Abstract
After nearly three decades with little change in the treatment for B-cell non-Hodgkin’s lymphoma, the addition of immunotherapy has had a profound effect on the treatment of this group of diseases. A more subtle addition to the armentarium has been the radiolabeled monoclonal antibodies, 90yttrium ibritumomab tiuxetan and 131iodine tositumomab. Unfortunately these drugs have been underutilized. This is, in part, because of the need for coordination between specialties, concern about long-term effects, possible limitations on the tolerance of subsequent therapies and, in part, because of reimbursement factors. In this review, the studies in relapsed and refractory disease are discussed and the very promising results reported from phase II studies using radioimmunotherapy as first-line. Potential mechanisms of resistance to monoclonal antibodies are postulated based on alterations in cell signaling pathways that have been observed in lymphoma cell lines resistant to rituximab. It is anticipated that as mechanisms of resistance are better understood for both unlabeled and labeled monoclonal antibodies, biomarkers will not only predict their efficacy but also lead to the development of therapies to overcome resistance.
Introduction
The non-Hodgkin’s lymphomas (NHL) are a diverse group of lymphoid neoplasms that collectively rank fifth in cancer incidence and mortality in the US (CitationParker et al 1996; CitationMüller et al 2005). The incidence and prevalence of NHL has risen 150% over the past few decades (CitationAisenberg 1995; CitationEdwards et al 2005) The incidence increases with age, as most patients are older than age 60 at diagnosis, and males overall are affected about 1.5 times more often than females. In 2007, it is estimated that there will be 63,000 new cases and over 18,000 deaths in the US from NHL (CitationNational Cancer Institute 2007).
NHL are categorized according to the World Health Organization (WHO) classification. The WHO system was developed to help standardize the diagnosis of lymphomas based on histologic, molecular, genetic, and immunologic criteria. The majority of adult lymphomas are B cell in origin; the most common subtypes are follicular lymphoma and diffuse large B-cell lymphoma which account for 25% and 35% of cases, respectively. Other less common subtypes of B-cell lymphoma include mantle cell lymphoma, small lymphocytic lymphoma, and marginal zone lymphoma (CitationHarris et al 2000; CitationWorld Health Organization 2001).
Approximately, 95% of B-cell lymphomas have a CD20 surface antigen. CD20 (membrane-spanning 4-domain, group A, member 1) is a nonglycosylated protein of 33 to 35 kDa expressed on the cell surface of human B lymphoctyes. The gene for CD20 (MS4A1) is switched on at the pre-B-cell stage of B-cell development, expressed throughout B-cell maturation, and lost during final maturation to plasma cells (CitationCartron et al 2004). The configuration of CD20, which is anchored in the cell membrane, protects antigen shedding. CD20 is expressed on the B-cell at a high density in excess of 100,000 copies per cell but does appear to vary by histologic subtype. The exact function of CD20 has not been fully elucidated. There is no ligand to CD20 that has been identified; and mice that are CD20-deficient do not demonstrate abnormal B-cell function. However, studies suggest that CD20 serves as a kinase in Ca2+ influx across plasma membranes and in doing so may have a role in regulating cell cycle progression (CitationGolay et al 1985). Anti-CD20 monoclonal antibodies have an effect on regulation of the cell cycle and induce a wide variety of signaling events which lead to the induction of apoptosis, as well as through a number of other potential mechanisms.
Rituximab is a chimeric murine/human monoclonal antibody produced by recombinant technology from the parent murine monoclonal antibody, ibritumomab. It binds specifically to the CD20 antigen with an apparent avidity 5–11 nM in vitro which is about one log greater than its parent murine antibody (CitationIDEC Pharmaceuticals 2001). It was engineered by fusing the light- and heavy-chain variable domains of 2B8 murine monoclonal anti-CD20 antibody and human kappa light-chain and gamma 1 heavy-chain constant regions (CitationReff et al 1994). In 1997 it became the first mono-clonal antibody for hematologic malignancies approved by the US Food and Drug Administration (FDA).
The anti-CD20 monoclonal antibody, rituximab, appears to have several mechanisms of action and their relative influence on these mechanisms may account for differences in sensitivity across hisotologic subtypes of B-cell lymphomas. In vitro studies indicate that rituximab induces complement-dependent cytotoxicity (CDC) in B-cell lym-phoma lines. This CDC lysis correlates in part with the level of CD20 expression but may also be dependent on the interaction with complement regulatory proteins (CitationGolay et al 2000, Citation2001; CitationTreon et al 2001). The increase in complement activation products which often occurs with the first infusion of rituximab has been described as “cytokine-release syndrome” (CitationWinkler et al 1999) with associated fever, rigors, headache, and rarely cardiovascular collapse.
Antibody-dependent cytotoxicity (ADCC) is another mechanism that results in tumor cell lysis. Binding of the antibody to the target antigen allows for the recruitment of effector cells such as NK cells and macrophages, which express Fc receptors. These cells are then directed toward the target cells inducing either phagocytosis or release of their cytotoxic granules to promote cell killing. There are several classes of Fc receptors. The presence of the FcγRIIIa genotype correlates with clinical and molecular responses in untreated follicular lymphoma confirming that ADCC is an important mechanism of action of rituximab (CitationCartron et al 2002; CitationWeng and Levy 2002, Citation2003). One of the effects of the immunomodulatory derivatives is to enhance N-K cell activity. It is of interest that preliminary data suggests potent synergy of the combination of thalidomide, a potential stimulator of N-K cell activity, and rituximab in relapsed mantle cell lymphoma. This possible synergy merits further investigation particularly as more potent immunomodulatory analogs become available (CitationKaufmann et al 2004).
Several studies have indicated that the maximal clinical response to rituximab may take several months suggesting additional mechanisms of action. One such hypothesis is that peptides from lysed lymphoma cells are presented to dendritic cells with the generation of specific cytolytic T lymphocytes (CitationKalergis and Ravetch 2002; CitationRafiq et al 2002; CitationSelenko et al 2002). While there are experimental data in murine models supporting this passive immunotherapy model, human data are lacking.
Mechanism of resistance to monoclonal antibody therapy are under investigation. The clinical observations are that previously untreated patients with low-grade and follicular lymphoma have a much higher overall response rate and complete response rate than patients with prior treatment, and that, patients will eventually become resistant or refractory to rituximab therapy. There are many possible explanations for this resistance. In vitro manipulation of the extracellular epitope of the CD20 loop alters the binding to anti-CD20 monoclonal antibodies, and thus, suggests that critical mutations in CD20 may be one mechanism of resistance (CitationBinder et al 2006). In another model using rituxiamb sensitive and resistant cell clones, surface CD20 expression has been demonstrated to be diminished in resistant line (CitationCzuczman et al 2004). Studies of the phenotypic cell signaling profiles of rituximab sensitive and resistant cell clones demonstrate that rituximab failed to chemosensitize rituximab-resistant clones which exhibited constitutively hyperactivation of NF-kB and ERK1/2 pathways, which leads to overexpression of resistance factors such as Bcl-2, Bcl-xL, and Mcl-1 (CitationJazirehi et al 2004, Citation2005, Citation2007). An understanding of altered cell signaling and resistance may have implications for combining agents which will reverse resistance.
At the same time that preclinical and clinical evaluation was taking place with unlabeled anti-CD20 monoclonal antibody, radiolabeling technologies made it possible to chelate radioactive isotopes to monoclonal antibodies, which retain their specificity and could provide targeted delivery of localized radiation (CitationChinn et al 1999). Anti-CD20 radioimmunoconjugates, which link the isotopes 90yttrium (90Y) and 131iodine (131I) to murine monoclonal antibodies, were subsequently approved by the US FDA for the treatment of relapsed, and refractory low-grade lymphoma in 2002 and 2003, respectively. Radioimmunoconjugates are an attractive therapeutic option for lymphomas presumably because of the inherent sensitivity of malignant lymphoma cells to ionizing radiation coupled with the mechanism of action associated with anti-CD20 binding. The popular thinking is that high energy isotopes will delivery targeted radiation. In the case of 90Y-ibritumomab tiuxetan, the radiolabel is a pure beta emitter with a short path length. This means that 90% of the dose is delivered within a range of 5 mm in tissue. This equates to a few hundred cell diameters and provides the thesis for a radiation crossfire effect in which cell kill can occur not only to cells with bound antibody but also cells at some distance which may not bind antibody and, hence, may be particularly beneficial in bulky or poorly vascularized tumors (CitationJacobs et al 2005; CitationSkvortsova et al 2005). In a small pilot clinical study, intratumoral distribution of monoclonal antibody and quantitative assessment of radioactivity was examined in patients’ nodal tissues sampled after therapy with 90Y-ibritumomab tiuxetan by autoradiography and measuring decay events. There was a general correlation between radioactivity in the tissue, isotopic localization to the cell surface membrane and response to therapy. A failure to bind to malignant lymphocytes would appear to be one explanation for lack to response to radioimmunotherapy (RIT) (CitationJacobs et al 2005) ().
Figure 1 Autoradiographic localization of 90Y-ibritumomab tiuxetan from lymph node on a patient sampled 4 days after treatment.
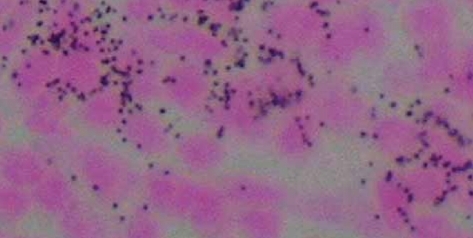
Several in vitro studies suggest that there are more complex mechanisms accounting for the additive or synergistic effects of radiation and monoclonal antibody therapy. When combining rituximab and external beam radiation there was a synergistic increase in expression of proteins involved in apoptosis and cell-cycle regulation (CitationSkvortsova et al 2005). In these in vitro studies, enhanced antiproliferative and apop-totic effects of rituximab and radiation were achieved through inhibition of c-myc and Bcl-xL expression. In another model evaluating the interaction between radiation and a monoclo-nal antibody, ionizing radiation was observed to trigger epidermal growth factor (EGF) import into the nucleus and as a consequence increase in DNA-repair kinase activity. By pre-treatment with anti-EGFR antibody DNA repair was inhibited and resulted in increased radiosensitivity of the treated cells (CitationMilas et al 2000; CitationDittman et al 2005). In another murine lymphoma model, I-131 labeled anti-MHC class II monoclonal antibody, while an excellent vector to deliver radiotherapy to a target, did not result in long-term clearance of tumor. However, by combining the radiation delivery antibody with an unlabeled anti-idiotype (CitationDu et al 2007) long-term tumor eradication was observed. Clinical data supporting an additive effect of radiation and monoclonal antibody come from a study in patients with relapsed/refractory B-cell lymphoma patients in which unlabeled tositumomab was compared with 131I-tositumomab. The overall response favored the radiola-beled monoclonal antibody 55% versus 19%. In addition 13 of 19 patients crossed over from the unlabeled antibody to the labeled antibody responded (CitationDavis et al 2004). Additional study is needed to elucidate more fully the effects of ionizing irradiation and monoclonal antibody to realize the potential of this therapy.
90Y-ibritumomab tiuxetan (Zevalin®) and 131I-tositumomab (Bexxar®) are FDA-approved for therapy for relapsed or refractory low-grade lymphoma with or without transfor- anti-CD20 antibody mation. Ibritumomab, the murine IgG1 that is the parent of the chimeric antibody rituximab, is covalently linked to the MX-DTPA linker-chelator tiuxetan, which provides a high-affinity chelation site for 90Y or for scanning purposes to 111indium (111In). As mentioned above, 90Y is a pure beta emitter with a half-life of 64 hours and a maximum energy of 2.3 MeV and a mean path length in soft tissue of 5.3 mm. With 90Y-ibritumomab tiuxetan, the radiation absorbed dose is primarily delivered to the region of binding with minimal free yttrium in the serum or cleared in the urine, thus allowing the prescribed dose to be based on a fixed amount of radioactivity per body weight. The dose of 90Y-ibritumomab tiuxetan is calculated by body weight in kilograms and is 0.4 mCi/kg for patients with a platelet count ≥150,000 × 109/mL or 0.3 mCi/kg for a platelet count between 100,000 and 149,000 × 109/mL. The maximum dose administered is 32 mCi. The 90Y-ibritumomab regimen can be conveniently administered over one week as an outpatient. On day 1, rituximab at 250 mg/m2 is infused and within 4 hours of completion, a tracer dose of 111In ibritumomab, 5 mCi, is administered over 10 minutes. Gamma scanning is performed once at 24–72 hours to confirm the expected biodistribution. On day 8, rituximab is repeated at 250 mg/m2 and the therapeutic dose of 90Y-ibritumomab tiuxetan is administered (CitationDillman 2002). Following treatment with 90Y-ibritumomab tiuxetan, patients do not require isolation and only minimal radiation precautions are required such as avoiding contact with bodily fluids for the first week after treatment (CitationWitzig et al 1999, Citation2003a; CitationDillman 2002; CitationWiseman et al 2002; CitationWhite 2004; CitationJohnston et al 2006).
Tositumomab, a murine IgG2a monoclonal antibody specific for CD20, is labeled with 131I. 131I is a beta and gamma emitter with a half-life of 8 days, maximum particle energy of 0.5 MeV and a path length of 0.8 mm. Because of individual patient variability in clearance of 131I-tositumomab, a dosimetric dose consisting of 10 mg of antibody labeled with approximately 5 mCi 131I is administered over 30 minutes. Using a NaI scintillation probe and gamma camera, serial total body radioactivity counts are obtained and total-body clearance kinetics are measured over three time points in order to calculate the millicuries to produce a total body therapeutic dose of 75 cGy for patients with a platelet count ≥ 150,000 × 109/mL and 65 cGy for patients with a platelet count of 100,000 to 149,000 × 109/mL. As the 131I-conjugated antibody is metabolized, free 131I metabolites are released into the bloodstream and rapidly excreted in the urine. Patients receive potassium iodide 120 mg daily, as thyroid blockage, administered from 24 hours prior to the dosimetric dose and continued for 14 days after the therapeutic administration. Institutional policies vary with regard to in-patient or out-patient administration of 131I-tositumomab (CitationVose et al 2000; CitationKaminski et al 2000, Citation2001).
The major toxicity with both radiolabeled antibodies is reversible myelosuppression. The hematologic toxicity tends to occur at 5–7 weeks after treatment and may take 2–4 weeks for recovery. With 90Y-ibritumomab tiuxetan, Grade 4 neutropenia, thrombocytopenia, and anemia occurred in 30%, 10% and 4% of patients, respectively (CitationWitzig et al 2003a). Growth factors, blood and platelet transfusion support are required in about 20% of patients (CitationDillman 2002). Hematologic toxicity increased with the degree of bone marrow involvement, number of prior regimens, and prior therapy with fludarabine. Two studies report treatment of patients with 90Y-ibritumomab tiuxetan and one report of 131I-tositumoab with prior high-dose therapy with manageable hematologic toxicity (CitationKaminski et al 2000; CitationVose et al 2003; CitationJacobs et al 2005). In a review of 349 patients treated with 90Y-ibritumomab tiuxetan for safety, the grade 3 or 4 non-hematologic toxicities occurred in 11% of patients; asthenia was most common and occurred in only 6 patients. Hospitalizations with infection during the treatment period occurred in 23 patients (7%) with 6 patient having neutropenic fever (CitationWitzig et al 2003a). Hematologic toxicities are similar with 131I- tositumomab. Human anti-mouse antibodies (HAMA) may be detected in the serum in approximately 2% of heavily pretreated patients with the 90Y-ibritumomab tiuxetan and 10% of heavily pretreated patients with the 131I-tositumomab (CitationKaminski et al 2001). However, HAMA were detected in 48 of 76 (63%) previously untreated patients with follicular lym-phoma following infusion with 131I-tositumomab (CitationKaminski et al 2005). The potential advantages of a HAMA or human anti-globulin antibody (HAGA) have not been generally appreciated and are not well studied after ibritumomab or tositumomab. However, following administration of RIT, the development of polyclonal HAGA directed against epitopes on the administered antibody may generate anti-idiotypic antibody that can mimic the tumor antigen and facilitate host recognition of the tumor. This may have a clinical benefit (CitationDeNardo et al 2003; CitationAzinovic et al 2006). Certain murine antibody-based assays such as prostate-specific antigen may be unreliable in HAMA positive patients. A major long-term concern with both radiolabeled antibodies has been the potential for myelodysplasia and acute leukemia. Thus far, it appears to be about 3%, which is the frequency of these toxicities in lymphoma patients receiving extensive chemotherapy (CitationCzuczman et al 2004; CitationBennett et al 2005). In the study of 76 previously untreated patients who received 131I-tositumomab, there have been no cases of MDS (CitationKaminski et al 2005). Another delayed toxicity of 131I-tositumomab is hypothyroidism, which may occur in a small number of patients despite pretreatment with potassium iodide (CitationKaminski et al 2000).
Patients relapsing after radioimmunotherapy can be treated feasibly with subsequent therapy. In 58 patient treated after 90Y-ibritumomab failure at a single institution, the median number of additional regimens was two (range one to seven). The therapies ranged from single agent chemotherapy to combination regimens to bone marrow transplant. The tolerability of salvage treatment seemed to be similar to the same salvage therapy in patients who did not receive ibritumomab (CitationAnsell et al 2002). Relapsed patients can safely receive radiation. In a recent report 20 of 26 sites in 19 patients responded to radiation after 90Y-ibritumomab tiuxetan. There were no unexpected toxicities (CitationJustice et al 2006).
Treatment of relapsed or refractory patients with follicular lymphoma with 90Y-ibritumomab tiuextan
There are multiple studies which demonstrate the efficacy of both ibritumomab tiuxetan and tositumomab in relapsed and rituximab-refractory follicular lymphoma (CitationKaminski et al 2001; CitationWitzig et al 2002a, Citationb). This review will focus mainly on the studies with 90Y-ibritumomab tiuxetan. The entry criteria in the pivotal studies with 90Y-ibritumomab tiuxetan required adequate bone marrow reserve as determined by peripheral counts showing absolute neutrophils of at least 1500 × 109/mL and a platelet count of >100,000 × 109/mL. Bone marrow criteria included a cellularity of at least 15% of the marrow space and less than 25% of the marrow space occupied by lymphoma. Patients with prior high-dose therapy with bone marrow rescue were excluded. Under these circumstances, it was established that for patients with a platelet count of 100,000 × 109/mL to 149,000 × 109/mL, a dose of 0.3 mCi/kg was well tolerated and that for patients with a platelet count >150,000 × 109/ml, a dose of 0.4 mCi/kg was well tolerated. The maximum dose for either group was 32 mCi/kg. The randomized, multicenter phase III study that compared 90Y-ibritumomab tiuxetan to rituximab included 143 patients. Eligible patients had prior chemotherapy but were rituximab naïve. There were 113 patients with follicular lymphoma, 17 patients with non-follicular low-grade lymphoma and 13 patients with transformed lymphoma. Patient characteristics were well balanced with Stage III/IV disease in 90% and mean and median prior treatments of 2 in each arm. The overall response rate (ORR), complete response rate and complete response rate unconfirmed (CR and CRu) were 80% and 34% for 90Y-ibritumomab tiuxetan and 56% and 20% for rituximab. Age did not predict response rate nor toxicity but patients with bulky disease faired less well. The estimated median duration of response for the follicular lymphoma patients was 18.5 months versus 12.1 months favoring the 90Y-ibritumomab arm (CitationWitzig et al 2002b). In a single arm study patients with rituximab refractory follicular lymphoma were treated with 90Y-ibritumomab tiuxetan. Rituximab refractory is defined as failure to respond to rituximab or relapse within 6 months of the last course of therapy. Fifty-four patients with a median number of 4 prior regimens had an overall and complete response rate of 74% and 15% respectively. The median duration of response was estimated to be 6.4 months (CitationWitzig et al 2002a) In an analysis of 1177 patients treated with 131I-tositumomab, overall response, complete response and duration of response decreased as the number of prior therapies increased (CitationGregory et al 2005). In , the response rate and duration of response in patients with follicular lymphoma are compared based on the prior therapy. The decrease in complete response and duration of response is striking most likely reflecting effect of prior therapy and not inherent differences in the efficacy of the radioimmunotherapies.
Table 1 Effect of prior treatment on response to radioimmunotherapy (RIT) in follicular lymphoma (FL)
First-line treatment of follicular lymphoma with RIT alone or in sequence
There is one study which has used radioimmunotherapy as first-line single agent therapy in follicular lymphoma. Seventy-six patients with follicular lymphoma, stage III and IV, and grade 1 or 2 histology received tositumomab. The overall response and complete response rates were 95% and 75% respectively. The ability to achieve a complete response was decreased in patients with a bulky mass of at least 5 cm and if bone marrow involvement was present. At the time of publication, the median follow-up was 5.1 years, the actuarial 5-year progression-free survival was 59% and the median progression-free survival was 6.1 years. The annualized rate of relapse was 25% during the first year and decreased to 13% in the second year, 12% in the third year, and 4.4% per year after 3 years (Kaminski et al 2003a). Non-hematologic toxicities were mild, mostly infusion-related and with low incidence of headache, arthralgias, and myalgias. In long-term follow-up 13% of patients required thyroid replacement. There were no cases of myelodysplastic syndrome or acute leukemia observed after a median follow-up of 5.1 years. Antimouse antibodies (HAMA) were detected in 63% of patients. The antibodies were detected a median of 3.3 weeks after the dosimetric dose of 131I-tositumomab and remained detectable for a median of 5.5 months. The development of high levels of HAMA within the first two weeks of the therapeutic dose was associated with fever, myalgias, arthralgia, or rash lasting 3–4 days without apparent sequelae. Thus, in selected patients, a single dose of 131I-tositumomab appears both safe and effective.
There are 6 studies that have employed chemotherapy followed by RIT as part of first-line therapy (CitationLink et al 2004; CitationShipley et al 2004; CitationLeonard et al 2005; CitationPress et al 2006; CitationZinzani et al 2006; CitationJankowitz et al 2007). As shown in , each of these studies reports a high complete response rate ranging from 67% to 90%. But, in addition, each of these studies suggests that the complete response rate nearly doubled after RIT. In evaluating these and future studies, it is essential to compare characteristics such as Follicular Lymphoma International Prognostic Index (FLIPI) score (CitationSolal-Céligny et al 2004), bulk of disease, histologic grade, and criteria for determination of complete response. In one study, 59 of 60 patients had a baseline positive PET scan. Restaging imaging included either a fused PET-CT or a PET and CT and followed the recently revised guidelines for staging NHL (CitationCheson et al 2007; CitationJuweid et al 2007). There is 10% discordance between using CT criteria for complete response or CRu and a negative PET scan. Also in this study, the majority of patients had marrow involvement, two-thirds had intermediate or high FLIPI score, and half the patients had masses greater than 5 cm and histologic grade 2 or 3. Combining PET and CT scans for evaluation of response, the complete response rate doubled following RIT (CitationJankowitz et al 2007). Some of these studies included measurement of BCL-2 rearrangement in blood or bone marrow. In these subset analyses, conversion of BCL-2 from positive to negative in blood and/or bone marrow correlated with prolonged remission. Several clinical trials suggest that patients achieving a molecular response have a significantly longer failure-free survival than those who did not (CitationCzuczman et al 2001; CitationLehy et al 2006). The implication is that the depth of complete response is greater in patients with a complete response who have converted from PRC positive BCL-2 in blood or bone marrow to PRC negative. While it is often cited that the t(14; 18) translocation of the BCL-2 proto-oncogene is expressed in blood or bone marrow in 80% of patients with follicular lymphoma, the detection rate in recent phase II studies has been substantially lower, 40%–70% (CitationKaminski et al 2005; CitationPress et al 2006; CitationJankowitz et al 2007). In future studies, it may be informative to correlate PET scan negativity with minimal residual disease as determined by BCL-2 assay. However, as yet there is not a standardized, uniformly accepted polymerase chain reaction (PCR) assay to measure BCL-2 rearrangement. As a look into the future, it seems likely to us that determination of cell signaling pathways will be predictive of response to monoclonal antibody therapy and be useful in delineating the mechanisms of synergy between targeted radiation and the effects of anti-CD20 therapy. For now, we await the results of the Intergroup S0016 which is a phase III randomized study comparing CHOP-R with CHOP followed by 131I-tositumomab in previously untreated patients with follicular lymphoma. The primary endpoint is progression-free survival and secondary outcomes are overall response rates and toxcities.
Table 2 First-line therapy in follicular lymphoma (FL) with sequential chemotherapy followed by radioimmunotherapy (RIT)
Marginal zone B cell lymphoma
This subtype of NHL accounts for about 8% of lymphomas and include nodal and extranodal marginal zone lymphoma, gastric mucosa-associated lymphoid tissue and splenic zone lymphoma. The immunophenotype of marginal lymphomas is distinct from other small lymphocytic lymphomas: the antigen profile for the marginal zone lymphomas include CD19, CD20, CD22 positive and CD5, CD10, CD23, and CD11C negative. In a phase II study including 34 patients with gastric and non-gastric marginal zone lymphoma of which 23 had no prior therapy, rituximab produced an overall and complete response rate of 73% and 44%, respectively. The median duration of response was 10.5 months. Studies with radioimmunotherapy are limited with these patients included in phase II studies of low-grade NHL. The randomized study of 90Y-ibritumomab versus rituximab included 9 patients on the ibritumomab arm with nonfol-licular low grade lymphoma. Two of these patients had marginal zone lymphomas and both achieved a complete response (CitationWitzig et al 2002b). A study using 131I-rituximab included 6 patients with marginal lymphoma who relapsed after prior therapy, all responded and 5 achieved a complete response (CitationLehy et al 2006).
Mantle cell lymphoma
Mantle cell lymphoma (MCL) is a distinct entity with characteristic histological appearance, phenotypic profile and chromosomal translocation, t(11; 14). The antigen profile includes CD20, CD19 and CD5 positive, and is BCL-2 and cyclin D1 positive but usually CD10 and BCL-6 negative. Like follicular lymphoma, mantle cell lymphoma is sensitive to radiation with local tumor being very responsive. About a third of patient with relapsed mantle cell lym-phoma will respond to radioimmunotherapy. In a study of 15 patients with heavily pretreated MCL (median number of 3 prior regimens and a range of 1 to 6), who were treated with 90Y-ibritumomab tiuxetan, there were 5 objective responses and a median response duration of 5.7 months (CitationOki et al 2004). A phase II trial from the European MCL Network treated relapsed mantle cell lymphoma patients with cytoreductive therapy followed by ibritumomab. A complete response occurred in 2 of 16 patients after chemotherapy with 7 of 14 partial responders achieving a complete response after RIT (CitationOki et al 2004). An ECOG phase II trial that recently completed accrual in previously untreated MCL patients treated with CHOP-R × 4 cycles followed by 90 Y-ibritumomab tiuxetan reported a complete response rate after CHOP-R of 14% with an increase to 45% after 90Y-ibritumomab tiuxetan (CitationSmith et al 2006). High-dose myeloablative radioimmunotherapy has been used alone and in combination with high-dose chemotherapy in relapsed mantle cell lymphoma. Seven patients with relapsed mantle cell lymphoma, all of who had received CHOP or CHOP-like regimens as first-line therapy, and also relapsed after high-dose chemotherapy with autologous stem cell transplantation were treated with a myeloablative dose of 131I C2B8 (rituximab) (CitationBehr et al 2002). Six patients achieved a complete response and with a median follow-up of 25 months, 5 patients are still in complete response at time of publication. Sixteen patients with relapsed mantle cell lymphoma with a median of three prior regimens received. 131I-tositumomab at a median dose of 510 mCi and 8–13 days later received high-dose etoposide/cyclophosphamide with autologous stem cells. Twelve patients had no progression of lymphoma 6–57 months after treatment with overall survival and progression-free survival at 3 years estimated to be 93% and 61%, respectively (CitationPress et al 2000). Another approach in relapsed mantle cell lymphoma is to sequence bortezomib with RIT to determine if drug resistance can be overcome. Additional clinical trials employing RIT will be necessary to determine the role of RIT in mantle cell lymphoma and to define optimal treatment strategies.
Diffuse large B-cell lymphoma
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous group of aggressive lymphomas which accounts for about 30% of all NHL (CitationArmitage and Weisenburger 1998). Gene expression array data have demonstrated that DLBCL is a heterogeneous group of diseases based on the tumor genotype (CitationAlizadeh et al 2000). At least 3 distinct genetic types were identified. The most common type, occurring in 40% of the cases, was associated with rearrangements of BCL-6 in the absence of other known genetic lesions. The second type involved the activation of BCL-2 (CitationGascoyne et al 1997). Cases with neither BCL-2 or BCL-6 rearrangements compromise the third genetic group of DLBCL. These genetic subgroups have prognostic relevance since the presence of BCL-2 confers a poor prognosis, while the presence of BCL-6 denotes a favorable prognosis (CitationYe et al 1993). DLBCL can arise de novo, or may transform from a low-grade lymphoma such as small B cell lymphoma or follicular lymphoma.
In the initial studies with 90Y-ibritumomab tiuxetan, eligible patients included transformed and intermediate grade diffuse large B-cell lymphoma. At least a partial response was observed in 11 of 23 patients (CitationWitzig et al 2002a). A series of patients at least 60 years old with relapsed or refractory DLBCL where treated with 90Y-ibritumomab tiuxetan and analyzed according to prior therapy. The overall response rate and median survival of approximately 50% and 22 months, respectively, and were similar in induction failures and relapse from complete remission if they did not have prior rituximab; however, the group of patients relapsing after chemotherapy and rituximab had a overall response rate and median survival of approximately 20% and 4.6 months (CitationMorschhauser et al 2007). This is of interest because it indicates that resistance to antibody is itself sufficient to largely abrogate the effects of radiation. Several studies with slightly different designs are evaluating chemotherapy-rituximab followed by 90Y-ibritumomab compared with chemotherapy-rituximab alone as first-line therapy.
Small lymphocytic lymphoma
Small lymphocytic lymphoma (SLL) is the nodal counterpart of chronic lymphocytic lymphoma. SLL cells have a lower density of CD20 than other B cell malignancies. In clinical trials, SLL has a lower response rate to rituximab than follicular lymphoma. The poor response rate to rituximab is particularly striking in patients who have failed prior therapy. Combining three series, 6 of 49 patients with prior therapy responded to single agent rituximab (CitationMcLaughlin et al 1998; CitationDavis et al 1999; CitationPiro et al 1999). This is contrasted to responses with single-agent therapy in 15 of 22 patients with no prior therapy (CitationHainsworth et al 2003). A small number of patients with SLL were included in the early trials with 90Y-ibritumomab. In one study 3 of 6 patients achieved a partial response (CitationWitzig et al 2002b), and in the study of patients with mild thrombocytopenia, SLL patients treated with 90Y-ibritumomab, the response rate in small lymphocytic lymphoma and transformed B-cell lymphoma was noted to be significantly lower than in follicular lymphoma (CitationWiseman et al 2002). In our personal experience, the response rate in SLL is poor and all the responses have been partial.
Radioimmunotherapy and high-dose chemotherapy with stem cells
There are several phase I/II and phase II studies combining either 90Y-ibritumomab tiuxetan or 131I-tositumomab either alone or with high-dose chemotherapy and stem cell rescue (CitationPress et al 2000; CitationGopal et al 2003; CitationWinter et al 2004; CitationNademanee et al 2005; CitationVose et al 2005). With 131I-tositumomab, the target dose of radiation to the critical normal organs such as lung, liver and kidneys was 2000–2700 cGy. Using the standard dose of 131I to achieve a total body dose of 75 cGy, depending upon dosimetry, the actual 131I dose varies from 1184 to 8510 MBq. By comparison, with the high dose regimen the actual 131I dose varies from 10064 to 31080 MBq. The regimens employing 90Y-ibritumomab tiuxetan deliver a target dose of 1000 cGy to highest normal organ with a median administered dose of 71.6 mCi (range 36.6–105 mCi). The maximum conventional dose of 90Y-ibritumomab tiuxetan is 32 mCi. These studies have shown that it is feasible to give high dose of RIT and further that there is no additional toxicity of combining high-dose RIT with high-dose chemotherapy with stem cell rescue. To date in phase I/II and phase II studies, overall response rates have been substantial in diffuse large B-cell, follicular lymphoma and mantle cell lymphoma. However, in the absence of larger, comparative studies it remains difficult to sort out the role of each component such as high-dose chemotherapy alone or with high- vs low-dose radioimmunotherapy.
Unusual adverse events
Anaphylactic reaction
The package inserts for RIT warn of allergic reactions such as angioedema, acute respiratory distress, hypoxia, bronchospasm, and even anaphylactic shock with first exposure to the monoclonal antibody component of the drug. There are a few series of cases in which responders to RIT have been re-treated. One report describes 32 patients who because of initial response to 131I-tositumomab, were re-treated with 131I-tositumomab tiuxetan. The overall and complete response rates to re-treatment were 52% and 25%, respectively. The median duration of compete response was 35 months. One patient experienced an anaphylactoid-type reaction associated with unlabeled tositumomab infusion during the therapeutic dose. In another phase I study in which 15 patients received a sequential second dose of 90Y-ibritumomab tiuxetan, there were no unexpected side effects reported (CitationWitzig et al 2003b). The overall response rate was 83%. We reported a case of an anaphylactic reaction in a 45-year-old man with an 8-year history of heavily treated follicular lymphoma who was receiving a second treatment with 90Y-ibritumomab tiuxetan. On day 1 of the re-treatment the ibritumomab tiuxetan regimen, the patient received rituximab followed by 111In-ibritumomab tiuxtan without any difficulty. His biodistribution scan was unremarkable. One week later, he returned for the therapeutic dose. Again, rituximab was administered without any reaction but after less than 1.0 ml of 90Y-ibritumomab tiuxetan, the patient complained of chest pain. He was observed to have shallow respirations, hypotension and incontinence. He responded to resuscitative measures. A HAMA was measured and was strongly positive.
The anaphylactic reaction of our patient represents an immunologic response from a clonal population of “memory” B-cells. Presumably, his primary immune response occurred with his first exposure to the dosimetric dose of 111In-ibritumomab tiuxetan. With his initial therapeutic dose 1 week later, he had no reaction. There is generally a lag of 5–7 days before antibody levels start to rise in the primary immune response (CitationGoldsby et al 2000). In a secondary immune response, the lag time before antibody levels start to rise is much shorter (1–2 days) and the levels are much higher (100 to 1000-fold higher) (CitationGoldsby et al 2000). Thus, when patients are re-treated with murine and chimeric antibodies, their secondary immune response will result in more rapid antibody formation. Our patient’s re-treatment dosimetric dose likely incited a secondary immune response with amplification of a HAMA-producing population of memory B-cells which prompted his robust immunologic response to his therapeutic dose 6 days later.
The median time that HAMA antibodies remained detectable in the serum of previously untreated patients using 131I-tositumomab was 5.5 months (range 1.1–25.1) (CitationKaminski et al 2005). Our patient was nearly 2 years out from his initial treatment with radioimmunotherapy, and he may or not have had detectable HAMA in his serum prior to his dosimetric dose, but he clearly had maintained his amnestic response. While this is an unusual reaction even in patients with HAMA, this case illustrates that even heavily pretreated patients may have a robust immune response, and it would be prudent to check HAMA levels in all patients prior to re-treatment with radioimmuno-therapy.50
Radiation fibrosis
A 75-year-old man with relapsed follicular non-Hodgkin lymphoma confined to a solitary lung mass was treated with 90Y-ibritumomab tiuxetan. Imaging with PET/CT showed a complete response 3 months after RIT. Thirteen months after RIT, his PET/CT scan showed an FDG-avid infiltrate in the area of the prior lung mass. Bronchoscopy failed to review evidence of recurrent lymphoma and all cultures were negative. The patient was followed over the next 2 years with serial PET-CT scans (). His latest scan 2 years after treatment showed persistent scarring in the area of previous disease with a marked decrease in SUV consistent with radiation fibrosis. Radiation-induced lung injury after therapy with 90Y-theraspheres was previously reported in the setting of intraarterial microspheres used to treat inoperable hepatic tumors. This occurs when there is a large shunt in the liver and the spheres become trapped in the small vessels of the lung. This is the first case in which radiation-induced radiographic changes are reported after RIT for lymphoma. In this case the area of fibrosis was limited to the site of prior lymphoma. Radiation-induced lung injury occurs after a latent period and is separated into 2 categories, which are acute and chronic phases (CitationMcDonald et al 1995; CitationLasky and Merrill 2005). Acute radiation pneumonitis occurs 1–3 months after exposure. Chronic radiation-induced lung injury consists of fibrosis which may be seen 6 months after exposure and can progress over years.
Figure 2 Panel 1 shows large left lung mass on CT scan with corresponding FDG-avidity, biopsy proven follicular lymphoma. Panel 2, 3 months later shows resolution of mass on CT scan and FDG-negative scan. Panel 3, 12 months after 90Y-ibritumomab tiuextan shows infiltrative mass on CT scan and FDG-avid area apart from the heart. Bronchoscopy was negative for recurrent lymphoma and cultures were negative. Panel 4, 24 months after treatment there is residual scarring with decreased FDG-avidity.
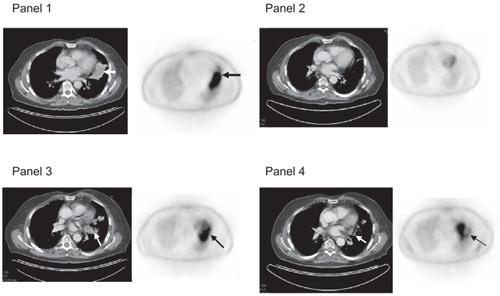
This patient represents an unusual case in that there was a solitary lung mass which appeared similar to a solid tumor without evidence of disseminated disease. For this reason, we suspect that he may have received a concentrated dose of radiation, at a low-dose rate. The 111In-ibritumomab tiuxetan with subsequent scanning with a gamma camera showed uptake in this mass and was used to estimate the radiation delivered to the region of interest (ROI) which we estimated to be a minimum of 980 rads. It is difficult to compare the physiologic effect of a dose of external beam radiation at 200 rads per fraction with that of continuous low-dose radiation which is delivered by RIT.
Prior case reports have indicated that PET imaging may reveal FDG uptake in radiation pneumonitis (CitationLin et al 2000; CitationAlavi et al 2002). PET positivity may be expected given the inflammatory nature of the disorder, including infiltration of leukocytes and pneumocyte proliferation (CitationAlavi et al 2002). It was critically important in this case not to assume that the PET-positive infiltrate was the result of recurrent lymphoma. Nearly 2 years elapsed before there was a significant fall in the FDG-avidity in the left lung.
Serum sickness
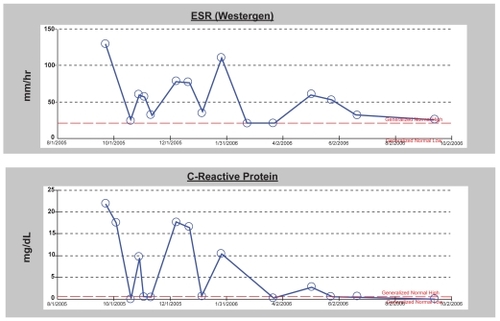
A previously healthy 47-year-old woman was treated for follicular lymphoma, grade III, with CHOP-R followed by 90Y-ibritumomab tiuxetan and rituximab. She developed a serum sickness-like illness 2 weeks after initiating 111In-ibritumomab and while she was receiving post-RIT rituximab with fever, myalgias, arthralgias, and pleural and pericardial effusions. Despite anti-inflammatory therapies her symptoms persisted for 10 months before ultimate resolution. Her symptoms responded to high dose steroids but with each attempt at tapering, inflammatory markers increased and symptoms recurred. The addition of methrotrexate and colchicine was ineffective. With the combination of indomethicin and prednisone, doses were gradually tapered and discontinued. In is shown a CT chest scan with pleural and pericardial effusions and the accompanying graph () is the time course of inflammatory markers.
Figure 3 CT scan with moderate pleural effusion and small pericardial effusion in patient with serum-sickness like syndrome. Both effusions rapidly cleared with steroid administration.
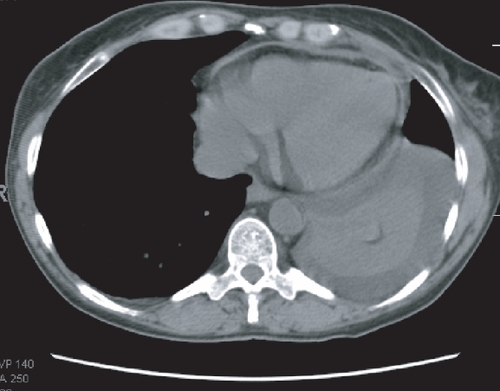
Figure 4 Graphs of sed rate and c-reative protein in patient with serum-sickness like syndrome following immunotherapy with ibritumomab aand rituximab. The rise in inflammatory markers corresponded with attempts at tapering of the dose of steroid.
The occurrence of serum sickness after therapy with rituximab has been previously reported in 19 patients. In general, symptoms consisted of fever, myalgias, and arthral-gias which responded promptly to corticosteroids. Serum sickness has been reported after rituximab in 2 patients with follicular lymphoma (CitationSeror et al 2007), although other reported cases occurred in patients previously diagnosed with autoimmune disorders. This type of reaction has not been reported after RIT. In this patient, we are unable to determine which is the more likely causative agent rituximab or ibritumomab. Theoretically, the antibody 90Y-ibritumomab may be more likely since it is a pure murine molecule, as opposed to rituximab, the majority of which is humanized. Serum testing was not definitive, as both HAMA and HACA were negative.
As monoclonal antibody use continues for various malignant and non-malignant diseases, and as 90Y-ibritumomab tiuxetan is used earlier in the course of disease in patients with lymphoma when patients are less immunocomprised, clinicians should be aware of this potential complication.
Summary
The development of anti-CD20 monoclonal antibody therapy has had a major impact on the treatment of B-cell NHL. It has been demonstrated in randomized clinical trials in patients with diffuse large B-cell lymphoma that the addition of rituximab to CHOP has improved the overall response rate and improved survival. What role of radioimmunotherapy will have on this subtype of lymphoma and in what setting remains to be defined. Current studies in previously untreated patients with stage I and II disease as well as in advanced disease may provide additional insight. Whether RIT will find a place in the treatment algorithm of mantle cell lymphoma similarly awaits additional trials and maturation of data from the ECOG study and others. In a summary of SWOG trials in follicular lymphoma comparing studies from 1974 to 1983 and 1988 to 1994 and from 1998 to 2000, it is apparent that the introduction of monoclonal antibody therapy has had a beneficial effect on progression-free survival and overall survival, however, it is as yet not possible to define differences between labeled and unlabeled antibody. Notwithstanding, it is clear that RIT can produce significant responses in patients with relapsed follicular lymphoma including those patients refractory to rituximab. The place of RIT as a first-line single agent or sequentially with chemotherapy needs to be determined by well-designed clinical trials. The results of the large randomized Intergroup study may not be available for several years. It is also likely that by further elucidating the interaction of labeled and unlabeled monoclonal antibody and defining the mechanism of action of these agents on cell signaling a scientific rationale for designing optimal use of these agents will become a reality. We would envision that there will be patterns of expression of cell signaling proteins that will be predictive of response or resistance to antibody therapy and, may also, provide insight into combination of agents which will enhance response or overcome resistance. For lymphomas, apoptosis seems to be a critical pathway for cell death and it is likely that radioimmunotherapy will be demonstrated to have a role in apoptotic cell death through cell signaling mechanisms.
References
- AisenbergAC1995Coherent view of non-Hodgkin’s lymphomaJ Clin Oncol132656757595720
- AlaviAGuptaNAlberiniJL2002Positron emission tomography imaging in nonmalignant thoracic disordersSemin Nucl Med3229332112524653
- AlizadehAEEisenMBDavisRE2000Distinct types of diffuse large B-cell lymphoma identified by gene expression profilingNature4035031110676951
- AnsellSMRistowKMHabermanTM2002Subsequent chemotherapy regimens are well tolerated after radioimmunotherapy with yttrium-90 ibritumomab tiuxetan for non-Hodgkin’s lymphomaJ Clin Oncol2038859012228209
- ArmitageJWeisenburgerD1998New approach to classifying non-Hodgkin’s lymphomas: Clinical features of the major histologic subtypesJ Clin Oncol1627809704731
- AzinovicIDeNardoGLLambornKR2006Survival benefit associated with human anti-mouse antibody (HAMA) in patients with B-cell malignanciesCancer Immunol Immunother551451816496145
- BehrTMGriesingerFRiggertJ2002High-dose myeloablative radio-immunotherapy of mantle cell non-Hodgkin lymphoma with the iodine-131-labeled chimeric anti-CD20 antibody C2B8 and autologous stem cell support. Results of a pilot studyCancer94Suppl13637211877767
- BennettJMKaminskiMSLeonardJP2005Assessment of treatment-related myelodysplastic syndromes and acute myeloid leukemia in patients with non-Hodgkin lymphoma treated with tositumomab and iodine I131 tositumomabBlood10545768215731177
- BinderMOttoFMertelsmannR2006The epitope recognized by rituximabBlood1081975816705086
- CartronGDacheauxLSallesG2002Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FeYRIIIa geneBlood99754811806974
- CartronGWatierHGolayJ2004From the bench to the bedside: ways to improve rituximab efficacyBlood10426354215226177
- ChesonBDPfistnerBJuweidME2007Revised response criteria for malignant lymphomaJ Clin Oncol255798617242396
- ChinnPCLeonardJERosenbergJ1999Preclinical evaluation of 90Y-labeled anti-CD20 monoclonal antibody for treatment of non-Hodgkin’s lymphomaInt J Oncol1510172510536187
- CzuczmanMGrillo-LopezAJMcLaughlinP2001Clearing of cells bearing the bcl-2 [t(14; 18)] translocation from blood and marrow of patients treated with rituximab alone or in combination with CHOP chemotherapyAnn Oncol121091411249036
- CzuczmanMSOlejniczakSGowdaAC2004Acquirement of rituximab resistance in lymphoma cell lines is associated with structural changes in the internal domain of CD20 regulated at the post-transcriptional level [abstract]Blood, (ASH Annual Meeting Abstracts)104 abstract 2280
- DavisTAKaminskiMSLeonardJP2004The radioisotope contributes significantly to the activity of radioimmunotherapyClin Cancer Res107792815585610
- DavisTAWhiteCAGrillo-LopezAJ1999Single-agent monoclonal antibody efficacy in bulky non-Hodgkin’s lymphoma: results of a phase II trial of rituximabJ Clin Oncol171851710561225
- DeNardoGLBradtBMMirickGR2003Human antiglobulin response to foreign antibodies: therapeutic benefitCancer Immunol Immunother523091612700946
- DillmanRO2002Radiolabeled anti-CD20 monoclonal antibodies for the treatment of B-cell lymphomaJ Clin Oncol2035455712177115
- DittmanKMayerCFehrenbacherB2005Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinaseJ Biol Chem28031182916000298
- DuYHoneychurchJGlennieM2007Microscopic intratumoral dosimetry of radiolabeled antibodies is a critical determinant of successful radioimmunotherapy in B-cell lymphomaCancer Res6713354317283171
- EdwardsBKBrownMLWingoPA2005Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatmentJ Natl Cancer Inst9714072716204691
- GascoyneRDAdomatSAKrajewskiS1997Prognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin’s lymphomaBlood90244519207459
- GolayJLazzariMFacchinettiV2001CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59Blood983383911719378
- GolayJZaffaroniLVaccariT2000Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysisBlood9539000810845926
- GolayJTClarkEABeverleyPC1985The CD20 (Bp35) antigen is involved in activation of B cells from the G0 to the G1 phase of the cell cycleJ Immunol13537958012415587
- GoldsbyRAKindtTJOsborneBA2000Kuby Immunology4th edW.H. Freeman and Company
- GopalAKGooleyTAMaloneyDG2003High-dose radioimmunotherapy versus conventional high-dose therapy and autologous hema-topoietic stem cell transplantation for relapsed follicular non-Hodgkin lymphoma: a multivariable cohort analysisBlood1022351712750161
- GregorySALeonardJPVoseJM2005Superior outcomes associated with earlier use: Experience with tositumomab and iodine I 131 tositumomab in 1,177 patients (pts) with low-grade, follicular, and transformed non-Hodgkin’s lymphoma (NHL)J Clin Oncol, (ASCO Annual Meeting Proceedings)2316S abstract 6561
- HainsworthJDLitchySMorrisseyL2003Rituximab as first-line and maintenance therapy for indolent non-Hodgkin’s lymphoma (NHL): Long-term follow-up of a Minnie Pearl Cancer Research Network Phase II trial [abstract]Blood102111116
- HarrisNLJaffeESDieboldJ2000The World Health Organization classification of hematological malignancies report of the Clinical Advisory Committee MeetingAirlie House, VirginiaNovember, 1997Mod Pathol1319320710697278
- IDEC Pharmaceuticals2001Yttrium–[90] ibritumomab tiuxetan (Zevalin™; IDEC-Y2B8) radioimmunotherapy regimenInvestigational Brochure Edition 6
- JacobsSAVidnovicNJoyceJ2005Full-dose 90Y ibritumomab tiuxetan therapy is safe in patients with prior myeloablative chemotherapyClin Cancer Res117146s50s16203814
- JankowitzRCDeMonacoNAFoonKA2007Phase II study of short course CHOP-rituximab (R) followed by ibritumomab tiuxetan (IT) as first-line treatment for follicular lymphoma (FL) [abstract]J Clin Oncol2518S
- JazirehiARHuerta-YepezSChengG2005Rituximab (chimeric anti-CD20 monoclonal antibody) inhibits the constitutive nuclear factor-(kappa)B signaling pathway in non-Hodgkin’s lymphoma B-cell lines: role in sensitization to chemotherapeutic drug-induced apoptosisCancer Res652647615665303
- JazirehiARVegaMIBonavidaB2007Development of rituximab-resistant lymphoma clones with altered cell signaling and cross-resistance to chemotherapyCancer Res67312708117283164
- JazirehiARVegaMIChatterjeeD2004Inhibition of the Raf-MEK1/2-ERK1/2 signaling pathway, Bci-xL down-regulation, and chemosensitization of non-Hodgkin’s lymphoma B cells by RituximabCancer Res6471172615466208
- JohnstonPBBondlyCMicallefI2006Ibritumomab tiuxetan for non-Hodgkin’s lymphomaExpert Rev Anticancer Ther6861916761929
- JusticeTEMartensonJAWisemanGA2006Safety and efficacy of external beam radiation therapy for non-Hodgkin lymphoma in patients with prior 90Y-ibritumomab tiuxetan radioimmunotherapyCancer107433816770786
- JuweidMEStroobantsSHoekstraOS2007Use of positron emission tomography for response assessment of lymphoma: Consensus of the imaging subcommittee of International Harmonization Project in LymphomaJ Clin Oncol25571817242397
- KalergisAMRavetchJV2002Inducing tumor immunity through the selective engagement of activating FcY receptors on dendritic cellsJ Exp Med1951653912070293
- KaminskiMSEstesJZasadnyKR2000Radioimmunotherapy with iodine 131I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experienceBlood9612596610942366
- KaminskiMSTuckMEstesJ2005131I-Tositumomab therapy as initial treatment for follicular lymphomaN Engl J Med352441915689582
- KaminskiMSZelenetzADPressOW2001Pivotal study of iodine I 131 tositumomab for chemotherapy refractory low-grade or transformed low-grade B-cell non-Hodgkin’s lymphomasJ Clin Oncol1939182811579112
- KaufmannHRafiqKWoehrerS2004Brief Report: Antitumor activity of rituximab plus thalidomide in patients with relapsed/refractory mantle cell lymphomaBlood10422697115166030
- LaskyJAMerrillWW2005Radiation-induced lung injury UpToDate Online, Version 14.1, vol. www.uptodate.com
- LehyMFSeymourJFHicksRJ2006Multicenter phase II clinical study of iodine-131-rituximab radioimmunotherapy in relapsed or refractory indolent non-Hodgkin’s lymphomaJ Clin Oncol2444182516940276
- LeonardJPColemanMKostakobluL2005Abbreviated chemotherapy with fludarabine followed by tositumomab and iodine I 131 tositu-momab for untreated follicular lymphomaJ Clin Oncol23569670416110029
- LinPDelaneyGChuJ2000Fluorine-18 FDG dual-head gamma camera coincidence imaging of radiation pneumonitisClin Nucl Med25866911079581
- LinkBKaminskiMSColemanM2004Phase II study of CVP followed by tositumomab and iodine I 131 tositumomab (Bexxar therapeutic regimen) in patients with untreated follicular non-Hodgkin’s lymphoma (NHL) [abstract]J Clin Oncol2214S
- McDonaldSRubinPPhillipsTL1995Injury to the lung from cancer therapy: clinical syndromes, measurable endpoints, and potential scoring systemsInt J Radiat Oncol Biol Phys3111872037713782
- McLaughlinPGrillo-LopezAJLinkBK1998Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program [abstract]J Clin Oncol1628259704735
- MilasLMasonKHunterN2000In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibodyClin Cancer Res6701810690556
- MorschhauserFIlldgeTHugloD2007Efficacy and safety of yttrium 90 ibritumomab tiuxetan in patients with relapsed or refractory diffuse large B-cell lymphoma not appropriate for autologous stem cell transplantationBlood11054817387223
- MüllerAMSIhorstGMertelsmannR2005Epidemiology of non-Hodgkin’s lymphoma (NHL): trends, geographic distribution, and etiologyAnn Hematol8411215480663
- NademaneeAFormanSMolinaA2005A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphomaBlood1063896902
- National Cancer Institute2007Cancer Topics: Non-Hodgkin lymphoma URL: www.cancer.gov
- OkiYProBDelpassandE2004A Phase II study of yttrium 90 (90Y) ibritumomab tiuxetan (Zevalin®) for treatment of patients with relapsed and refractory mantle cell lymphoma (MCL) [abstract]Blood104111614976060
- ParkerSLTongTBoldenS1996Cancer statistics, 1996Ca465278548526
- PiroLDWhiteCAGrillo-LopezAJ1999Extended rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin’s lymphomaAnn Oncol106556110442187
- PressOWEaryJFGooleyTA2000A Phase I/II trial of iodine-131-tositumomab (anti-CD20), etoposide, cyclophosphamide and autologous stem cell transplantation for relapsed B-cell lymphomasBlood9629344211049969
- PressOWUngerJMBrazielRM2006Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131 tositumomab for previously untreated follicular non-Hodgkin’s lymphomna: Five-year follow-up of Southwest Oncology Group Protocol S9911J Clin Oncol244143916896003
- RafiqKBergtoldAClynesR2002Immune complex-mediated antigen presentation induces tumor immunityJ Clin Immunol110719
- ReffMECarnerKChambersKS1994Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20Blood83435457506951
- SelenkoNMajdicOJagerU2002Cross-priming of cytotoxic T cells promoted by apoptosis-inducing tumor cell reactive antibodiesJ Clin Immunol221243012078853
- SerorRSordetCGuillevinL2007Tolerance and efficacy of rituximab and changes in serum B cell biomarkers in patients with systemic complications of primary Sjogren’s syndromeAnn Rheum Dis66351716950808
- ShipleyDLSpigelDRCarrellDL2004Phase II trial of rituximab and short duration chemotherapy followed by 90Y-ibritumomab tiuxetan as first-line treatment for patients with follocular lymphoma: A Minnie Pearl Cancer Research Network phase II trial [abstract]J Clin Oncol2214S71514665612
- SkvortsovaRPopperBSkvortsovS2005Pretreatment with Rituximab enhances radiosensitivity of non-Hodgkin’s lymphoma cellsJ Radiat Res46241815988143
- SmithMRChenHGordonL2006Phase II study of rituximab + CHOP followed by 90Y-ibritumomab tiuxetan in patients with previously untreated mantle cell lymphoma: An Eastern Cooperative Oncology Group Study (E1499) [abstract]J Clin Oncol2418S
- Solal-CélignyPRoyPColombatP2004Follicular lymphoma international prognostic indexBlood10412586515126323
- TreonSPMitsiadesCMitsiadesN2001Tumor cell expression of CD59 is associated with resistance to CD20 serotherapy in patients with B-cell malignanciesJ Immunother2426371
- VoseJMBiermanPEnkeC2005Phase I trial of iodine-131 tositu-momab with high-dose chemotherapy and autologous stem-cell transplantation for relapsed non-Hodgkin’s lymphomaJ Clin Oncol23461715534357
- VoseJMBiermanPJLynchJC2003Phase I clinical trial of Zevalin (90Y-ibritumomab) in patients with B-cell non-Hodgkin’s lymphoma (NHL) with relapsed disease following high-dose chemotherapy and autologous stem cell transplantation (ASCT) [abstract]Blood102111116
- VoseJMWahlRLSalehM2000Multicenter Phase II study of iodine-131 tostumomab for chemotherapy-relapsed/refractory low-grade and transformed low-grade B-cell non-Hodgkin’s lymphomasJ Clin Oncol1813162310715303
- WengWKLevyR2002Rituximab-induced antibody-dependent cellular cytotoxicity (ADCC) in vollicular non-Hodgkin’s lymphoma [abstract]Blood, (ASH Annual Meeting Abstracts)100 abstract 590
- WengWKLevyR2003Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphomaJ Clin Oncol2139404712975461
- WhiteCA2004Radioimmunotherapy in non-Hodgkin’s lymphoma: focus on 90Y-ibritumomab tiuxetan (Zevalin®)J Exp Ther Oncol43051615844660
- WinklerUJensenMManzkeO1999Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (Rituximab, IDEC-C2B8)Blood9422172410498591
- WinterJNInwardsDJSpiesS200490Y Ibritumomab Tiuxetan (Zevalin®; 90YZ) doses higher than .4 mCi/kg may be safely combined with high-dose beam and autotransplant: The role for dosimetry [abstract]Blood, (ASH Annual Meeting Abstracts)104 abstract 1162
- WisemanGAGordonLIMultaniPS2002Ibritumomab tiuxetan radioimmunotherapy for patients with relapsed or refractory non-Hodgkin lymphoma and mild thrombocytopenia: a phase II multicenter trialBlood9943364212036859
- WitzigTEFlinnIWGordonLI2002aTreatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphomaJ Clin Oncol203269
- WitzigTEGordonLICabanillasF2002bRandomized controlled trial of yttrium-90 labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphomaJ Clin Oncol2024536312011122
- WitzigTEWhiteCAGordonLI2003Safety of yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non-Hodgkin’s lymphomaJ Clin Oncol2112637012663713
- WitzigTEWhiteCAWisemanGA1999Phase I/II trial of IDEC-Y2B8 radioimmunotherapy for treatment of relapsed or refractory CD20 (+) B-cell non-Hodgkin’s lymphomaJ Clin Oncol17379380310577851
- WitzigTEWisemanGAGeyerSM2003A Phase I trial of two-sequential doses of Zevalin™ radioimmunotherapy for relapsed low-grade B-cell non-Hodgkin’s lymphoma [abstract]Blood10211406a.1116
- World Health Organization2001Classification of tumours, pathology and genetics of tumours of haematopoietic and lymphoid tissuesLyonIARC Press
- YeBHListaFLoCF1993Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphomaScience262747508235596
- ZinzaniPLPulsoniABaloccoM2006A Phase II trial of FM (oral fludarabine and mitoxantrone) chemotherapy followed by yttrium 90 (90Y) ibritumomab tiuxetan (Zevalin®) for previously untreated follicular lymphoma (FL) patients [abstract]Blood1081111116543467