for the use of AC in ruminants. It is contraindicated to administer AC with mineral oil, as the adsorptive properties of charcoal diminish. Dairy calves should be fed at least 3 hours apart from charcoal administration as feeding interferes with adsorptive properties.
Administration of a purgative agent will decrease the GI transit time and subsequently time for absorption. Commonly used parenteral cathartics in cattle include magnesium sulfate (Epsom salt) and sodium sulfate (Glauber’s salt). Their cathartic action results from osmotically mediated water retention, stimulating GI peristalsis. Magnesium also may stimulate release of cholecystokinin, leading to accumulation of intraluminal electrolytes and fluid and increasing intestinal motility.Citation120 Epsom salt can be administered from 250 to 500 mg/kg body mass in the AC slurry. Magnesium sulfate should be used with caution in animals with cardiac abnormalities or myocardial injury. If affected cattle already have diarrhea, there is no need to administer a cathartic agent. Mineral oil is a lubricant laxative, commonly used in large animals for the treatment of GI constipation and fecal impaction. Its use is discouraged in intoxications because of questionable efficiency as adsorbent for toxicants and adverse effect on AC ().
Intoxicated cattle often need supportive therapy irrespective of specific toxin. Depending on electrolyte and hydration status of the animal, intravenous fluid therapy may be warranted. On farm, fluid therapy is possible and should be encouraged by the livestock veterinarian. Thiamine (vitamin B1) should be administered to anorexic cattle and those with neurologic deficits, for prevention or treatment of PEM.
If a specific diagnosis has been made, possible antidotes should be administered as soon as possible. The food animal veterinarian should keep in mind that most of the antidotes are not approved for the use in cattle, and extra-label drug use will be required in addition to establishment of milk and meat withdrawal times. Veterinarians are encouraged to familiarize themselves with regulations and laws applicable in their practicing region. In the United States, the Food Animal Residue Avoidance Databank provides a useful resource.
Conclusion
The present study demonstrated that in cattle, overexposures to minerals, metals, and poisonous plants are the predominant causes of intoxications in California. While poisonings were only confirmed in 13.5% of the 1199 cases submitted as suspect intoxications, all cases of unexplained deaths or high morbidity events should be investigated as to a possible toxic etiology. Proper sample collection from the animal and environment is crucial for a diagnostic work-up. Information regarding exposure, chronology of events and types of clinical signs, blood and chemistry changes, treatment initiated, and response to treatment should be recorded and sent to the veterinary diagnostic laboratory to narrow necessary testing. While newer analytical methodologies allow broad-based screening of appropriate samples in cases in which exposure to a specific toxicant has not been identified, no single comprehensive test for all possible toxicants exists.
Treatment of suspect poisonings is initially symptomatic and supportive but becomes more targeted with diagnosis. Decontamination of the GI tract using activated charcoal is common practice for managing acute poisonings in cattle, even though few data exist on the adsorptive capacity. While decontamination is commonly initiated prior to a confirmed laboratory diagnosis, it is important to consider that some toxicants, such as metals, are not adsorbed by activated charcoal. Food animal practitioners and veterinary toxicologists can provide consultation about toxic rule-outs for a case, diagnostic testing, treatment of affected animals, and prevention of additional cases. In addition, the possibility of residues in food animal products must be considered when dealing with a poisoning case in cattle, which may require consultation with appropriate regulatory agencies.
Acknowledgments
We thank all pathologists, diagnosticians, and technicians of the California Animal Health and Food Safety Laboratory for their expertise and valuable contributions to the diagnostic investigations of cases.
Disclosure
The authors report no conflicts of interest in this work.
References
- GaleyFDDiagnostic toxicology for the small animal veterinarianCalif VetSep-Oct1994710
- California Department of Food and AgricultureReporting animal diseases http://www.cdfa.ca.gov/ahfss/animal_health/Disease.htmlAccessed June 26, 2012
- GiorgiMNasoBLaboratory diagnostic examinations in veterinary toxicologyVet Res Commun200428Suppl 1103106
- GaleyFDDiagnostic toxicologyPlumleeKHClinical Veterinary ToxicologySt Louis, MOMosby20042223
- OsheimDLRossPFNelsonHAPCB residues in feedlot steers. II. Tissue levelsBull Environ Contam Toxicol19822867167176809083
- RogersGMCapucilleDJPooreMHMassJSmallwoodJEGrowth performance of cattle following percutaneous liver biopsy utilizing a Schackelford–Courtney biopsy instrumentBovine Pract200135177184
- ZhangDBJElswickRKMillerWGBaileyJLEffect of serum-clot contact time on clinical chemistry laboratory resultsClin Chem1998446132513339625060
- MinnickPDBraseltonWEMeerdinkGLSlankerMRAltered serum element concentrations due to laboratory usage of Vacutainer tubesVet Hum Toxicol19822464134147179713
- FrankELHughesMPBanksonDDRobertsWLEffects of anticoagulants and contemporary blood collection containers on aluminum, copper, and zinc resultsClin Chem20014761109111211375301
- SatoISeraKSuzukiTKobayashiHTsudaSEffects of formalin-preservation on element concentrations in animal tissuesJ Toxicol Sci200631319119516960429
- BedwellCLHamarDWHoestereyMLSondermanJPOddeKGComparison of 4 Methods for Forage Nitrate AnalysisJ Vet Diagn Invest1995745275308580177
- CasteelSWJohnsonGCMillerMAAmaranthus retroflexus (Redroot Pigweed) poisoning in cattleJ Am Vet Med Assoc19942047106810708045809
- OzmenOMorFUnsalANitrate poisoning in cattle fed Chenopodium album hayVet Hum Toxicol2003452838412678293
- MuirheadDPropionibacterium appears capable of reducing nitrate, nitrite toxicitiesFeedstuffs199264401213
- KempAGeurinkJHHaalstraRTMalesteinANitrate poisoning in cattle. 2. Changes in nitrite in rumen fluid and methemoglobin formation in blood after high nitrate intakeNeth J Agr Sci19772515162
- EdwardsGFosterALiveseyCUse of ocular fluids to aid postmortem diagnosis in cattle and sheepIn Pract20093112225
- BoermansHJDiagnosis of nitrate toxicosis in cattle, using biological fluids and a rapid ion chromatographic methodAm J Vet Res19905134914952316929
- BrightSJPostLOVeterinary antidotes and availability: an update2008 Available from: http://www.abvt.org/public/docs/antidoteupdate08.pdfAccessed June 27, 2012
- BrightSJMurphyMJSteinschneiderJCLovellRAPostLOTreatment of animal toxicoses: a regulatory perspectiveVet Clin North Am Food Anim Pract2011272481512x21575782
- InoueTKaibaraMSakurai-YamashitaYKawanoMIshimaruTTaniyamaKIncreases in serum nitrite and nitrate of a few-fold adversely affect the outcome of pregnancy in ratsJ Pharmacol Sci200495222823315215647
- JortaniSAHelmRAValdesRInhibition of Na,K-ATPase by oleandrin and oleandrigenin, and their detection by digoxin immunoassaysClin Chem19964210165416588855150
- McLainPLEffects of cardiac glycosides on spontaneous efferent activity in vagus and sympathetic nerves of catsInt J Neuropharmacol1969843793875820455
- GaleyFDHolstegeDMPlumleeKHDiagnosis of oleander poisoning in livestockJ Vet Diagn Invest1996833583648844581
- GaleyFDHolstegeDMJohnsonBJSiemensLToxicity and diagnosis of oleander (Nerium oleander) poisoning in livestockGarlandTBarrACToxic plants and other natural toxicantsNew York, NYCAB International1998215219
- TorERFiligenziMSPuschnerBDetermination of oleandrin in tissues and biological fluids by liquid chromatography-electrospray tandem mass spectrometryJ Agric Food Chem200553114322432515913289
- TiwaryAKPoppengaRHPuschnerBIn vitro study of the effectiveness of three commercial adsorbents for binding oleander toxinsClin Toxicol2009473213218
- CamphausenCHaasNAMattkeACSuccessful treatment of oleander intoxication (cardiac glycosides) with digoxin-specific Fab antibody fragments in a 7-year-old child: case report and review of literatureZ Kardiol2005941281782316382383
- WiedenfeldHPlants containing pyrrolizidine alkaloids: toxicity and problemsFood Addit Contam Part A Chem Anal Control Expo Risk Assess201128328229221360374
- StegelmeierBGardnerDDavisTZLivestock poisoning with pyrrolizidine-alkaloid–containing plants (Senecio, Crotalaria, Cynoglossum, Amsinckia, Heliotropium, and Echium spp.)Rangelands20093113537
- YeeSBKinserSHillDASynergistic hepatotoxicity from coexposure to bacterial endotoxin and the pyrrolizidine alkaloid monocrotalineToxicol Appl Pharmacol2000166317318510906281
- MorrisPO’NeillDTannerSSynergistic liver toxicity of copper and retrorsine in the ratJ Hepatol19942157357427890887
- NewbernePMChanWCRogersAEInfluence of light, riboflavin, and carotene on the response of rats to the acute toxicity of aflatoxin and monocrotalineToxicol Appl Pharmacol19742822002084136821
- HincksJRKimHYSegallHJMolyneuxRJStermitzFRCoulombeRAJrDNA cross-linking in mammalian cells by pyrrolizidine alkaloids: structure-activity relationshipsToxicol Appl Pharmacol1991111190981949039
- HolstegeDMSeiberJNGaleyFDRapid multiresidue screen for alkaloids in plant-material and biological samplesJ Agric Food Chem1995433691699
- EdgarJAColegateSMBoppreMMolyneuxRJPyrrolizidine alkaloids in food: a spectrum of potential health consequencesFood Addit Contam Part A Chem Anal Control Expo Risk Assess201128330832421360376
- WangXHowellCPChenFYinJJiangYGossypol – a polyphenolic compound from cotton plantAdv Food Nutr Res20095821526319878861
- SmithHAThe pathology of gossypol poisoningAm J Pathol195733235336513402891
- HudsonLMKerrLAMaslinWRGossypol toxicosis in a herd of beef calvesJ Am Vet Med Assoc19881929130313053391858
- ReiserRFuHCThe mechanism of gossypol detoxification by ruminant animalsJ Nutr19627621521814491326
- LindseyTOHawkinsGEGuthrieLDPhysiological responses of lactating cows to gossypol from cottonseed meal rationsJ Dairy Sci19806345625737189761
- HassanMESmithGWOttRSReversibility of the reproductive toxicity of gossypol in peripubertal bullsTheriogenology20046161171117915037004
- ZelskiRZRothwellJTMooreREKennedyDJGossypol toxicity in preruminant calvesAust Vet J199572103943988599575
- RiscoCAHolmbergCAKutchesAEffect of graded concentrations of gossypol on calf performance: toxicological and pathological considerationsJ Dairy Sci19927510278727981430484
- RiscoCAChenowethPJLarsenREThe effect of gossypol in cottonseed meal on performance and on hematological and semen traits in postpubertal Brahman bullsTheriogenology199340362964216727345
- HronRJKimHLCalhounMCFisherGSDetermination of (+)-, (−)-, and total gossypol in cottonseed by high-performance liquid chromatographyJ Am Oil Chem Soc1999761113511355
- CallawayTREdringtonTSRychlikJLIonophores: their use as ruminant growth promotants and impact on food safetyCurr Issues Intest Microbiol200342435114503688
- EricksonGEMiltonCTFanningKCInteraction between bunk management and monensin concentration on finishing performance, feeding behavior, and ruminal metabolism during an acidosis challenge with feedlot cattleJ Anim Sci200381112869287914601891
- DuffieldTFBaggRNUse of ionophores in lactating dairy cattle: a reviewCan Vet J200041538839410816832
- WentinkGHVenteJPMonensin poisoning in dairy cattle. Report of a case (author’s transl)Tijdschr Diergeneeskd198110612623625 Dutch7196096
- WardropeDDMacleodNSSloanJROutbreak of monensin poisoning in cattleVet Rec1983112245605616879977
- PotterELVanDuynRLCooleyCOMonensin toxicity in cattleJ Anim Sci1984586149915116378866
- Van VleetJFAmstutzHEWeirichWERebarAHFerransVJClinical, clinicopathologic, and pathologic alterations in acute monensin toxicosis in cattleAm J Vet Res19834411213321446650960
- VargaASchoberKEHollomanCHStrombergPCLakritzJRingsDMCorrelation of serum cardiac troponin I and myocardial damage in cattle with monensin toxicosisJ Vet Intern Med20092351108111619656284
- LitwakKNMcMahanALottKALottLEKoenigSCMonensin toxicosis in the domestic bovine calf: a large animal model of cardiac dysfunctionContemp Top Lab Anim Sci2005443454915934724
- HosieBDRolloDGNutritional myopathy in cattle associated with monensin toxicosisVet Rec198511651321333984177
- HuangMRumbeihaWKBraseltonWEJohnsonMRapid quantification of ionophores in feeds by liquid chromatography-tandem mass spectrometryJ Vet Diagn Invest201123595696121908354
- LangstonVCGaleyFDLovellRABuckWBToxicity and therapeutics of monensin: a reviewVet Med198580107584
- PlumbDCTetracyclineHCLPlumbDCPlumb’s Veterinary Drug Handbook5th edAmes, IOWiley-Blackwell Publishing2005741745
- SpearsJWMicronutrients and immune function in cattleProc Nutr Soc200059458759411115794
- SteffenDJCarlsonMPCasperHHCopper toxicosis in suckling beef calves associated with improper administration of copper oxide bolusesJ Vet Diagn Invest1997944434469376443
- GaleyFDMaasJTronstadRJCopper toxicosis in two herds of beef calves following injection with copper disodium edetateJ Vet Diagn Invest1991332602631911999
- BlakleyBRBerezowskiJASchieferHBArmstrongKRChronic copper toxicity in a dairy cowCan Vet J198223619019217422152
- SullivanJMJanovitzEBRobinsonFRCopper toxicosis in veal calvesJ Vet Diagn Invest1991321611641892934
- BohmanVRPooleSCKvasnickaWGTronstadRJCollinsonRWThe toxicology and composition of bovine tissues after parenteral administration of high levels of copper saltsVet Hum Toxicol19872943073123629910
- BidewellCADrewJRPayneJHSayersARHigginsRJLiveseyCTCase study of copper poisoning in a British dairy herdVet Rec20121701846422562897
- BradleyCHCopper poisoning in a dairy herd fed a mineral supplementCan Vet J199334528729217424221
- MinervinoAHBarrêto JúniorRAFerreiraRNClinical observations of cattle and buffalos with experimentally induced chronic copper poisoningRes Vet Sci200987347347819487001
- PerrinDJSchieferHBBlakleyBRChronic copper toxicity in a dairy herdCan Vet J199031962963217423660
- Lopez-AlonsoMCrespoAMirandaMCastilloCHernandezJBeneditoJLAssessment of some blood parameters as potential markers of hepatic copper accumulation in cattleJ Vet Diagn Invest2006181717516566259
- MeltonLATracyMLMollerGScreening trace elements and electrolytes in serum by inductively-coupled plasma emission spetrometryClin Chem1990362472502302768
- PuschnerBThurmondMCChoiYKInfluence of age and production type on liver copper concentrations in calvesJ Vet Diagn Invest200416538238715460319
- SharpeRTLiveseyCTLead poisoning in cattle and its implications for food safetyVet Rec20061593717416844817
- BaarsAJvan BeekHVisserIJLead intoxication in cattle: a case reportFood Addit Contam1992943573641493885
- WaldnerCCheckleySBlakleyBPollockCMitchellBManaging lead exposure and toxicity in cow-calf herds to minimize the potential for food residuesJ Vet Diagn Invest200214648148612423030
- DwivediSKDeySSwarupDLead in blood and milk from urban Indian cattle and buffaloVet Hum Toxicol19953754714728592841
- OskarssonAJorhemLSundbergJNilssonNGAlbanusLLead poisoning in cattle – transfer of lead to milkSci Total Environ19921112–383941539131
- YabeJNakayamaSMIkenakaYMuzanduKIshizukaMUmemuraTUptake of lead, cadmium, and other metals in the liver and kidneys of cattle near a lead-zinc mine in Kabwe, ZambiaEnviron Toxicol Chem20113081892189721590713
- OskarssonAJorhemLSundbergJNilssonNGAlbanusLLead poisoning in cattle – transfer of lead to milkSci Total Environ19921112–383941539131
- MavangiraVEvansTJVillamilJAHahnAWChigerweMTylerJWRelationships between demographic variables and lead toxicosis in cattle evaluated at North American veterinary teaching hospitalsJ Am Vet Med Assoc2008233695595918795860
- Krametter-FroetscherRTataruchFHauserSLeschnikMUrlABaumgartnerWToxic effects seen in a herd of beef cattle following exposure to ash residues contaminated by lead and mercuryVet J200717419910516753317
- OzmenOMorFAcute lead intoxication in cattle housed in an old battery factoryVet Hum Toxicol200446525525615487647
- BakerJCLead poisoning in cattleVet Clin North Am Food Anim Pract1987311371473552148
- MirandaMLopez-AlonsoMGarcia-PartidaPVelascoJBeneditoJLLong-term follow-up of blood lead levels and haematological and biochemical parameters in heifers that survived an accidental lead poisoning episodeJ Vet Med A Physiol Pathol Clin Med200653630531016901275
- Lead poisoning in cattle associated with car batteries and sump oilVet Rec2011169717317621961156
- O’HaraTMBennettLMcCoyCPJackSWFlemingSLead poisoning and toxicokinetics in a heifer and fetus treated with CaNa2 EDTA and thiamineJ Vet Diagn Invest1995745315378580178
- LittlePBSorensenDKBovine polioencephalomalacia, infectious embolic meningoencephalitis, and acute lead poisoning in feedlot cattleJ Am Vet Med Assoc196915512189219035392156
- SpearsJWLloydKEFryRSTolerance of cattle to increased dietary sulfur and effect of dietary cation-anion balanceJ Anim Sci20118982502250921383030
- HaydockDSulfur-induced polioencephalomalacia in a herd of rotationally grazed beef cattleCan Vet J2003441082882914601680
- BekeGJHironakaRToxicity to beef cattle of sulfur in saline well water: a case studySci Total Environ199110132812902038662
- McKenzieRACarmichaelAMSchibrowskiMLDuiganSAGibsonJATaylorJDSulfur-associated polioencephalomalacia in cattle grazing plants in the Family BrassicaceaeAust Vet J2009871273219178473
- DahmeEBilzerTDirksenGNeuropathology of manure gas poisoning (H2S poisoning) in cattleDtsch Tierarztl Wochenschr1983908316320 German6354670
- FelixTLWeissWPFluhartyFLLoerchSCEffects of copper supplementation on feedlot performance, carcass characteristics, and rumen sulfur metabolism of growing cattle fed diets containing 60% dried distillers grainsJ Anim Sci20129082710271622393033
- GouldDHPolioencephalomalaciaJ Anim Sci19987613093149464912
- MillerWJZinc nutrition of cattle: a reviewJ Dairy Sci1970538112311354918951
- GrahamTWThurmondMCCleggMSAn epidemiologic study of mortality in veal calves subsequent to an episode of zinc toxicosis on a California veal calf operation using zinc sulfate-supplemented milk replacerJ Am Vet Med Assoc198719010129613013583883
- Lopez AlonsoMBeneditoJLMirandaMCastilloCHernandezJShoreRFArsenic, cadmium, lead, copper and zinc in cattle from Galicia, NW SpainSci Total Environ20002462–323724810696725
- GrahamTWGoodgerWJChristiansenVThurmondMCEconomic losses from an episode of zinc toxicosis on a California veal calf operation using a zinc sulfate-supplemented milk replacerJ Am Vet Med Assoc198719066686713570916
- RunnegarMTKongSBerndtNProtein phosphatase inhibition and in vivo hepatotoxicity of microcystinsAm J Physiol19932652 Pt 1G224G2308396333
- PuschnerBGaleyFDJohnsonBBlue-green algae toxicosis in cattleJ Am Vet Med Assoc1998213111605160715719838962
- GaleyFDBeasleyVRCarmichaelWWKleppeGHooserSBHaschekWMBlue-green algae (Microcystis aeruginosa) hepatotoxicosis in dairy cowsAm J Vet Res1987489141514203116892
- MereishKASolowREffect of antihepatotoxic agents against microcystin-LR toxicity in cultured rat hepatocytesPharm Res1990732562592339099
- OrrPTJonesGJHunterRABergerKExposure of beef cattle to sub-clinical doses of Microcystis aeruginosa: toxin bioaccumulation, physiological effects and human health risk assessmentToxicon200341561362012676440
- OrrPTJonesGJHunterRABergerKDe PaoliDAOrrCLAIngestion of toxic Microcystis aeruginosa by dairy cattle and the implications for microcystin contamination of milkToxicon200139121847185411600147
- FiligenziMSPoppengaRHTiwaryAKPuschnerBDetermination of alpha-amanitin in serum and liver by multistage linear ion trap mass spectrometryJ Agric Food Chem20075582784279017371042
- PuschnerBWegenastCMushroom poisoning cases in dogs and cats: diagnosis and treatment of hepatotoxic, neurotoxic, gastroenterotoxic, nephrotoxic, and muscarinic mushroomsVet Clin North Am Small Anim Pract2012422375387viii22381186
- YeeMMWoodsLWPoppengaRHPuschnerBAmanitin intoxication in two beef calves in CaliforniaJ Vet Diagn Invest201224124124422362963
- LindellTJWeinbergFMorrisPWRoederRGRutterWJSpecific inhibition of nuclear RNA polymerase II by alpha-amanitinScience197017039564474494918258
- PuschnerBRoseHHFiligenziMSDiagnosis of Amanita toxicosis in a dog with acute hepatic necrosisJ Vet Diagn Invest200719331231717459866
- RiffkinGGHuckerDAMcLoedIKWater deprivation in agisted cattleAust Vet J198157115325337342939
- HoganJPPetherickJCPhillipsCJThe physiological and metabolic impacts on sheep and cattle of feed and water deprivation before and during transportNutr Res Rev2007201172819079858
- PringleJKBerthiaumeLMHypernatremia in calvesJ Vet Intern Med19882266703221359
- TruemanKFClagueDCSodium chloride poisoning in cattleAust Vet J19785428991655985
- OsweilerGDCarrTFSandersonTPCarsonTLKinkerJAWater deprivation – sodium ion toxicosis in cattleJ Vet Diagn Invest1995745835858580196
- GuitartRCroubelsSCaloniFAnimal poisoning in Europe. Part 1: Farm livestock and poultryVet J2010183324925419359202
- GiorgiMMengozziGMalicious animal intoxications: poisoned baitsVet Med (Praha)2011564173179
- PassMAStewartCAdministration of activated charcoal for the treatment of lantana poisoning of sheep and cattleJ Appl Toxicol1984452672696512165
- KobelWSumnerDDCampbellJBHudsonDBJohnsonJLProtective effect of activated charcoal in cattle poisoned with atrazineVet Hum Toxicol19852731851884024447
- JoubertJPSchultzRAThe treatment of Moraea polystachya (Thunb) KER-GAWL (cardiac glycoside) poisoning in sheep and cattle with activated charcoal and potassium chlorideJ S Afr Vet Assoc19825342492537182498
- PlumbDCCharcoal, activatedPlumb’s Veterinary Drug HandbookAmes, IOWiley-Blackwell Publishing2005216217
- PasrichaPJTreatment of disorders of bowel motility and water flux; antiemetics; agents used in biliary and pancreatic diseaseBruntonLLLazoJSParkerKLThe Pharmacological Basis of Therapeutics11th edNew York, NYMcGraw-Hill Professional2005989995