Abstract
Avian bornavirus (ABV) matrix (M) genes were detected by RT-PCR on brain tissue obtained from 192 mute swans harvested from several Northeastern states. A RT-PCR product was detected in 45 samples. Sequencing of the PCR products confirmed the presence of ABV belonging to the ‘goose’ genotype. The prevalence of positive samples ranged from 28% in Michigan to 0% in northern New York State. Two Rhode Island isolates were cultured. Their M, N, and X-P gene sequences closely matched recently published sequences from Canada geese.
Introduction
Mute swans (Cygnus olor) are native to Europe and Asia. They were introduced into North America in the late 1870s as decorative waterfowl.Citation1 Some birds escaped or were intentionally released and became established along the East Coast of the United States. Today they are resident along the East Coast, around the Great Lakes, and into the Pacific Northwest.Citation2 There are localized populations inland throughout the Northeast and Midwest. As an introduced species, mute swans have significant negative effects on native waterfowl populations and habitat. They consume large amounts of aquatic vegetation and disrupt natural food chains. They are aggressive towards many native waterfowl, other wetland and shore birds as well as people and may pose a danger to pets and children. For these reasons, states are permitted to control nuisance mute swan populations. In some states, control is based on the capture and euthanasia of birds. We took advantage of this resource to examine the brains from euthanized birds for the presence of avian bornavirus (ABV).
Avian bornavirus was first described in parrots suffering from Proventricular Dilatation disease (PDD).Citation3,Citation4 This neurologic disease of psittacines affects the enteric ganglia and results in paralysis of the gastrointestinal tract, food impaction, and proventricular dilatation. While ABV has been consistently isolated from PDD-affected birds, surveys have shown that many ABV-infected parrots remain healthy while shedding the virus in their droppings and choana.Citation5
The origins of these psittacine strains of ABV are unknown but recent surveys have demonstrated that ABV is common in wild Canada geese (Branta canadensis) across North America.Citation6,Citation7 The presence of ABV has been demonstrated by RT-PCR on goose droppings while the virus has been isolated from goose brains. ABV has also been demonstrated in the brains of encephalitic Canada geese and trumpeter swans (Cygnus buccinator) in Ontario.Citation6 Payne et al found the virus in apparently healthy Canada geese.Citation7 These goose isolates belong to their own characteristic genotype and do not appear to be closely related to psittacine isolates. They are however more closely related to Borna disease virus, a virus that causes neurologic disease in horses and sheep in central Europe. Borna disease virus has been detected in the droppings of mallards (Anas platyrhynchos) and jackdaws (Corvus monedula) in Europe.Citation8
In order to further examine the distribution of avian Bornavirus in free-living species, we screened brains from mute swans (Cygnus olor) obtained during the removal of nuisance flocks in the Northeastern United States. We report the widespread presence of ABV in these birds.
Materials and methods
Sample collection
The samples were collected by the Wildlife Services division of the United States Department of Agriculture-Animal and Plant Health Inspection Service (USDA-APHIS). One hundred and ninety-two swan heads were obtained from flocks culled in Michigan, New Jersey, New York, and Rhode Island. Birds were removed under wildlife damage management between April 2008 and August 2011. Their health status was recorded and the birds euthanized with CO2. The heads were removed and frozen until transported to our laboratory. Samples were taken from both fore and hind brain after removal of the calvarium from 40 swan heads. The remaining 152 brain tissue samples were removed by syringe aspiration after thawing using an 18-gauge needle inserted into the foramen magnum. Approximately 1 mL of brain tissue was obtained from each bird in this way.
RT-PCR
RNA purification
Frozen brain samples were excised and homogenized in lysis buffer. Total RNA was isolated using the Quiagen RNeasy Minikit based on the manufacturer’s instructions and total RNA was eluted in 40 µL of elution buffer. First strand cDNA was generated using the Applied Biosystems® High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), with 10 µL RNA and random primers. The final composition of the cDNA reaction mixture was 2 µL of 10X buffer, 2 µL of 10X random primers, 0.8 µL of 10 mM dNTP mix, 1 µL RNAse inhibitor, and 1 µL reverse transcriptase in a volume of 20 µL.
PCR assays
A primer set (PM1) targeting a 350 nt region of the M gene was used as the initial screen for ABV sequences. This primer set was previously used by Payne et al for the characterization of ABV in Canada geese.Citation7 Primer sequences were: PM1Forward 5′GGTAATTGTTCCTGGATGGC3′ and PM1Reverse 5′ACACCAATGTTCCGAAGACG 3. PCR conditions were as follows: Initial denaturation, 94°C for 2 minutes, followed by 35 cycles of 94°C, 30 seconds, 55°C, 30 seconds, and 72°C, 30 seconds followed by a final extension of 5 minutes at 72°C. A primer set (5′-TGTTGCGGTAACAACCAACC-3′ and 5′-ACACCAATGTTCCGAAGACG-3′) was used to amplify the N, X, P, and partial M genes from the two swan isolates. PCR conditions were as follows; initial denaturation, 94°C for 2 minutes, followed by 35 cycles of 94°C for 1 minute, 55°C for 1 minute, and 72°C for 2 minutes, followed by a final extension of 5 minutes at 72°C. PCR assays included multiple reagent controls (no added cDNA). Reaction products were analyzed by agarose gel electrophoresis and purified using a commercially available gel extraction kit (Qiagen, Venlo, the Netherlands). Purified PCR products were sequenced by the Gene Technologies Laboratory at Texas A&M University. Sequences were assembled using Sequencher® version 4.1 sequence analysis software, Gene Codes Corporation, (Ann Arbor, MI, USA) and Geneious Pro 5.1.7 software (Auckland, New Zealand).
Virus isolation: Two swan brain samples were used to infect a duck embryo fibroblast culture as described previously.Citation9 Following three blind passages, the presence of ABV was determined by RT-PCR using an M gene probe. When growth was detected, primer sets for targeting the N, X, P, and partial M genes were employed to examine the sequences of these isolates.
Immunofluorescence assays: These were conducted according to the method of Gray et al.Citation9
Western blotting: This was conducted according to the method of Villanueva et al.Citation10
Phylogenetic analysis: A phylogeny was constructed using a 1500 nt genome region that encompassed partial N, complete X and P, and partial M sequences. The evolutionary history was inferred using the Maximum Likelihood method based on the HKY model.Citation11 A bootstrap consensus tree was inferred from 1000 replicates.Citation12 Evolutionary analyses were conducted in MEGA5.Citation13
Results
Detection of ABV RNA in brain samples
Total RNA from 192 brain tissue samples was extracted and tested by RT-PCR using the primer set described above. Overall, 45 samples (23%) yielded a PCR product of appropriate size. There was no difference in apparent prevalence between brain tissues removed by dissection and tissues removed by needle aspiration. Twenty of these PCR products were sequenced and all were shown to be closely related to previously reported goose isolates. Of the positive brain samples, 26/96 (27%) were obtained from Michigan; 18/81 (22%) from Rhode Island, and one of one from New Jersey. None of the 14 samples from upper New York State were positive. Testing of ten swan samples from each of ten Michigan counties bordering Lake Huron indicated that the proportion of positive samples ranged from 10% to 50%.
ABV brain isolates
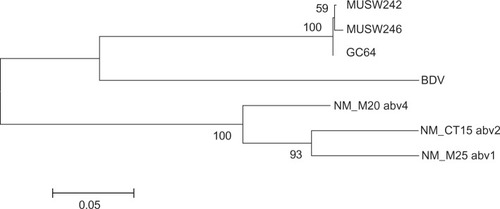
Two RT-PCR-positive brain samples from Washington County, Rhode Island, were inoculated into primary cultures of duck embryo fibroblasts (DEF). After the third passage, ABV was detected by RT-PCR, indirect immunofluorescence, and western blotting. The N, X, P, and partial M genes of two swan isolates showed at least 99.4% nt sequence identity with each other and 95.5% identity to a Canada goose genotype.Citation7 They clustered with the Canada goose isolates in the phylogenetic tree (). These swan bornaviruses shared 69% nt identity with a prototype Borna disease virus (BDV) strain. (These sequences have Genbank Accession numbers, JQ687270 and JQ687271.)
Figure 1 Phylogeny generated using an 1100 nt fragment of bird and mammal bornaviruses that includes part of the N gene, the N/X intergenic region, the entire X gene, and part of the P gene.
Discussion
This survey indicates that ABV infection is widespread among mute swans in the Northeastern United States. The virus could be detected in the brains of up to 50% of birds sampled in some Michigan counties. It is likely that transmission of this virus between birds is mainly by the fecal–oral route since 4/219 oropharyngeal/cloacal swab samples were RT-PCR positive (Tizard I, unpublished data, 2012). Given their environment and behavior, droppings from infected swans could readily contaminate their habitat including both water and nearby shorelines. The survival time of this virus in the environment remains unknown. We have observed that domestic ducks can acquire ABV infection by consuming the droppings of other, naturally infected psittacine birds (Tizard I, unpublished data, 2012). The close similarity of swan ABV isolates to Canada goose isolates and the fact that both species occupy similar habitats, suggests that ABV may circulate between these two species and possibly other adjacent waterfowl in the United States and Canada.
The significance of this infection is unclear. Five of the samples examined in this study were obtained from birds reported to be ’sick‘ at the time of capture but none of these had neurologic disease. At the time, their sickness was attributed either to parasitic worm infestations or to lead poisoning. No necropsies were performed on any of the birds. This raises the question of whether ABV can cause disease in swans and if so, when, and under what circumstances? We have examined brain samples from Canada geese presenting with neurologic illness and have demonstrated the presence of the goose genotype of ABV in their brains (Tizard I, unpublished data 2012). Indeed previous investigations have suggested that a PDD-like illness and/or bornavirus infection occurs in geese and swans. For example, Daoust et al reported on two cases of disease in Canada geese in which the lesions closely resembled classical PDD.Citation14 More recently, Smith et alCitation6,Citation15 used immunohistochemistry as well as PCR to demonstrate the presence of ABV in the brains of several Canada geese and two trumpeter swans (Cygnus buccinator) presenting with an ill-defined neurologic disease. However, these prior reports did not suggest that PDD was a common disease in these birds, nor that healthy birds served as carriers of ABV. Given the apparently high background prevalence of infection in these waterfowl, it is unsurprising that some birds with neurologic disease are infected with ABV. It does not however prove causation. It is also clear from this data that most ABV-infected swans appear to be healthy.
Acknowledgments
The authors would like to thank the wildlife disease biologists with Wildlife Services’ National Wildlife Disease Program, the many other Wildlife Services staff that contributed assistance in the field, and the Wildlife Services Wild Bird Tissue Archive for samples from the influenza swab collection. This study was supported by the Richard M Schubot endowment at Texas A&M University.
Disclosure
The authors report no conflicts of interest in this work.
References
- AllinCCChaskoGGHusbandTPMute swans in the Atlantic flyway: a review of the history, population growth, and management needsTrans NE Sect Wildlife Soc1987443247
- CobbJSHarlanMMMute swan (Cygnus olor) feeding and territoriality affects diversity and density of rooted aquatic vegetationAmer Zool198020882
- KistlerALGanczAClubbSRecovery of divergent avian bornaviruses from cases of proventricular dilatation disease: Identification of a candidate etiologic agentVirol J200858818671869
- HonkavuoriKSShivaprasadHLWilliamsBLNovel Borna virus in psittacine birds with proventricular dilatation diseaseEmerg Infect Dis200814121883188619046511
- PayneSShivaprasadHLMirhosseiniNUnusual and severe lesions of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) acting as healthy carriers of avian bornavirus (ABV) and subsequently infected with a virulent strain of ABVAvian Pathol2011401152221331944
- DelnattePBerkvensCKummrowMNew genotype of avian bornavirus in wild geese and trumpeter swans in CanadaVet Rec20111694108
- PayneSCovaledaCJianhuaGDetection and characterization of a distinct Bornavirus lineage from healthy Canada geese (Branta canadensis)J Virol201185120531205621900161
- BergMJohanssonMMontellHBergALWild birds as a possible natural reservoir of Borna disease virusEpidemiol Infect2001127117317811561971
- GrayPLSuchodolskiPWigleWUse of avian Bornavirus isolates to produce proventricular dilatation disease in conuresEmerg Infect Dis20101647347920202423
- VillanuevaIGrayPMirhosseiniNThe diagnosis of proventricular dilatation disease: Use of a Western blot assay to detect antibodies against avian Borna virusVet Microbiol201014319620120036080
- HasegawaMKishinoHYanoTDating the human-ape splitting by a molecular clock of mitochondrial DNAJ Mol Evol19852221601743934395
- FelsensteinJConfidence limits on phylogenies: An approach using the bootstrapEvolution198539478379128561359
- TamuraKPetersonDPetersonNStecherGNeiMKumarSMEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methodsMol Biol Evol201128102731273921546353
- DaoustPYJulianRJYasonCVArtsobHProventricular Impaction Associated with Nonsuppurative Encephalomyelitis and Ganglioneuritis in 2 Canada GeeseJ Wildlife Dis1991273513517
- SmithDBerkvensCKummrowMIdentification of avian bornavirus in the brains of Canada geese (Branta canadensis) and trumpeter swans (Cygnus buccinator) with non-suppurative encephalitisProc Wildlife Dis AssnPuerto Iguazu, Argentina5 2010