Abstract
We describe a new vaccination method called modified vaccination technique (MVT). The technique is able to achieve downregulation of pathogenic autoimmune events leading to a chronic progressive disorder in rats called slowly progressive Heymann nephritis. Downregulation of immunopathological events is achieved by injections of immune complex (IC) made up of the target native antigen (ag) and specific naturally occurring immunoglobulin M (IgM) antibody (ab) directed against it. Repeated injections of IC maintain high levels of specific circulating IgM autoantibodies (aabs) against the kidney ag. The developing physiologic IgM aabs assist in the catabolism of both modified and unmodified renal ags from the circulation. No disease-causing renal ags in the circulation results in no stimulation of pathogenic immunoglobulin G aab producing cell lines. Such specific targeted therapy leads to termination of disease-causing processes and reestablishment of tolerance. The MVT can be employed both prophylactically and therapeutically with equal effectiveness. A redirected immune response is achieved by specifically stimulating the animals’ own IgM-producing cell lines with the injected ICs, resulting in a natural cure. Such ICs are nontoxic and nonirritant and cause no side effects. We surmise that the MVT, employing the appropriate components in each instance, can also be used to treat human ailments.
Introduction
The reason why we cannot specifically treat experimental autoimmune disorders and naturally occurring autoimmune diseases in humans is that we still do not fully understand the autoimmune events that take place and contribute to the maintenance or loss of tolerance to self (CitationWeigle et al 1967; CitationWeir 1969; CitationTung 1994; CitationDrakesmith et al 2000; CitationManz et al 2002). Hence autoimmune diseases are still not treated specifically, but rather with immunosuppressive agents. Not only are immunosuppressive agents nonspecific in action, most often resulting in serious side effects, but they also fail to cure the disease, since the underlying pathogenic immune events can continue despite the control of symptoms (CitationBen Yehuda et al 1988; CitationGolbus and McCune 1994).
It should be acknowledged that not every autoimmune process is harmful. In effect, most of the autoimmune process related events occurring throughout life are physiologic (CitationGrabar 1965, Citation1983; CitationWeir et al 1966; CitationWeir 1966; CitationCasali and Notkins 1989; CitationNawata et al 1990; CitationChen et al 1995). Physiologic autoimmune events contribute in a major way towards the catabolism of intracytoplazmic components released into the circulation from cells at the end of their life span. In a physiological sense, we are not per se “tolerant” to our intracytoplazmic components (CitationWeir and Elson 1969), since specific immunoglobulin M (IgM) autoantibodies (aabs) are designated from birth to react with these components and assist in their removal once they are released into the intravascular space (CitationSolvason et al 1992). Such events prevent toxic accumulation of tissue breakdown products and/or their chemical modification by intrinsic or foreign agents (CitationYung et al 1995). Chemically modified autoantigens (aags) can initiate pathogenic autoimmune disease-causing responses (CitationTotoritis and Rubin 1985).
The development of an autoimmune disease therefore results in most instances not from some malfunctioning of the immune system against normal self components but from abnormal presentation of self or self-like molecules to the cells of the immune system (ie, as modified self or by molecular mimicry) (CitationBarabas and Lannigan 1969; CitationFujinami and Oldstone 1985; CitationWilson et al 2000; CitationLenz et al 2001; CitationBarabas et al 2004c). This thesis presumes that normal intracytoplazmic components will not produce autoimmune disease per se but in the meantime does not preclude their participation in lesion development. The following explanation will help to clarify these statements.
Issues relating to altered self antigen initiated events
If a native antigen (ag) from the target organ is exposed and becomes modified, eg, by a chemical agent (CitationSchoen and Trentham 1981; CitationTotoritis and Rubin 1985; CitationYung et al 1995; CitationRich 1996) in the intravascular space, or if a modified self-like ag is administered repeatedly (CitationBarabas et al 2003, Citation2004c), then the following events can occur:
If the modified ag initiated events are short-lived, then a limited pathogenic autoimmune disease process will result in minimal functional and morphological alterations in the target organ (CitationTotoritis and Rubin 1985).
If the modified ag initiated events are maintained, then a progressive autoimmune disease process will ensue by the developing pathogenic immunoglobulin G (IgG) aabs and cause major morphological and functional change in the target organ resulting in a diagnosable autoimmune disease (CitationHeymann et al 1959; CitationMendrick et al 1980).
Pathogenic autoimmune disease-causing events can take place because the pathogenic aab (resulting from the stimulation of IgG aab producing cell lines by the modified native ag) is able to cross-react with both native and modified aags present in the circulation and in the target organ (eg, in the kidney it reacts with fixed glomerular kidney ags and forms immune complexes (ICs) during the course of the autoimmune disease).
Genetic predisposition can also affect autoimmune disease development. Certain patients are unable to respond with sufficiently strong immune responses to bacterial or viral infections. Under such circumstances, bacterial or viral breakdown products linger on at the site of infection, and sustained tissue damage occurs whereby cells are injured and intracytoplazmic components are released. In this local milieu the ideal conditions are thus created for the modification of self ags. If a low-grade chronic infection persists, then pathogenic IgG aab production (occurring alongside IgM aab production) can commence. If such events are sufficiently prolonged then serious morphologic and functional alterations can take place in the target organ and lead to a chronic progressive autoimmune disease (CitationWucherpfennig 2001).
Genetic susceptibility could also be due to physiologic immune response deficiency where an infection is not involved. For example, an individual’s immune system might be unable to produce sufficient amounts of specific IgM aabs to clear released intracytoplazmic ags from the intravascular space; under such conditions aags can become modified by agents (chemicals, drugs, toxic compounds, etc) present in the circulation and initiate pathogenic IgG aab response against the modified self. If pathogenic IgG aabs are continuously produced then damage to a target organ can cause a low-grade slowly progressive autoimmune disease.
Issues relating to normal self ag initiated events
Although normal aags will not themselves produce an autoimmune disease, they can contribute to disease establishment and progression for two reasons. First, the normal aags within an organ can become the primary targets of pathogenic autoimmune processes (CitationBarabas et al 2004a), and second, they can contribute to IC deposits in affected organs (eg, in the glomeruli) (CitationBarabas et al 2003).
In our studies we found that the physiologic IgM aabs and pathogenic IgG aabs cross-react with both normal and modified ags (CitationBarabas et al 2004b):
Pathogenic aabs reacting with normal aags in the target organ cause autoimmune disease (CitationBarabas et al 2003), and when reacting with modified aags in the circulation, maintain increased pathogenic IgG aab production.
Nonpathogenic IgM aabs also react with both native and modified ags in the circulation and assist in their catabolism.
Heightened IgM aab production is maintained alongside IgG aab production during an autoimmune disease state both by native aags per se that are released into the intravascular space (CitationBarabas et al 2003) and by developing ICs (made up of native aags and specific IgM aabs). Thus, while pathogenic IgG aab production attempts to increase the severity of insult against a target, there is a concurrent attempt by the IgM aabs to slow down pathogenic autoimmune processes (CitationBarabas et al 2006c).
Despite the normal functioning of the IgM mechanism, as long as the modified ag is present and maintains pathogenic IgG aab production, tissue damage will proceed towards chronic progressive changes that result in morphological and functional alterations. But tipping the balance in favor of increased IgM aab production, in order to neutralize the circulating modified and native ags that contribute to pathogenesis, can halt the autoimmune disease altogether (CitationBarabas and Lafreniere 2005).
Recent attempts to treat autoimmune disorders
So far research scientists have not come up with a specific immune-inducing technique that could prevent or treat endogenous source ag derived chronic ailments (such as autoimmune disorders and cancer). This might be due to the very complex science that describes autoimmune related diseases. Indeed, consensus as to the etiology and pathogenesis of autoimmune diseases still has not been reached (CitationTung 1994; CitationTheofilopoulos 1995; CitationGarza et al 2000; CitationLernmark 2001; CitationLudewig et al 2001; CitationSherman 2001; CitationLafaille and Mathis 2002). Consequently, diverse opinions and research efforts continue to contribute to the probative and problem-solving work to find a cure. Under these circumstances the use of immunosuppressive agents is still advocated to treat patients with chronic ailments. In recent years treatment with monoclonal abs has achieved approval but most of such products are not specific in their action and cause side effects. Another treatment modality, the oral presentation of tissue-derived ags, has in experimental animals and even in humans with autoimmune disorders had a degree of beneficial influence on autoimmune disease-causing events, but has not resulted in a cure (CitationRamiya et al 1997; CitationWeiner 1997, Citation2000; CitationFaria and Weiner 1999; CitationBilsborough and Viney 2002).
The form of presentation of the target aag in producing (and also in preventing and treating) an autoimmune disorder can influence outcomes even to the extent of determining final states of disease or no disease. A detailed schematic of the events involved is given in and . Under certain circumstances native aags and injected disease-related ags can start or worsen disease progression (CitationPeakman and Dayan 2001), for example when an aag released from the intracytoplazmic environment or an injected disease-related ag is modified by a modifying agent present in the individual’s intravascular space (). Such modifying agents can be drugs (CitationYung and Richardson 1994) or their metabolic products, or toxic agents derived from infectious microbes etc (CitationGuilherme and Kalil 2004). Peptides of microbial proteins, exhibiting sequence similarity or identity to self peptides through molecular mimicry (CitationWucherpfennig 2001), can also activate autoreactive T-cells and cause autoimmune disease. Under these unusual circumstances autoimmune disease-initiating processes can take place; however, under normal circumstances “native” intracytoplazmic ags liberated into the circulation will not cause pathogenic IgG aab response.
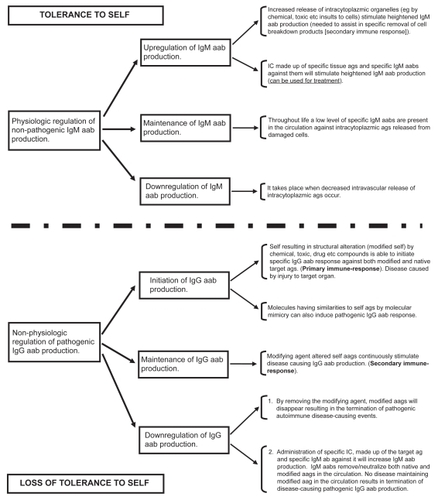
Figure 1 Autoimmune processes initiated and maintained by native and modified autologous antigens.
Removal of the inciting agent that modifies the autologous ags, if it can be identified (it could be a drug, infectious agent etc);
Increase in the specific IgM aab response against the target ags capable of removing both modified and native ags from the circulation; or
Preferably both 1 & 2.
“Native” aags initiate and maintain physiologic IgM aab response throughout life, assisting in the removal of intracytoplasmic aags released into the intravascular space.
Abbreviations: aab, autoantibody; aag, autoantigen; ag, antigen; AID, autoimmune disease; C, complement; IC, immune complex; IgG, immunoglobulin G; IgM, immunoglobulin M; MW, molecular weight.
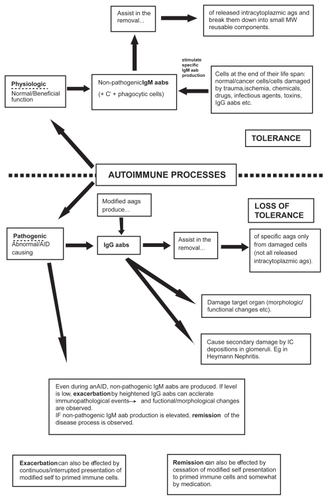
Figure 2 Possible fate of released autoantigens.
Abbreviations: aab, autoantibody; aag, autoantigen; IC, immune complex; IgG, immunoglobulin G; IgM, immunoglobulin M; MW, molecular weight.
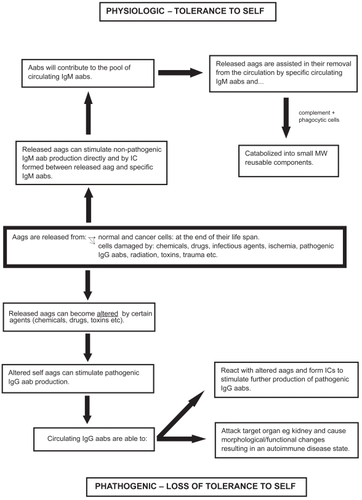
Figure 3 Downregulation/upregulation of nonpathogenic and pathogenic autoimmune processes.
Abbreviations: aab, autoantibody; ag, antigen; IC, immune complex; IgG, immunoglobulin G; IgM, immunoglobulin M; MW, molecular weight.
A new vaccination technique for the prevention and treatment of an experimental autoimmune kidney disease
Heymann nephritis (HN) and slowly progressive Heymann nephritis (SPHN) are pathogenic IgG aab initiated and mediated autoimmune diseases (CitationHeymann et al 1959; CitationBarabas et al 2004c). Classical HN is established in susceptible strains of rats by repeated IP injections of nephritogenic ags incorporated into Freund’s complete adjuvant [FCA] (CitationBarabas and Lannigan 1969). After two to four injections of the preparation, progressive proteinuria, severe morphological changes in the kidney, and in the circulation pathogenic IgG aab against the brush-border region of the renal proximal convoluted tubules commences. Attempts to treat HN by various agents before or after the induction of the disease have proved to be unsuccessful (CitationBarabas et al 1969, Citation1970; CitationKupor et al 1976; CitationCattran 1988; CitationMatsukawa et al 1992; CitationPenny et al 1998; CitationYokoyama et al 1999; CitationHasegawa et al 2001;). The futility of these attempts to downregulate immunopathological events has been due to at least two factors:
Kidney ag injected in FCA established an irreversibly progressive disease process with the development of pathogenic IgG aabs (CitationAndres et al 1986).
Agents used to circumvent disease development or treat the progressive disease did not act specifically to achieve downregulation or termination of the ab-mediated response against kidney-directed immune insults (CitationBarabas et al 1969, Citation1970; CitationBaker et al 1989; CitationPenny et al 1998; CitationHasegawa et al 2001; CitationSpicer et al 2001).
We realized that in order to influence immunopathological events we needed to establish a new model of HN that was slowly progressive. We established two models of SPHN. One was produced by using alum instead of FCA as the adjuvant in the injected renal ag (CitationBarabas et al 2003). The other, which perhaps mimics most closely the development of a slowly progressive autoimmune disease in humans, was established by repeated injections of a chemically modified renal ag preparation (CitationBarabas et al 2004c). The two SPHN models illustrate the process by which immunological mishaps result in pathogenic autoimmune events that cause harm in target organs and lead to morphological and functional changes.
The next challenge was to find a way to correct such immunological mishaps. To do so we have developed a new vaccination technique, and have successfully employed it in both SPHN models, before and after the initiation of the disease. This modified vaccination technique (MVT), which is a hybrid of active and passive immunization programs, has proven itself capable of redirecting immune responses (CitationBarabas et al 2004b, Citation2006b, Citation2006c).
In our SPHN experimental autoimmune disorder models, the MVT achieved specific downregulation of disease initiating and maintaining events (). We produced specific ICs made up of the disease-causing native kidney tubular ag and specific IgM abs directed against it at slight ag excess (CitationBarabas et al 2004b, Citation2006b, Citation2006c). Injections of these ICs at weekly intervals maintained high levels of circulating nonpathogenic IgM aabs. The increased production of IgM aabs neutralized both modified and native ags by removing them from the circulation and preventing them from playing a part in pathogenic autoimmune events. Since the modified ag was no longer available to stimulate pathogenic IgG aab production, the pathogenic IgG aab producing cell lines were silenced. The unmodified (native) ag, normally located in the glomeruli of the kidney (CitationKerjaschki and Farquhar 1983; CitationCornish et al 1984) and also present in the circulation (CitationMakker and Singh 1982; CitationSingh and Makker 1986; CitationSingh and Schwartz 1986), did not remain a target of pathogenic aabs or a contributor to further deposition of IC. The absence of altered or native ags in the circulation meant downregulation of pathogenic autoimmune events ().
We maintain that such downregulation of pathogenic autoimmune events can only be achieved with our MVT. The MVT is effective in both pre- and post-treatment protocols, ie, both in preventing autoimmune disease development (CitationBarabas et al 2006b) and in terminating ongoing auto-immune disease processes (CitationBarabas et al 2004b, Citation2006c). The MVT, via the injection of ICs with predetermined immune inducing components, produces the same class of immunoglobulin (in our case specific IgM aabs directed against the nephritogenic ags both modified and native) with the same specificity against the target ag as resides in the inoculum. The technique provides a specific redirected immune response without influencing normal immune events in any way. The ICs are nontoxic and nonirritant in both short- and long-term applications. The technique achieves total downregulation of pathogenic autoimmune events resulting in regained tolerance. However, since memory cells are retained for pathogenic aab production, pathogenic autoimmune events could start up again if modified ags are reintroduced.
Summary and concluding remarks
Vaccination over the last 200 years has prevented the occurrence and spread of often lethal infectious and contagious diseases (diphtheria, small pox) in our population. However, in spite of vaccination’s development, many diseases initiated and maintained by exogenous ags (HIV/AIDS, malaria, TB) and all of those caused by endogenous ags (autoimmune diseases, cancer) are still not controlled or treated either by prophylactic or therapeutic vaccination programs. Numerous attempts, especially within the last few years, attest to the desire to come up with effective vaccinations to deal with chronic ailments (CitationPeakman and Dayan 2001; CitationSchijns 2001; CitationAndre 2003; CitationReed and Campos-Neto 2003; CitationSlingluff, Jr. and Speiser 2005; CitationTang and Bluestone 2006). The fact that we still have not come up with specific vaccination technologies, despite enormous investments of money, manpower, government resources, etc, confirms that the search for a cure is not easy. However, we are inching towards solutions. Lately there have been many publications that provide hope, not only for better drugs, but also for other treatment methods able to influence immune response outcomes (CitationPeakman and Dayan 2001; CitationStauss 2001; CitationMaloney et al 2002; CitationMelief et al 2002; CitationMorris et al 2003; CitationClynes 2005; CitationPolakis 2005). We have substantial evidence demonstrating that a normally functioning immune system can exert protective immune responses against exogenous source ags (bacteria, viruses, etc) but often not so protective immune responses against harmful endogenous source ags. Sorting out physiologic and pathogenic immune activity against endogenous source ags – ie, events that can maintain, break, or reestablish tolerance – would provide us the chance to understand how to manipulate immune responses, eg, for tolerance reestablishment in an autoimmune disease. (In cancer, we would require the opposite. We would want a specific immune response against cells bearing cancer-specific ags, for the elimination of these cancer cells from the host’s internal environment).
So far autoimmune diseases have been generally treated with immunosuppressive agents (CitationBarabas et al 1969, Citation1970; CitationBolton et al 1974; CitationCattran 1988). These medications nonspecifically suppress immune functions and predispose patients to infections. The aim in the last few years has been to find better solutions or techniques to downregulate autoimmune disease-causing immune events. By observing the “natural” and “pathogenic” events that take place in normal and experimental autoimmune disease induced animals, one can investigate physiologic and pathogenic autoimmune responses in health and in disease. Extensive literature documents how aags released from the intracellular environment are assisted in their removal by specific IgM aabs (CitationWeir et al 1966; CitationElson and Weir 1967; CitationCasali and Notkins 1989; CitationAvrameas 1991; CitationChen et al 1995; CitationCoutinho et al 1995; CitationBarabas et al 2003). These aabs are physiologic and specific in their action against native and corresponding modified native aags. Weir and associates have shown that specific naturally occurring IgM aabs play a significant role in the catabolism of intracytoplazmic ags released from normal cells (CitationPinckard and Weir 1966; CitationWeir et al 1966) at the end of their lifespan and following injury by cytotoxic and infectious agents, burns, hypoxia, trauma, etc. Such observations led naturally to the suggestion that these aabs played a role in the maintenance of tolerance to self ags (CitationWeir and Elson 1969), ie, by efficiently removing intracytoplazmic waste.
Several experiments have revealed promptly increased IgM aab production (since animals/humans are not per se “tolerant” to escaped intracytoplazmic cell components) following massive release of aags from the intracellular environment [secondary ab response] (CitationPinckard and Weir 1966; CitationWeir 1966; CitationBarabas et al 2003). The elevated level of circulating IgM aabs assist in the removal of released aags. However, if the circulating released aags are not efficiently cleared in the shortest possible time, they can become modified by agents present in the circulation such as drugs or their breakdown products, toxic agents, etc. In order to prevent toxic or modified-self aag accumulation in the intravascular space, we can increase IgM aab production more effectively (ie, not relying on IgM aab stimulation by released aags alone) by administering ICs made up of aags and specific IgM abs directed against them at slight ag excess. These ICs, as mentioned above, will enhance the production of the same class of immunoglobulin with the same specificity against the target ag as resides in the inoculum.
The implementation of our MVT is most appropriate during a pathogenic autoimmune event, such as in our experimental autoimmune kidney disease (CitationBarabas et al 2004b, Citation2006a, Citation2006b, Citation2006c), for the following reasons:
During an autoimmune disease process both pathogenic IgG aabs and nonpathogenic IgM aabs are produced. Pathogenic aabs cause harm and nonpathogenic aabs attempt to remove both native and modified self ags thereby aiming to terminate autoimmune disease-causing events.
The pathogenic IgG aab production is maintained by modified self-like ags.
The nonpathogenic IgM aab production is maintained by native aags.
Tipping the balance between IgG aab and nonpathogenic IgM aab production in favor of IgM aab response is achieved by injections of suitably assembled ICs made up of the target ag and specific IgM ab against it. The resulting increase in IgM aab in the circulation assists in the removal of both native and modified ags and prevents further production of pathogenic IgG aabs.
In certain autoimmune diseases the inciting agent also has to be removed to achieve termination of pathogenic aab production.
We have shown that by redirecting an immune response we can prevent an experimental autoimmune kidney disease-causing process from beginning (CitationBarabas et al 2006b) and also terminate it with equal effectiveness when the disease is established (CitationBarabas et al 2004b, Citation2006c). This solution is achieved by the MVT that we have developed. The vaccination technique can evoke a specific predetermined immune response of our choosing by stimulating appropriate ab-producing cell lines, as long as the appropriate vaccine components are present. The injected components are nontoxic and nonirritant and do not cause any disturbance in the normal functioning of the immune system. The MVT achieves by ab information transfer the production in the vaccinated recipients of the same class of immunoglobulin (ie, ab) with the same specificity against the target ag as resides in the inoculum. According to our assessment, the immune system should be able to correct any mishaps that might occur (associated, eg, with autoimmune diseases, cancer, chronic infections, etc) provided the cells of the immune system are afforded the right presentation of the offending ag.
We predict that this MVT will in time be the vaccination technique of choice for both the prevention and with equal effectiveness the treatment of most of the presently untreatable diseases caused by exogenous and endogenous source ags.
Acknowledgments
We acknowledge the assistance of our research assistant, Zoltan B Kovacs, in computer-related work.
References
- AndreFE2003Vaccinology: past achievements, present roadblocks and future promisesVaccine21593512531323
- AndresGBrentjensJRCaldwellPR1986Formation of immune deposits and diseaseLab Invest55510202945966
- AvrameasS1991Natural autoantibodies: from ‘horror autotoxicus’ to ‘gnothi seauton’Immunol Today1215491715166
- BakerPJOchiRFSchulzeM1989Depletion of C6 prevents development of proteinuria in experimental membranous nephropathy in ratsAm J Pathol135185942672823
- BarabasAZColeCDBarabasAD2006aEffect of rat kidney fraction 3 (rKF3) antigen and specific IgM antibody against rKF3 on the progression of slowly progressive Heymann nephritisPathol Int565162916930332
- BarabasAZColeCDBarabasAD2006bReduced incidence of slowly progressive Heymann nephritis in rats immunized with a modified vaccination techniqueClin Dev Immunol13172416603441
- BarabasAZColeCDBarabasAD2004aPresence of immunoglobulin M antibodies around the glomerular capillaries and in the mesangium of normal and passive Heymann nephritis ratsInt J Exp Pathol852011215312125
- BarabasAZColeCDBarabasAD2003Production of a new model of slowly progressive Heymann nephritisInt J Exp Pathol842455814748744
- BarabasAZColeCDBarabasAD2004bDown-regulation of pathogenic autoantibody response in a slowly progressive Heymann nephritis kidney disease modelInt J Exp Pathol853213415566429
- BarabasAZColeCDBarabasAD2004cProduction of Heymann nephritis by a chemically modified renal antigenInt J Exp Pathol852778515379960
- BarabasAZColeCDBarabasAD2006cDownregulation of a pathogenic autoantibody response by IgM autoantibodies directed against the nephritogenic antigen in slowly progressive Heymann nephritisPathol Int561819016634963
- BarabasAZJamesKLanniganR1969Preliminary observations on the effect of heterologous anti-lymphocytic globulin on autologous immune complex nephritis in ratsClin Exp Immunol5419275359960
- BarabasAZLafreniereR2005Antigen-specific down-regulation of immunopathological events in an experimental autoimmune kidney diseaseAutoimmun Rev45657016214097
- BarabasAZLanniganR1969Auto-immune nephritis in ratsJ Pathol97537434187535
- BarabasAZNagiAHLanniganR1970The effect of cortisone treatment on autologous immune complex glomerulonephritis in ratsBr J Exp Pathol5154164099592
- Ben YehudaOTomerYShoenfeldY1988Advances in therapy of auto-immune diseasesSemin Arthritis Rheum17206203072680
- BilsboroughJVineyJL2002Getting to the guts of immune regulationImmunology1061394312047743
- BoltonWKSpargoBALewisEJ1974Chronic autologous immune complex glomerulopathy: effect of cyproheptadineJ Lab Clin Med836957044821854
- CasaliPNotkinsAL1989CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoireImmunol Today1036482482031
- CattranDC1988Effect of ciclosporin on active Heymann nephritisNephron4814283344054
- ChenZJWheelerJNotkinsAL1995Antigen-binding B cells and polyreactive antibodiesEur J Immunol25579867533091
- ClynesR2005Immune complexes as therapy for autoimmunityJ Clin Invest11525715630438
- CornishJBarabasAZLanniganR1984Modified immunofluorescent antibody test: demonstration of nephritogenic antigen in glomeruli of ratsDiagn Immunol213366388980
- CoutinhoAKazatchkineMDAvrameasS1995Natural autoantibodiesCurr Opin Immunol781288679125
- DrakesmithHChainBBeverleyP2000How can dendritic cells cause autoimmune diseaseImmunol Today212141710782051
- ElsonCJWeirDM1967Chemotaxis of polymorphs induced by tissue antigens and normal serum in rats: a possible clearance mechanismClin Exp Immunol258186064346
- FariaAMWeinerHL1999Oral tolerance: mechanisms and therapeutic applicationsAdv Immunol7315326410399007
- FujinamiRSOldstoneMB1985Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunityScience230104352414848
- GarzaKMChanSMSuriR2000Role of antigen-presenting cells in mediating tolerance and autoimmunityJ Exp Med1912021710839816
- GolbusJMcCuneWJ1994Lupus nephritis. Classification, prognosis, immunopathogenesis, and treatmentRheum Dis Clin North Am20213428153400
- GrabarP1965Some considerations of the problem of auto-antibody formationTex Rep Biol Med23278845890855
- GrabarP1983Autoantibodies and the physiological role of immunoglobulinsImmunol Today433740
- GuilhermeLKalilJRoseNRShoenfeldY2004Rheumatic fever: how streptococcal throat infection triggers an autoimmune diseaseInfection and autoimmunityAmsterdamElsevier B.V32131
- HasegawaYKaneokaHTanakaT2001Suppression of experimental membranous glomerulonephritis in rats by an anti-MHC class II antibodyNephron882334011423754
- HeymannWHackelDBHarwoodS1959Production of the nephritic syndrome in rat by Freund’s adjuvant and rat kidney suspensionProc Soc Exp Biol Med100660413645677
- KerjaschkiDFarquharMG1983Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis ratsJ Exp Med157667866337231
- KuporLRLowanceDCMcPhaulJJJr1976Single and multiple drug therapy in autologous immune complex nephritis in ratsJ Lab Clin Med872736128575
- LafailleJJMathisD2002Immunological Yin-YangCurr Opin Immunol147413
- LenzDCLuLConantSB2001A Chlamydia pneumoniae-specific peptide induces experimental autoimmune encephalomyelitis in ratsJ Immunol1671803811466406
- LernmarkA2001Autoimmune diseases: are markers ready for predictionJ Clin Invest1081091611602614
- LudewigBJuntTHengartnerH2001Dendritic cells in autoimmune diseasesCurr Opin Immunol136576211677086
- MakkerSPSinghAK1982Biochemical analysis of nephritogenic rat kidney tubular membrane fraction (FX1A) [abstract]Federation of American Societies for Experimental Biology 66th Annual Meeting New OrleansLouisianaApril 15–23 1982411159
- MaloneyDGSmithBRoseA2002Rituximab: mechanism of action and resistanceSemin Oncol292911842383
- ManzRAArceSCasseseG2002Humoral immunity and long-lived plasma cellsCurr Opin Immunol145172112088688
- MatsukawaWHaraSYoshidaF1992Effects of a new immunosuppressive agent, FK506, in rats with active Heymann nephritisJ Lab Clin Med119116231371298
- MeliefCJVan Der BurgSHToesRE2002Effective therapeutic anticancer vaccines based on precision guiding of cytolytic T lymphocytesImmunol Rev1881778212445291
- MendrickDLNobleBBrentjensJR1980Antibody-mediated injury to proximal tubules in Heymann nephritisKidney Int18328437007708
- MorrisECBendleGMStaussHJ2003Prospects for immunotherapy of malignant diseaseClin Exp Immunol1311712519379
- NawataYStallAMHerzenbergLA1990Surface immunoglobulin ligands and cytokines differentially affect proliferation and antibody production by human CD5+ and CD5− B lymphocytesInt Immunol2603141703783
- PeakmanMDayanCM2001Antigen-specific immunotherapy for autoimmune disease: fighting fire with fireImmunology104361611899420
- PennyMJBoydRAHallBM1998Permanent CD8(+) T cell depletion prevents proteinuria in active Heymann nephritisJ Exp Med1881775849815255
- PinckardRNWeirDM1966Antibodies against the mitochondrial fraction of liver after toxic liver damage in ratsClin Exp Immunol133434958207
- PolakisP2005Arming antibodies for cancer therapyCurr Opin Pharmacol5382715951239
- RamiyaVKLanMSWasserfallCH1997Immunization therapies in the prevention of diabetesJ Autoimmun10287929218756
- ReedSGCampos-NetoA2003Vaccines for parasitic and bacterial diseasesCurr Opin Immunol154566012900279
- RichMW1996Drug-induced lupus. The list of culprits growsPostgrad Med10029988795660
- SchijnsVE2001Induction and direction of immune responses by vaccine adjuvantsCrit Rev Immunol21758511642615
- SchoenRTTrenthamDE1981Drug-induced lupus: an adjuvant diseaseAm J Med71587018236
- ShermanLA2001Greater complexity, greater opportunityCurr Opin Immunol136378
- SinghAKMakkerSP1986Circulatory antigens of Heymann nephritis. I. Identification and partial characterizationImmunology57467723485565
- SinghAKSchwartzMM1986Circulatory antigen of Heymann nephritis. II. Isolation of a 70,000 MW antigen from normal rat serum which cross-reacts with Heymann nephritis antigenImmunology5945183539772
- SlingluffCLJrSpeiserDE2005Progress and controversies in developing cancer vaccinesJ Transl Med31815862126
- SolvasonNChenXShuF1992The fetal omentum in mice and humans. A site enriched for precursors of CD5 B cells early in developmentAnn N Y Acad Sci65110201376027
- SpicerSTHaHBoydRA2001Il-4 therapy prevents the development of proteinuria in active Heymann nephritis by inhibition of Tc1 cellsJ Immunol16737253311564788
- StaussHJ2001Benign autoimmunity to combat malignancyClin Exp Immunol1251211472418
- TangQBluestoneJA2006Regulatory T-cell physiology and application to treat autoimmunityImmunol Rev2122173716903917
- TheofilopoulosAN1995The basis of autoimmunity: Part I. Mechanisms of aberrant self-recognitionImmunol Today169087888073
- TotoritisMCRubinRL1985Drug-induced lupus. Genetic, clinical, and laboratory featuresPostgrad Med78149613875843
- TungKS1994Mechanism of self-tolerance and events leading to autoimmune disease and autoantibody responseClin Immunol Immunopathol73275827955555
- WeigleWONakamuraRMSpiegelbergHL1967Autoimmunity and termination of immunological unresponsivenessArch Pathol84647584167987
- WeinerHL1997Oral tolerance: immune mechanisms and treatment of autoimmune diseasesImmunol Today18335439238837
- WeinerHL2000Oral tolerance, an active immunologic process mediated by multiple mechanismsJ Clin Invest106935711032852
- WeirDM1966The immune response after tissue injuryPathol Eur1108185990934
- WeirDM1969Altered antigens and autoimmunityVox Sang16304135800589
- WeirDMElsonCJ1969Antitissue antibodies and immunological tolerance to selfArthritis Rheum12254604182396
- WeirDMPinckardRNElsonCJ1966Naturally occurring anti-tissue antibodies in rat seraClin Exp Immunol1433425338951
- WilsonCTiwanaHEbringerA2000Molecular mimicry between HLA-DR alleles associated with rheumatoid arthritis and Proteus mirabilis as the Aetiological basis for autoimmunityMicrobes Infect214899611099935
- WucherpfennigKW2001Mechanisms for the induction of autoimmunity by infectious agentsJ Clin Invest108109710411602615
- YokoyamaHGoshimaSWadaT1999The short- and long-term outcomes of membranous nephropathy treated with intravenous immune globulin therapy. Kanazawa Study Group for Renal Diseases and HypertensionNephrol Dial Transplant1423798610528661
- YungRLJohnsonKJRichardsonBC1995New concepts in the pathogenesis of drug-induced lupusLab Invest73746598558836
- YungRLRichardsonBC1994Drug-induced lupusRheum Dis Clin North Am2061867512273