Abstract
Alcohol consumption is an established risk factor for breast cancer. Nonetheless, the mechanism by which alcohol contributes to breast tumor initiation or progression has yet to be definitively established. Studies using cultured human tumor cell lines have identified signaling molecules that may contribute to the effects of alcohol, including reactive oxygen species and other ethanol metabolites, matrix metalloproteases, the ErbB2/Her2/Neu receptor tyrosine kinase, cytoplasmic protein kinases, adenylate cyclase, E-cadherins, estrogen receptor, and a variety of transcription factors. Emerging data suggest that the epidermal growth factor receptor (EGFR) tyrosine kinase may contribute to breast cancer genesis and progression. Here we integrate these findings and propose three mechanisms by which alcohol contributes to breast cancer. A common feature of these mechanisms is increased EGFR signaling. Finally, we discuss how these mechanisms suggest strategies for addressing the risks associated with alcohol consumption.
Alcohol and other breast cancer risk factors
One in eight American women will be stricken with breast cancer, which is the second leading cause of cancer-related mortality among American women.Citation1 Approximately 15% of those breast cancer patients who receive surgery and chemotherapy will experience a recurrence of the disease. This is a major contributor to the fact that one-third of those who develop breast cancer will ultimately succumb to the disease.Citation2 Thus, breast cancer remains an important public health and societal issue and more information is needed concerning the causes of breast cancer and risk factors associated with the disease.
In 50% of breast cancers the only apparent risk factors are gender and age.Citation1,Citation2 In the other half of breast cancers the risk factors include genetic factors, environmental factors, age of menarche and menopause, and reproductive history.Citation2 The latter factors underscore the contributions of female reproductive hormones to breast cancer risk.Citation2
ErbB family receptor tyrosine kinases and their ligands, members of the epidermal growth factor (EGF) family of peptide growth factors, also play significant roles in breast cancer pathogenesis and progression. The ErbB2 receptor tyrosine kinase, also known as HER2/Neu, is overexpressed in a significant fraction of breast tumors and this overexpression correlates with the absence of steroid hormone receptor expression and with poor disease prognosis.Citation3–Citation5 Constitutive ErbB2 signaling causes mammary malignancies in transgenic mouse model systemsCitation6,Citation7 and causes malignant growth transformation in cell culture models of breast cancer.Citation8,Citation9 Moreover, agents that target ErbB2 are moderately effective at controlling ErbB2-positive breast tumors when these agents are administered as part of a combination chemotherapy regimen.Citation10
Although EGF receptor (EGFR) overexpression is observed in breast tumors,Citation11 the potential roles that EGFR signaling may have in the development or progression of breast cancer are not as well-characterized as those played by ErbB2.Citation12 Nonetheless, EGFR expression and signaling have been postulated to play roles in the genesis, maintenance, or progression of breast tumors. EGFR appears to be of particular relevance to breast cancer stem cells, triple negative tumors, and basal-type tumors. For example, breast cancer stem cells that exhibit elevated EGFR signaling display resistance to endocrine therapyCitation13 as well as uncontrolled self-renewal and unlimited proliferation.Citation14 Approximately 60% of basal-type and triple-negative tumors display elevated EGFR expression. Basal-type and triple-negative breast tumors are typically devoid of estrogen receptor (ER) and HER2 (ErbB2/Neu) expression and are therefore resistant to existing targeted therapies. Thus, EGFR is an attractive target in these types of breast tumors.Citation15 Indeed, a combination of carboplatin and the EGFR tyrosine kinase inhibitor cetuximab (Erbitux) synergistically inhibits proliferation of basal-type breast cancer cell lines.Citation16
Endogenous ligands for ErbB receptors also appear to play important roles in human breast cancers. For example, the EGFR ligand amphiregulin stimulates breast tumor cell motilityCitation17 and is sufficient and necessary for tumor cell expression of parathyroid hormone-related protein (PTHrP), an important contributor to tumor cell invasion and colonization of bone.Citation18,Citation19 Reduced expression of matrix metalloproteases (MMPs) in SCP20 human breast tumor cells is associated with decreased osteoclastic stimulation by these cells and reduced amphiregulin release by these cells. These data suggest that amphiregulin/EGFR signaling is coupled to bone colonization of breast tumor cells and can be regulated by matrix metallproteases. Indeed, the EGFR antagonistic antibody cetuximab or the EGFR tyrosine kinase inhibitor gefinitib inhibits bone metastasis by SCP20 cells in mouse xenograft assays.Citation20 Moreover, the fact that EGFR ligands can stimulate ErbB2 signaling through heterodimerization with EGFRCitation21 suggests that EGFR and its ligands may play a significant role in at least some ErbB2-dependent breast tumors. Indeed, pharmacological agents that target EGFR or its ligands continue to be investigated as potential breast cancer therapeutics.Citation22–Citation24
Several environmental factors appear to contribute to breast cancer risk, including ionizing radiation, pesticides, and diet.Citation2 A number of studies have identified an association between moderate alcohol consumption and increased breast cancer risk.Citation25–Citation29 For example, average consumption of 14 or more drinks per week in a five-year period prior to breast cancer diagnosis increased breast cancer risk by 82%.Citation30
Alcohol may exert its effects via several mechanisms
There appear to be multiple mechanisms by which alcohol may contribute to breast malignancies or may modulate the behavior of mammary epithelial and tumor cells in vivo and in vitro. Alcohol is metabolized in a variety of tissues, including the breast. Alcohol dehydrogenase converts ethanol to acetaldehyde, which can form adducts of DNA and can cause DNA cross-links and G:A transitions at A:T base pairs. Indeed, acetaldehyde displays weak mutagenic and carcinogenic activity.Citation29,Citation31 Xanthine oxidoreductase and aldehyde oxidase are found in breast tissue and metabolize acetaldehyde to reactive oxygen species (ROS), including the superoxide anion free radical (O2 ·−), the neutral hydroxide free radical (OH·), and hydrogen peroxide (H2O2). These ROS can contribute to breast malignancies via a variety of mechanisms, including DNA mutation, base deletion, and single and double strand breaks.Citation29,Citation31
Additional mechanisms may underlie the contributions of ethanol to breast malignancies. Alcohol at a concentration as low as 0.06% stimulates the expression of estrogen receptor alpha (ERα) and the estradiol biosynthesis enzyme aromatase in human breast cancer cell lines.Citation32,Citation33 In fact, moderate alcohol consumption of 0.7 g/kg is associated with an elevated estrogen concentration in the plasma of postmenopausal women.Citation34,Citation35 In cell line model systems alcohol concentrations of 0.06% to 0.6% stimulate transcription from the estrogen response elementCitation36 and increased transcription of the progesterone receptor and pS2, two genes whose transcription is regulated by ER.Citation37 Alcohol stimulation of ER-dependent gene expression may account for the observation that alcohol stimulates proliferation in ER-positive MCF7 human breast tumor cells but has no effect on the proliferation of ER-negative MDA-MB-231 and BT-20 human breast tumor cell lines.Citation33 However, it is has yet to be demonstrated that changes in ER-dependent gene expression underlie the effects of alcohol on the malignant phenotypes of breast tumor cells.
Alcohol induces other intracellular signaling events. Increased expression of the c-Fos transcription factor is observed in NIH 3T3 cells one hour after treatment with 0.2% alcohol. This increase persists up to 96 hours after treatment with alcohol.Citation38 Likewise, alcohol concentrations of 1.3% to 5.1% stimulate phosphorylation of the c-Jun NH2-terminal protein kinase (JNK), the p38 mitogen-activated protein kinase (p38 MAPK), and phosphatidylinositol 3-kinase (PI3K).Citation39,Citation40 Finally, alcohol concentrations as low as 0.12% inhibit expression of the adhesion molecule E-cadherin and cause an increase in cell migration.Citation41 E-cadherin is a tumor suppressor and loss of E-cadherin expression contributes to tumor progression by enhancing metastatic phenotypes.Citation42
Metalloproteases are zinc-dependent endopeptidases. These enzymes are expressed in an inactive, secreted, or transmembrane precursor form. The interaction of a zinc ion with three histidine residues and a cysteine residue (cysteine “switch” residue) hold the metalloprotease in an inactive conformation. Metalloproteases can be activated through cleavage by convertases such as furin. ROS can activate metalloproteases by oxidizing the cysteine switch residue, thereby disrupting its interaction with the zinc ion and allowing the enzyme to adopt an active conformation.Citation43 Active tumor cell metalloproteases degrade components of the extracellular matrix, such as gelatin and collagens, thereby contributing to tumor cell invasiveness and metastasis.Citation44,Citation45 In breast cancer cell lines, alcohol concentrations of 1.3% to 5.1% stimulate the expression and secretion of MMPs 2 and 9.Citation39,Citation46,Citation47 Thus, it is not surprising that alcohol stimulates invasiveness and anchorage-independent proliferation of MCF7 human mammary tumor cells.Citation32,Citation41 Moreover, small interfering RNAs (siRNAs) specific for MMP2 or small molecule inhibitors of MMP2 reduce the effect of alcohol on anchorage-independent proliferation.Citation39
Alcohol appears to stimulate metalloprotease activity via a variety of mechanisms. As discussed earlier, alcohol is metabolized to ROS,Citation48–Citation50 which then can stimulate metalloprotease activity.Citation51,Citation52 Thus, it is reasonable to postulate that ROS and their regulation of metalloproteases mediate at least some of the effects of alcohol on the malignant phenotypes of breast tumor cells. Indeed, ROS scavengers inhibit alcohol stimulation of metalloprotease activity and alcohol stimulation of tumor cell invasiveness.Citation39
Alcohol may stimulate tumor cell aggressiveness through increased EGFR signaling
Experiments in fruit fies indicate that increased signaling of EGFR through Erk, but not through p38 or JNK, inhibits the sedative effects of alcohol. Moreover, the sedative effects of alcohol exposure in fruit fies or alcohol consumption by rats appear more rapidly in animals treated with the EGFR tyrosine kinase inhibitor erlotinib. These data suggest that EGFR signaling modulates pathways that couple alcohol to its behavioral effects.Citation53 In subsequent paragraphs we will discuss numerous other observations that support the hypothesis that EGFR signaling couples alcohol to biological responses, particularly malignant phenotypes of breast tumor cells.
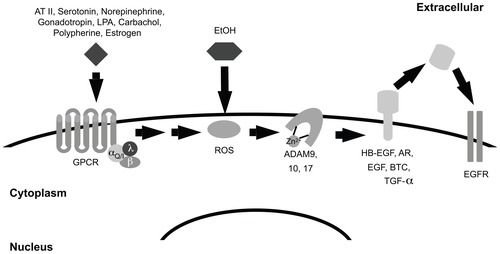
Most EGF family peptide growth factors are expressed as transmembrane precursors. Active metalloproteases cleave these precursors, releasing a mature, soluble form of the growth factor that binds ErbB receptors and stimulates their signaling.Citation43 Agonists for numerous serpentine G protein-coupled receptors (GPCRs) regulate the cleavage of EGF family precursors by metalloproteases, thereby enabling GPCR agonists to stimulate ErbB receptor signaling and coupling to biological responses.Citation51,Citation54 For example, MMP9 and MMP2 are required for agonists of the Gq-coupled gonadotropin receptor to stimulate EGFR tyrosine phosphorylation and induction of the downstream transcription factors c-Fos and c-Jun.Citation51,Citation55 Similarly, the GPCR agonists lysophosphatidic acid (LPA) and carbachol stimulate cleavage and maturation of the precursor form of amphiregulin (AR) by the metalloprotease ADAM17, resulting in enhanced cell migration and increased DNA synthesis.Citation17,Citation56 Phenylpherine stimulates cleavage of the precursor form of heparin-binding EGF-like growth factor (HB-EGF) by MMP7, leading to arterial vasoconstriction.Citation51,Citation57 GPCR transactivation of EGFR signaling also stimulates the activity of the c-Jun NH2-terminal protein kinase (JNK), the p38 mitogen-activated protein kinase (p38 MAPK), and phosphatidylinositol 3-kinase (PI3K).Citation51 It should be reiterated that the activity of JNK, MAPK, PI3K, c-fos, and c-jun increases upon treatment with alcohol,Citation39,Citation40,Citation51,Citation55 suggesting that alcohol may activate signaling pathways that are also activated by Gαq-coupled or Gαi-coupled GPCRs and that may include EGFR and its effectors. Indeed, angiotensin (AT) stimulation of the Gαq-coupled AT receptor I leads to ROS production, which in turn stimulates ADAM17-dependent cleavage of HB-EGF.Citation51 Recall that alcohol is metabolized to ROS. Consequently, we postulate that alcohol metabolism to ROS and subsequent stimulation of metalloprotease activity by ROS could result in increased processing of EGF family precursor proteins and increased EGFR signaling (). Indeed, preliminary data from our laboratory indicates that 1% alcohol stimulates MCF7 human breast tumor cells to release AR into the culture medium.
Figure 1 Alcohol may mimic G-protein coupled receptor transactivation of EGFR. Signaling by Gq/i-coupled receptors contributes to the production of reactive oxygen species (ROS). These ROS oxidize a cysteine residue in the catalytic cleft of the ADAM, thereby disrupting the interaction between the zinc ion and the ADAM protein. Disruption of this interaction causes the ADAM protein to adopt an active conformation. Active ADAM proteins can then cleave the precursor form of EGF family growth factors. The cleaved factors bind EGFR and stimulate its signaling activity. Because alcohol causes intracellular accumulation of ROS, it is predicted to stimulate EGFR signaling.
Abbreviations: AT II, angiotensin II; LPA, lysophosphatidic acid; EtOH, ethanol; GPCR, G-protein coupled receptor; ROS, reactive oxygen species; HB-EGF, heparin-binding, EGF-like growth factor; AR, amphiregulin; EGF, epidermal growth factor; BTC, betacellulin; TGFalpha, transforming growth factor alpha; EGFR, EGF receptor.
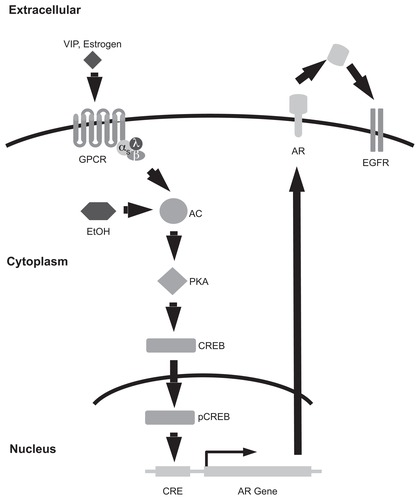
Alcohol concentrations of 0.1% to 0.3% stimulate the transcription of genes whose promoter contains a cyclic AMP (cAMP) response element (CRE).Citation37 Indeed, alcohol stimulates the activity of some adenylate cyclases (ACs), apparently by stabilizing the enzyme in the active conformation.Citation58,Citation59 This leads to increased cAMP production, protein kinase A (PKA) activity, phosphorylation of the CRE binding protein (CREB) by PKA, and increased transactivation of CRE promoters by phosphorylated CREB. Because the amphiregulin promoter contains a CRE,Citation17 it is reasonable to speculate that alcohol may stimulate EGFR signaling through a cAMP-dependent increase in amphiregulin transcription (). Indeed, vasoactive intestinal peptide (VIP), which stimulates cAMP production through a Gαs-coupled GPCR, also stimulates EGFR tyrosine phosphorylation in colonic epithelial cells.Citation60
Figure 2 Alcohol may mimic G-protein coupled receptor stimulated transcription of amphiregulin, an EGF family growth factor. Signaling by Gs-coupled receptors stimulates adenylate cyclase (AC) production of cAMP. This increases phosphorylation of the transcription factor CREB (cAMP response element binding protein) by protein kinase A (PKA). CREB promotes the transcription of the EGF family growth factor amphiregulin (AR), resulting in increasing EGFR signaling. Alcohol stabilizes AC in the active conformation, resulting in increased PKA activity. Thus, alcohol is predicted to stimulate AR transcription and EGFR signaling.
Abbreviations: VIP, vasoactive intestinal peptide; GPCR, G-protein coupled receptor; EtOH, ethanol; AC, adenylate cyclase; PKA, protein kinase A; CRE, cyclic AMP response element; CREB, CRE-binding protein; pCREB, phosphorylated CREB; AR, amphiregulin; EGFR, EGF receptor.
As discussed earlier, alcohol stimulates transcriptional activation by the estrogen receptor. The details of the mechanism by which this occurs remain unclear. Nonetheless, alcohol stimulates ERα and aromatase expressionCitation32,Citation33 and estrogen stimulates ER-dependent TGFα and amphiregulin gene expression.Citation61–Citation63 Thus, alcohol may stimulate biological responses in breast tumor cells through ERα stimulation of EGFR ligand expression and EGFR signaling. This hypothesis is consistent with our preliminary observation that 1% alcohol stimulates MCF7 human breast tumor cells to express AR and release it into the culture medium.
Estrogen can also rapidly modulate cellular signaling pathways in the cytosol and at the membrane in the absence of any direct effect of ER on gene expression.Citation64,Citation65 Some of these nongenomic responses are mediated by a GPCR that stimulates Gαi, resulting in activation of matrix metalloproteases and cleavage of the proform of an EGF family growth factor ().Citation64 Other effects appear to be mediated by Gαs, resulting in elevated PKA activity and cAMP accumulation ().Citation65 As discussed earlier, this could result in elevated amphiregulin expression via the CRE present in the amphiregulin promoter.
EGFR signaling can modulate ER-dependent signaling.Citation64 EGFR can couple to the PI3K/Akt pathway, leading to IKK phosphorylation of the estrogen receptor and modulation of ER-dependent gene expression.Citation66 EGFR signaling can also couple to increased Erk and Jnk activity, resulting in the phosphorylation of various transcription factors, including CREB-binding protein (CBP). Phosphorylated CBP can dimerize with estrogen receptor, leading to ER-dependent gene transcription.Citation64 Thus, EGFR signaling may lie upstream or downstream of ER in alcohol-induced signaling pathways.
As discussed earlier, alcohol inhibits expression of the cell adhesion tumor suppressor protein E-cadherin, leading to an increase in cell migration.Citation41 EGFR signaling is coupled to inactivation of the E-cadherin/β-catenin complex in tumor cell lines, thereby contributing to metastatic phenotypes.Citation67–Citation70 In fact, treatment of lung cancer cell lines with EGFR monoclonal antibodies results in increased E-cadherin expression.Citation71 Thus, increased EGFR signaling may be responsible for the decrease in E-cadherin expression observed following alcohol treatment.
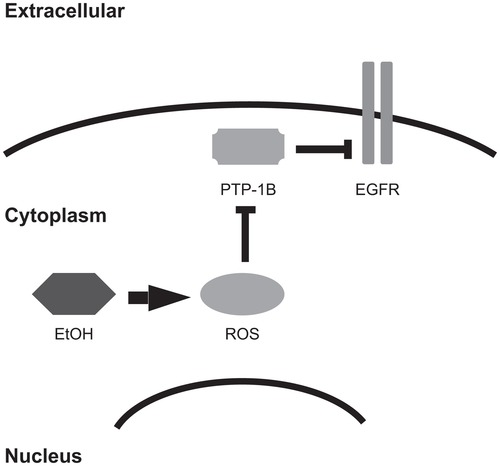
A hallmark of EGFR signaling is phosphorylation of EGFR on cytoplasmic tyrosine residues. This phosphorylation creates docking sites for effector binding and coupling. Thus, dephosphorylation of these tyrosine residues by phosphatases negatively regulates EGFR coupling to effector proteins and biological responses.Citation72–Citation74 There is emerging evidence suggesting that alcohol enhances EGFR signaling by inactivating phosphatases that catalyze EGFR dephosphorylation (). Recall that alcohol is metabolized to ROS in breast tissue. These ROS can oxidize a conserved, essential cysteine residue in the catalytic domain of phosphatases, thereby disrupting the catalytic activity of the phosphatases.Citation49,Citation75,Citation76 Therefore, it is plausible to postulate that alcohol may stimulate increased EGFR signaling through ROS-mediated inactivation of the phosphatases that dephosphorylate EGFR tyrosine residues. Indeed, it has been suggested that ultraviolet light and other cellular stresses that contribute to the production of intracellular ROS cause increased EGFR signaling via this mechanism.Citation77
Figure 3 Alcohol may inhibit phosphatases that negatively regulate EGFR. The protein tyrosine phosphatase PTP-1B inhibits EGFR signaling by catalyzing the dephosphorylation of EGFR tyrosine residues. ROS can oxidize a catalytic cysteine residue of protein phosphatases, thereby inactivating them and leading to increased EGFR signaling. Because alcohol causes intracellular accumulation of ROS, it is predicted to stimulate EGFR signaling.
Abbreviations: EtOH, ethanol; ROS, reactive oxygen species; PTP-1B, protein tyrosine phosphatase 1B; EGFR, EGF receptor.
Agonist binding to EGFR can cause EGFR heterodimerization with ErbB2, leading to phosphorylation of both receptor molecules and coupling of both receptors to downstream signaling events. Because ErbB2 does not possess soluble agonists, this is an important mechanism by which ErbB2 signaling can be regulated.Citation78 This heterodimerization of ErbB2 with EGFR may contribute to the effect alcohol has on breast tumor cell behavior. Indeed, this model is supported by the observation that ErbB2 overexpression potentiates the effect of alcohol on invasiveness and other malignant phenotypes of breast cancer cell lines.Citation39–Citation41,Citation79 ErbB2 overexpression is also associated with increased transcription of MMP2 and MMP9Citation39,Citation47 and siRNA knockdown of endogenous ErbB2 overexpression is associated with reduced MMP activity.Citation39
Finally, amphiregulin induces EGFR coupling to increased expression of MMP2 and MMP9 in breast tumor cell lines; antisense knockdown of amphiregulin expression in the NS2T2A1 breast tumor cell line results in decreased expression of MMP2.Citation17 Thus, alcohol stimulation of MMP expression and activity as described in this review may establish a feed forward mechanism that features increased amphiregulin stimulation of EGFR/ErbB2 signaling. This signaling is then coupled to increased expression of MMP2 and MMP9, which contributes to further increases in signaling by the amphiregulin/EGFR/ErbB2 axis and deregulated cellular proliferation, motility, and invasiveness.
Here we postulate three mechanisms by which alcohol may contribute to breast tumor genesis, progression, or aggressiveness. Additional experimentation is necessary to decipher which, if any, of these mechanisms are relevant. These experiments should proceed with an understanding that these mechanisms are not likely to be mutually exclusive. Indeed, at different stages during breast cancer progression distinct mechanisms may be relevant.
The mechanisms discussed here may have important implications in understanding breast cancer progression and potential therapies. Given that alcohol may stimulate estrogen receptor, EGFR, and ErbB2/Her2/Neu signaling, even moderate alcohol consumption may affect the outcome of breast cancer patients whose tumors express these proteins. Moreover, given the central role that ROS and estrogen may have in mediating the effects of alcohol on breast tumor genesis, progression, or aggressiveness, antioxidants and antiestrogens may hold value in blocking the negative effects of alcohol on breast cancer.
Acknowledgments
We acknowledge support from the National Cancer Institute (R01CA114209 to DJR), the US Army Breast Cancer Research Program (DAMD17-00-1-0416 to DJR), the American Cancer Society (IRG-58-006), the Indiana Elks Foundation, the Purdue University Center for Cancer Research, and the Oncological Sciences Center of Purdue University Discovery Park. The authors report no conflicts of interest in this work.
References
- DraperLBreast cancer: trends, risks, treatments, and effectsAaohn J20065410445451 quiz 452–44317059162
- DeVitaVTHellmanSRosenbergSACancer: principles and practice of oncology5th edPhiladelphiaLippincott-Raven1997
- Dean-ColombWEstevaFJHer2-positive breast cancer: herceptin and beyondEur J Cancer200844182806281219022660
- WangSCZhangLHortobagyiGNHungMCTargeting HER2: recent developments and future directions for breast cancer patientsSemin Oncol2001286 Suppl 18212911774202
- HoriguchiJKoibuchiYIijimaK Co-expressed type of ER and HER2 protein as a predictive factor in determining resistance to antiestrogen therapy in patients with ER-positive and HER2-positive breast cancerOncol Rep20051451109111616211272
- AndrechekERMullerWJDevelopmental timing of activated erbB2 expression plays a critical role in the induction of mammary tumorsCell Cycle2004391111111315326381
- Ursini-SiegelJSchadeBCardiffRDMullerWJInsights from transgenic mouse models of ERBB2-induced breast cancerNat Rev Cancer20077538939717446858
- AmundadottirLTLederPSignal transduction pathways activated and required for mammary carcinogenesis in response to specific oncogenesOncogene19981667377469488037
- SheffieldLGC-Src activation by ErbB2 leads to attachment-independent growth of human breast epithelial cellsBiochem Biophys Res Commun1998250127319735325
- ParkJWNeveRMSzollosiJBenzCCUnraveling the biologic and clinical complexities of HER2Clin Breast Cancer20088539240118952552
- ChrysogelosSADicksonRBEGF receptor expression, regulation, and function in breast cancerBreast Cancer Res Treat199429129408018962
- RampaulRSPinderSENicholsonRIGullickWJRobertsonJFEllisIOClinical value of epidermal growth factor receptor expression in primary breast cancerAdv Anat Pathol200512527127316210923
- O’BrienCSHowellSJFarnieGClarkeRBResistance to endocrine therapy: are breast cancer stem cells the culprits?J Mammary Gland Biol Neoplasia2009141455419252972
- IschenkoISeeligerHSchafferMJauchKWBrunsCJCancer stem cells: how can we target them?Curr Med Chem200815303171318419075661
- AndersCCareyLAUnderstanding and treating triple-negative breast cancerOncology (Williston Park)2008221112331239 discussion 1239–1240, 124318980022
- HoadleyKAWeigmanVJFanC EGFR associated expression profiles vary with breast tumor subtypeBMC Genomics2007825817663798
- WillmarthNEEthierSPAmphiregulin as a novel target for breast cancer therapyJ Mammary Gland Biol Neoplasia200813217117918437539
- GilmoreJLScottJABouizarZ Amphiregulin-EGFR signaling regulates PTHrP gene expression in breast cancer cellsBreast Cancer Res Treat2008110349350517882547
- LorchGGilmoreJLKoltzPF Inhibition of epidermal growth factor receptor signalling reduces hypercalcaemia induced by human lung squamous-cell carcinoma in athymic miceBr J Cancer200797218319317533397
- LuXWangQHuG ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasisGenes Dev200923161882189419608765
- WangZZhangLYeungTKChenXEndocytosis deficiency of epidermal growth factor (EGF) receptor-ErbB2 heterodimers in response to EGF stimulationMol Biol Cell19991051621163610233167
- DiermeierSHorvathGKnuechel-ClarkeRHofstaedterFSzollosiJBrockhoffGEpidermal growth factor receptor coexpression modulates susceptibility to Herceptin in HER2/neu overexpressing breast cancer cells via specific erbB-receptor interaction and activationExp Cell Res2005304260461915748904
- PatelDBassiRHooperAPrewettMHicklinDJKangXAnti-epidermal growth factor receptor monoclonal antibody cetuximab inhibits EGFR/HER-2 heterodimerization and activationInt J Oncol2009341253219082474
- XiaWMullinRJKeithBR Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathwaysOncogene200221416255626312214266
- KeyJHodgsonSOmarRZ Meta-analysis of studies of alcohol and breast cancer with consideration of the methodological issuesCancer Causes Control200617675977016783604
- LongneckerMPAlcoholic beverage consumption in relation to risk of breast cancer: meta-analysis and reviewCancer Causes Control19945173828123780
- Smith-WarnerSASpiegelmanDYaunSS Alcohol and breast cancer in women: a pooled analysis of cohort studiesJAMA199827975355409480365
- ZhangSMLeeIMMansonJECookNRWillettWCBuringJEAlcohol consumption and breast cancer risk in the Women’s Health StudyAm J Epidemiol2007165666767617204515
- DumitrescuRGShieldsPGThe etiology of alcohol-induced breast cancerAlcohol200535321322516054983
- BerstadPMaHBernsteinLUrsinGAlcohol intake and breast cancer risk among young womenBreast Cancer Res Treat2008108111312017468952
- SeitzHKStickelFMolecular mechanisms of alcohol-mediated carcinogenesisNat Rev Cancer20077859961217646865
- EtiqueNChardardDChesnelAMerlinJLFlamentSGrillier-VuissozIEthanol stimulates proliferation, ERalpha and aromatase expression in MCF-7 human breast cancer cellsInt J Mol Med200413114915514654987
- SingletaryKWFreyRSYanWEffect of ethanol on proliferation and estrogen receptor-alpha expression in human breast cancer cellsCancer Lett2001165213113711275361
- PurohitVModerate alcohol consumption and estrogen levels in postmenopausal women: a reviewAlcohol Clin Exp Res19982259949979726268
- PurohitVCan alcohol promote aromatization of androgens to estrogens? A reviewAlcohol200022312312711163119
- FanSMengQGaoB Alcohol stimulates estrogen receptor signaling in human breast cancer cell linesCancer Res200060205635563911059753
- EtiqueNFlamentSLecomteJGrillier-VuissozIEthanol-induced ligand-independent activation of ERalpha mediated by cyclic AMP/PKA signaling pathway: an in vitro study on MCF-7 breast cancer cellsInt J Oncol20073161509151817982678
- VolmMEfferthTGrabnerPPommerenkeEWInduction of P-glycoprotein, glutathione S-transferase, catalase, c-FOS and C-ERBB1 in rodent cell lines after exposure to doxorubicin, ethanol and caffeineInt J Oncol199561879221556506
- KeZLinHFanZ MMP-2 mediates ethanol-induced invasion of mammary epithelial cells over-expressing ErbB2Int J Cancer2006119181616450376
- MaCLinHLeonardSSShiXYeJLuoJOverexpression of ErbB2 enhances ethanol-stimulated intracellular signaling and invasion of human mammary epithelial and breast cancer cells in vitroOncogene200322345281529012917629
- MengQGaoBGoldbergIDRosenEMFanSStimulation of cell invasion and migration by alcohol in breast cancer cellsBiochem Biophys Res Commun2000273244845310873626
- JeanesAGottardiCJYapASCadherins and cancer: how does cadherin dysfunction promote tumor progression?Oncogene200827556920692919029934
- SandersonMPDempseyPJDunbarAJControl of ErbB signaling through metalloprotease mediated ectodomain shedding of EGF-like factorsGrowth Factors200624212113616801132
- DuffyMJMaguireTMHillAMcDermottEO’HigginsNMetalloproteinases: role in breast carcinogenesis, invasion and metastasisBreast Cancer Res20002425225711250717
- VihinenPKahariVMMatrix metalloproteinases in cancer: prognostic markers and therapeutic targetsInt J Cancer200299215716611979428
- EtiqueNGrillier-VuissozIFlamentSEthanol stimulates the secretion of matrix metalloproteinases 2 and 9 in MCF-7 human breast cancer cellsOncol Rep200615360360816465419
- LuoJRole of matrix metalloproteinase-2 in ethanol-induced invasion by breast cancer cellsJ Gastroenterol Hepatol200621Suppl 3S65S6816958676
- AlbanoEAlcohol, oxidative stress and free radical damageProc Nutr Soc200665327829016923312
- DasSKVasudevanDMAlcohol-induced oxidative stressLife Sci200781317718717570440
- DeyACederbaumAIAlcohol and oxidative liver injuryHepatology2006432 Suppl 1S63S7416447273
- OhtsuHDempseyPJEguchiSADAMs as mediators of EGF receptor transactivation by G protein-coupled receptorsAm J Physiol Cell Physiol20062911C1C1016769815
- FischerOMHartSGschwindAPrenzelNUllrichAOxidative and osmotic stress signaling in tumor cells is mediated by ADAM proteases and heparin-binding epidermal growth factorMol Cell Biol200424125172518315169883
- CorlABBergerKHOphir-ShohatG Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanol-induced behaviorsCell2009137594996019464045
- DelcourtNBockaertJMarinPGPCR-jacking: from a new route in RTK signalling to a new concept in GPCR activationTrends Pharmacol Sci2007281260260718001849
- RoelleSGrosseRAignerAKrellHWCzubaykoFGudermannTMatrix metalloproteinases 2 and 9 mediate epidermal growth factor receptor transactivation by gonadotropin-releasing hormoneJ Biol Chem200327847473074731812963732
- GschwindAHartSFischerOMUllrichATACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cellsEMBO J200322102411242112743035
- HaoLDuMLopez-CampistrousAFernandez-PatronCAgonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathwayCirc Res2004941687614656925
- HarrisRATrudellJRMihicSJEthanol’s molecular targetsSci Signal2008128re718632551
- YoshimuraMPearsonSKadotaYGonzalezCEIdentification of ethanol responsive domains of adenylyl cyclaseAlcohol Clin Exp Res200630111824183217067346
- BertelsenLSBarrettKEKeelySJGs protein-coupled receptor agonists induce transactivation of the epidermal growth factor receptor in T84 cells: implications for epithelial secretory responsesJ Biol Chem200427986271627914660604
- BatesSEDavidsonNEValveriusEM Expression of transforming growth factor alpha and its messenger ribonucleic acid in human breast cancer: its regulation by estrogen and its possible functional significanceMol Endocrinol1988265435553047554
- CiarloniLMallepellSBriskenCAmphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland developmentProc Natl Acad Sci U S A2007104135455546017369357
- KenneyNJSaekiTGottardisM Expression of transforming growth factor alpha antisense mRNA inhibits the estrogen-induced production of TGF alpha and estrogen-induced proliferation of estrogen-responsive human breast cancer cellsJ Cell Physiol199315634975148360257
- LevinERBidirectional signaling between the estrogen receptor and the epidermal growth factor receptorMol Endocrinol200317330931712554774
- LevinERPietrasRJEstrogen receptors outside the nucleus in breast cancerBreast Cancer Res Treat2008108335136117592774
- BiswasDKIglehartJDLinkage between EGFR family receptors and nuclear factor kappaB (NF-kappaB) signaling in breast cancerJ Cell Physiol2006209364565217001676
- Al MoustafaAEYenLBenlimameNAlaoui-JamaliMARegulation of E-cadherin/catenin complex patterns by epidermal growth factor receptor modulation in human lung cancer cellsLung Cancer2002371495612057867
- Cowden DahlKDSymowiczJNingY Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent e-cadherin loss in ovarian carcinoma cellsCancer Res200868124606461318559505
- JawhariAUFarthingMJPignatelliMThe E-cadherin/epidermal growth factor receptor interaction: a hypothesis of reciprocal and reversible control of intercellular adhesion and cell proliferationJ Pathol1999187215515710365089
- LuZGhoshSWangZHunterTDownregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasionCancer Cell20034649951514706341
- Al MoustafaAEYansouniCAlaoui-JamaliMAO’Connor-McCourtMUp-regulation of E-cadherin by an anti-epidermal growth factor receptor monoclonal antibody in lung cancer cell linesClin Cancer Res19995368168610100722
- HarrisDLJoyceNCProtein tyrosine phosphatase, PTP1B, expression and activity in rat corneal endothelial cellsMol Vis20071378579617563729
- IshinoYZhuCHarrisDLJoyceNCProtein tyrosine phosphatase-1B (PTP1B) helps regulate EGF-induced stimulation of S-phase entry in human corneal endothelial cellsMol Vis200814617018253097
- MattilaEPellinenTNevoJVuoriluotoKArjonenAIvaskaJNegative regulation of EGFR signalling through integrin-alpha1beta1-mediated activation of protein tyrosine phosphatase TCPTPNat Cell Biol200571788515592458
- BarrettWCDeGnoreJPKengYFZhangZYYimMBChockPBRoles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1BJ Biol Chem199927449345433454610574916
- RobinsonKAStewartCAPyeQN Redox-sensitive protein phosphatase activity regulates the phosphorylation state of p38 protein kinase in primary astrocyte cultureJ Neurosci Res199955672473210220113
- CarpenterGEmployment of the epidermal growth factor receptor in growth factor-independent signaling pathwaysJ Cell Biol1999146469770210459005
- RieseDJ2ndSternDFSpecificity within the EGF family/ErbB receptor family signaling networkBioessays199820141489504046
- AyeMMMaCLinHBowerKAWigginsRCLuoJEthanol-induced in vitro invasion of breast cancer cells: the contribution of MMP-2 by fibroblastsInt J Cancer2004112573874615386367