Abstract
Although the advent of infliximab has changed the treatment paradigm and goals in inflammatory bowel diseases (IBD), it does not provide a cure for IBD and recent evidence has demonstrated that the immunogenicity of this chimeric anti-TNF antibody is associated with secondary loss of response and intolerance. In ulcerative colitis (UC) the efficacy of infliximab was demonstrated in two large clinical trials, but long-term maintenance efficacy data are lacking. Novel biological agents have entered clinical development and pioneering trials have been reported in the last two years. For Crohn’s disease (CD) two anti-TNF agents, the fully human IgG1 anti-TNF monoclonal adalimumab and the humanized pegylated Fab-fragment certolizumab-pegol and the humanized anti α4 integrin IgG4 antibody both have demonstrated efficacy as maintenance agents. Adalimumab has been approved to treat active rheumatoid arthritis, psoriatric arthritis, and ankylosing spondylitis, and recently moderate-to-severe luminal CD has been added as an indication for this agent both by the FDA and EMEA. Further evidence is needed to establish the therapeutic potential of adalimumab in fistulizing CD and in UC. The benefit to risk ratio of anti-TNF agents in refractory IBD is clearly positive and since most of the toxicity is class specific, adalimumab is expected to have a safety profile similar to that of infliximab except for adverse events related to infusions.
Introduction
Inflammatory bowel diseases (IBD) are chronically relapsing intestinal inflammatory conditions with a typical onset in young adulthood and with an unpredictable disease course, which may lead to debilitating complications (CitationPodolsky et al 2002). Crohn’s disease (CD) and ulcerative colitis (UC) are the two main phenotypes of IBD. These chronic immune mediated disorders are characterized by abdominal symptoms (diarrhea, abdominal pain rectal bleeding) and systemic manifestations (arthralgias, fatigue, dermatologic, and biliary complications). The most pertinent difference between the two illnesses is the disease location. CD typically occurs in the terminal ileum (terminal enteritis) and right-sided colon. However, lesions can appear anywhere in the gut and the disease frequently affects different parts of the intestine with skip areas of uninvolved mucosa (regional enteritis). UC, on the other hand, universally involves the rectum and extends over a variable distance in the colon. Two thirds of the patients have only left sided colonic disease with sparing of the right colon. Also, CD is a transmural disease leading to fistulas and intestinal strictures, whereas UC affects only the colonic mucosa and submucosa. Patients with CD are usually diagnosed in puberty or young adulthood, which means that this disease is associated with a high psychological and socio-economic and burden. The diagnosis is based on the clinical presentation, on findings at ileocolonoscopy and at enteroclysis, and on histological findings in mucosal biopsies. The initial course of CD and UC is notably unpredictable at diagnosis. Some patients have smoldering ileal disease with few symptoms until they develop a fibrotic stricture, but others have signs of severe systemic inflammation and invalidating diarrhea with abdominal cramps necessitating an early aggressive medical management. However, most patients (60%–70%) will eventually develop protracted disease needing immune-suppressive agents and/or surgery (CitationBeaugerie et al 2006). Complications include obstructive strictures and internal fistulas leading to bowel resection and perianal fistulizing disease with secondary loss of anal sphincter function. Surgery is unavoidable in more than 50% of patients with CD, but given the rapid disease recurrence after a surgical bowel anastomosis and the risk of short bowel syndrome, conservative bowel-sparing surgery is the gold standard.
CD and UC are relatively infrequent with an estimated incidence of 5 in 100,000 in the US, Canada, Australia, and Northwestern Europe. Southern America, Southern Europe, and the developing world appear to have a much lower incidence. However, since CD is a life-time illness, peak IBD prevalences of 1 in 250 have been reported in Scandinavia. At present most patients need to be treated long term (several decades), which increases the importance of an optimal benefit to risk ratio whatever treatment is applied.
The advent of the anti-tumor necrosis factor (TNF) agent infliximab has dramatically changed our concept of treating refractory IBD, particularly CD. Although infliximab has proven to induce clinical response and remission with rapid onset of mucosal healing, to spare steroids, to improve perianal disease, and to increase quality of life, there is a considerable unmet medical need in both CD and UC. More humanized anti-TNF agents are prime candidates to fill the gap. This review will focus on the therapeutic potential of the human anti-TNF antibody adalimumab in active CD.
Current treatment and unmet medical need
The paradigm of treatment in CD has been revolutionized by the introduction of two classes of drugs. The advent of corticosteroids 4 decades ago enabled physicians and surgeons to bring patients to full disease remission without symptoms. However, although corticosteroids are used universally in immune-mediated disorders, they are double-edged swords. Steroids have high efficacy at inducing remission (up to 80% in steroids naïve patients) but the side effect profile does not allow long-term treatment. Moreover, up to 20% of patients lose the response to steroids over time (CitationMunkholm et al 1994). Therefore, the advent of the biological agent, infliximab (Remicade®), a chimeric monoclonal anti-TNF inhibitor, again dramatically changed the paradigm of CD treatment. Even if infliximab does not bring the propect of cure, and surgery is still desperately needed for patients with fibrostenosis and uncontrolled inflammatory or fistulizing disease, this drug has accomplished many goals for a good medical therapy in IBD (). Indeed, data from numerous clinical trials and from clinical practice in large academical centers have demonstrated that infliximab induces and maintains remission in luminal, inflammatory disease and that the improvement enables tapering of steroids (CitationTargan et al 1997; CitationHanauer et al 2002). Moreover, repeated administration of infliximab results in fistula healing (as judged by the cessation of drainage from cutaneous orifices) and maintenance therapy results in durable fistula healing (CitationPresent et al 1999; CitationSands et al 2004).
Table 1 Treatment goals for state novel therapies in Crohn’s disease
Although many of these goals can also be achieved with azathioprine and probably also with methotrexate, these immunomodulators are characterized by a slow onset of action, and therefore are only useful to maintain remission. Furthermore, virtually all clinical trials have convincingly shown that anti-TNF agents are efficacious in most patients failing adequate courses of immunomodulators such as azathioprine (CitationTargan et al 1997; CitationPresent et al 1999; CitationHanauer et al 2002; CitationSands et al 2004).
In general 50%–70% of patients report clinical improvement when treated with infliximab and 40% achieve remission. However, being a chimeric antibody, infliximab is not devoid of immunogenicity and this is most likely the main mechanism underlying secondary loss of response to infliximab (CitationBaert et al 2003). Antibodies to infliximab (ATIs) are associated with acute and delayed hypersensitivity reactions and with secondary loss of response (CitationBaert et al 2003; CitationFarrell et al 2003). Several treatment strategies such as systematic maintenance therapy, concomitant immunosuppression, and prophylactic systemic steroids decrease the incidence of ATI formation (CitationBaert et al 2003; CitationFarrell et al 2003; CitationRutgeerts et al 2004; CitationHanauer et al 2004). Even when the treatment is optimized to avoid anti-drug antibodies either by administering the antibody in scheduled maintenance or by concomitant immunosuppressive therapy, preliminary reports indicate that 40%–50% of patients need an adjustment in dose or treatment schedule to avoid intermittent symptoms and 20% of patients lose their response over time (CitationSiemanowski et al 2006; CitationVan Assche et al 2006). Also infliximab is administered as a 2-hour infusion which means that patients treated long term need to visit an infusion center regularly (, ).
Table 2 Unmet needs in medical therapy for IBD
Table 3 Inflammatory pathways in IBD targeted by biological therapies
Development of adalimumab
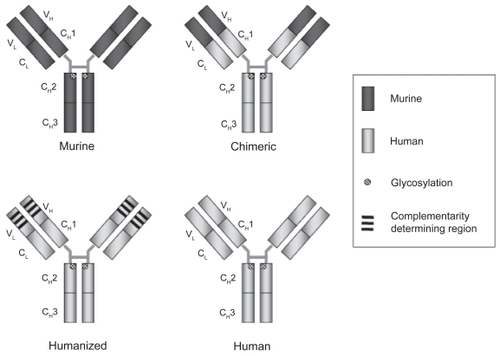
Adalimumab is a fully human IgG1 antibody and the first of these new generation monoclonal antibodies to be approved in human medicine (). Guided selection on phage display was used to construct the antibody by Knoll and Abbott with assistance from Cambridge antibody technology. Using the murine anti-TNF monoclonal Ab MAK195 as a template, the genes for the VL and VH variable regions of the antibody were paired to human DNA sequences of the immunoglobulin repertoire. The hybrid gene encodes monomeric proteins containing the antigen binding protein. The phage display technique was than applied to select for high affinity variable regions and the affinity was further increased in multiple “mix and match” steps of recombination, which closely resemble the biological process of affinity maturation underlying the high specificity of our adaptive immune system. As a final step in development the variable regions were linked to human constant regions, and immortalized Chinese hamster ovary (CHO) cells were than transfected with this complete expression vector to produce a fully human IgG1 monoclonal antibody with a molecular weight of 148 kDa (CitationSalfield et al 2000; CitationOsbourn et al 2005). Hence, adalimumab, is devoid of murine amino acid sequences. However, fully human “designed” monoclonals can be different from naturally occurring immunoglobulines in amino acids sequences and in glycoprotein content, and the complex proteins may undergo structural changes in solution or in the human tissue. As a consequence, they may be recognized as a “non-self” epitope by the human adaptive immune system and generate human anti human antibodies (HAHAs). In the case of adalimumab these HAHAs are referred to as anti adalimumab antibodies.
Figure 1 Humanization of therapeutic antibodies. In general the immunogenicity of therapeutic antibodies has decreased with advances in humanization. The efficacy of an antibody is determined by affinity, avidity, and antibody isotype. This is independent of the degree of humanization.
Efficacy of adalimumab in IBD
Luminal CD
Adalimumab (Humira®, Abott) has been commercially available for the treatment of rheumatoid arthritis since 2002 and more recently for spondylarthropathy, psoriatric arthritis, and CD. Clinical efficacy in CD is inferred from open label experience (CitationSandborn et al 2004; CitationYoudim et al 2004; CitationHinojosa et al 2007), from data in several placebo-controlled trials (CitationHanauer et al 2006; CitationColombel et al 2007; CitationSandborn et al 2007a, Citationb), and from in vitro data on the induction of apoptosis (CitationShen et al 2005; CitationShen et al 2006) (, ).
Table 4 Randomized controlled trials with adalimumab in Crohn’s disease
Two open label trials assessed the therapeutic potential of adalimumab in patients with CD initially responding to infliximab and who had lost response or had become intolerant to this antibody. Sandborn et al reported on a trial enrolling 24 patients, who were dosed with 80 mg adalimumab at week 0 and 40 mg 2 weeks later (CitationSandborn et al 2004). The open label trial ran for 12 weeks with doses of 40 mg every 2 weeks. Adalimumab was well tolerated by all patients regardless of previous reactions to infliximab and efficacy was suggested with 59% of patients having 70 point CD activity index (CDAI) drop response rates at week 12. After week 4 patients had the option of increasing stepping up to 40 mg of adalimumab weekly, and 79% (19/24) decreased the interval to once per week. CitationYoudim et al (2004) reported on a smaller trial in only 7 infliximab-intolerant patients using a similar dosing strategy (CitationSandborn et al 2004). Of these, 5 out of 7 had high titers of antibodies to infliximab when they had stopped treatment with this antibody earlier in their disease course. Also, in this trial adalimumab was well tolerated with only injection site reactions. In one patient clear endoscopic and histologic healing of the colonic mucosa was demonstrated after 8 weeks in the trial.
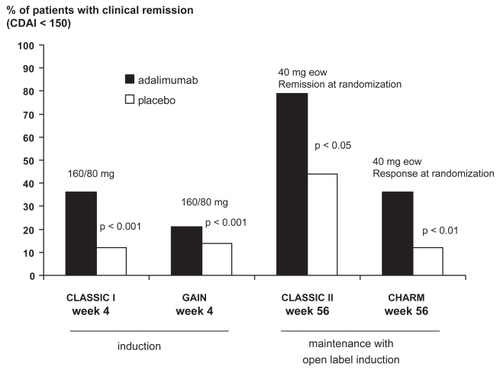
In the Clinical Assessment of Adalimumab Safety and Efficacy Studied as Induction therapy in CD (CLASSIC I), a 4-week multicenter, randomized, placebo-controlled dose-ranging study the efficacy of adalimumab in 299 patients with active luminal CD was demonstrated particularly at the highest dose level (remission 160 mg week 0 and 80 mg week 2 36%, placebo 12%) (CitationHanauer et al 2006). Response as defined by a decrease of at least 70 points in the CDAI was also significantly increased at lower dose levels (adalimumab 40/20 mg: 54%, 80/40 mg: 59% [p < 0.05], 160/80 mg 59% [p < 0.01], placebo 37%), but remission and improvement of at least 100 points in the CDAI was observed only at the highest dose level. Patients who had completed CLASSIC I, were eligible to enter CLASSIC II with open label induction (40 mg adalimumab every other week [eow] twice) and subsequently at 4 weeks randomization of patients in remission (at weeks 0 and 4) to adalimumab 40 mg eow or weekly or to placebo for 56 weeks. A total of 276 patients chose to be enrolled, and 55 achieved remission and were randomized (CitationSandborn et al 2007b). Patients not in remission (n = 204) were offered open label adalimumab 40 mg eow. Patients in both the randomized and open label arm had the option of stepping up the dose in case of a relapse from placebo to adalimumab 40 mg eow or from eow adalimumab 40 mg to no change initially and every week later on. In the randomized arm, 1 year (week 56) remission rates were significantly higher in both adalimumab groups (40 mg eow: 79%, 15/19, 40 mg weekly: 83%, 15/18, p < 0.05 for both compared with placebo 44%, 8/18). Patients with concomitant immunosuppressives use were not more likely to be in remission at week 56. In the open label arm, 131 of the 204 patients completed treatment through week 56 and of the original group of 204, 93 (46%) were in remission at that time point. Reason for discontinuation included adverse events (23/73), lack of efficacy (18/73), and withdrawal of consent (14/73). Step up from eow to weekly adalimumab 40 mg occurred in 60/131 (46%) patients at some point before week 56.
The open label induction (80 mg at week 0, and 40 mg at week 2) Crohn’s Trial of the Fully Human Antibody Adalimumab for Remission Maintenance (CHARM) randomized clinical responders and non-responders to receive adalimumab 40 mg SC eow, 40 mg. weekly, or placebo for 56 weeks (CitationColombel et al 2007). Co-primary endpoints were the proportion of randomized responders who were in remission at both week 26 and week 56. Patients in the two adalimumab groups were more likely to be in remission after 6 months (week 26: adalimumab 40 mg eow 40% (n = 172) and 47% (n = 157) vs 17% (n = 170) for placebo) and after 1 year (week 56: 36% and 41% vs 12% for placebo, p < 0.001). Again, no evidence for an increased efficacy of a higher dosage schedule with 40 mg adalimumab weekly was found in this trial. However, in patients without concomitant immunosuppresives the group treated weekly had 50% (18/36) remission rates vs 33% (12/36) eow, but this difference was not significant. Of note, among all randomized patients 35% withdrew before the end of treatment. Also, 50% of all randomized patients (251/505) left the randomized double-blind cohort and received adalimumab 40 mg eow or weekly. Factors predictive of response were not found, but of note higher numeric values were observed for remission at week 56 in anti-TNF naïve patients (42% adalimumab 40 mg eow, 48% weekly) compared to those treated with anti-TNF agents before (31% adalimumab 40 mg eow, 34% weekly). Sustained clinical remission with full discontinuation of steroids (for at least 90 days) was achieved more often in patients with active treatment (adalimumab 40 mg 29% eow and 20% weekly, placebo 5%, p < 0.001 and p < 0.01).
Subsequently, the GAIN (Gauging Adalimumab Efficacy in Infliximab Nonresponders) trial was designed to assess the efficacy and safety of adalimumab in the patient group with loss of response to or intolerance of infliximab. This short-term (4 weeks) induction-of-remission trial compared adalimumab 160 mg at week 0 and 80 mg at week 2 with placebo (CitationSandborn et al 2007a). A total of 325 moderately to severely ill CD patients were randomized. Of these 57% had stopped infliximab for intolerance and 52% for loss of response (13% experienced both problems). At week 4 significantly more patients in the adalimumab group achieved remission (21% vs 7%, delta 14%, p < 0.001). Improvement of the CDAI by at least 70 and 100 points was also significantly better with adalimumab than with placebo. For the 70 point or more improvement this was observed as early as 1 week from baseline after one dose of 160 mg adalimumab SC (35% vs 21% for placebo, p < 0.01) and for both endpoints at both week 2 and week 4. Parallel to what was seen in CLASSIC I the IBD-questionnaire (IBDQ, disease specific measure of quality of life) scores were significantly higher in the actively treated group (p < 0.001, 150 vs 139). Subgroup analysis of efficacy results showed that only concomitant steroid therapy was associated with better efficacy (p = 0.01). In contrast, neither intolerance of nor loss of response to infliximab, nor the combination of both, nor the presence of ATIs determined the efficacy of adalimumab in this trial. At this time the precise implication of the apparent synergistic effect of concomitant steroids (and not of immunosuppressives) is unclear.
Perianal fistulizing CD
Enterocutaneous fistulas, more particularly in the perianal region, are an invalidating complication of CD and notably refractory to non-biological therapy. Infliximab has shown to be beneficial both in induction and in maintenance of fistula orifice closure. For adalimumab the evidence is limited to open label trial data and secondary endpoints from trials in luminal CD. In the open label trial reported by CitationSandborn et al (2004) 3/9 (33%) patients with draining fistulas achieved closure of all draining orifices at week 12 after treatment with adalimumab 80 mg and 40 mg eow or weekly thereafter. A Spanish open label collaborative trial in 50 patients who had lost their response to or had become intolerant of infliximab enrolled 22 patients with fistulizing disease (CitationHinojosa et al 2007). Patients were treated with adalimumab 160 mg sc at baseline and with 80 mg at week 2. Of these patients 23% (5/22) achieved cessation of drainage from all fistula orifices (fistula remission) and 41% (9/22) had 50% or more of the orifices closed (fistula response) at week 4. In GAIN, which also specifically enrolled patients who had previously failed infliximab, 45/325 patients had draining enterocutaneous or perianal fistulas at baseline (CitationSandborn 2007a). In this trial the fistula response and remission rates were lower and no significant improvement over placebo was observed (fistula remission: adalimumab 160/80 mg: 8%, 2/25; placebo 5%, 1/20, NS). In CHARM, with open label induction and a long-term blinded maintenance arm, significantly more patients in the combined adalimumab groups achieved fistula remission (week 26 30%; week 56 33%) than in the placebo group (13% week 26 and week 56, p < 0.05) (CitationColombel et al 2007). At present it is not possible to position adalimumab between other approved treatments for fistulizing disease, in particular relative to infliximab. Due to the conflicting results, a specific controlled trial with clinical and biological endpoints such as magnetic resonance imaging for patients with fistulizing disease is warranted.
Ulcerative colitis
Data for UC are limited to one open-label trial conducted in France. Ten patients were treated with 160 mg of adalimumab sc followed by 80 mg after 2 weeks. At 4 weeks, only 4 out of 10 patients had improved and in patients with more severe colitis (6/10) no responses were noted (CitationPeyrin-Biroulet et al 2007). Controlled data in this indication are clearly needed.
Other anti-TNF agents in clinical development
CDP-870, certolizumab pegol (Cimzia®, UCB/Celltech) a humanized Fab antibody fragment to tumor necrosis factor linked to polyethylene glycol (PEG) for subcutaneous administration, suggested efficacy in CD patients with elevated C-reactive protein at baseline (>10 mg/L) in an initial randomized trial (CitationSchreiber et al 2005). In the two subsequent trials, Precise 2 with an open label induction phase and placebo controlled maintenance, and Precise 1 with a placebo arm for both induction and maintenance certolizumab pegol, was more efficacious than placebo at maintaining clinical response and remission over 26 weeks of therapy (CitationSandborn et al 2007c). However, in the Precise 1 trial certolizumab pegol failed to induce clinical remission and was of modest benefit for the induction of clinical response (7%–11% delta over placebo) (CitationSandborn 2007c).
Centocor also developed a fully human anti-TNF antibody, golimumab (CNTO-148). Phase II trials in rheumatoid arthritis have been completed and phase I trials have been initiated for CD. Etanercept, a p75 TNF receptor construct, also marketed to treat rheumatoid arthritis, failed to show efficacy in refractory CD and is no longer in development for this indication (CitationSandborn et al 2001). Also, the soluble p55 TNF receptor Onercept (Serono®) has been developed for the treatment of refractory CD. A phase II randomized control trial, however, failed to show clinical efficacy and further development has been discontinued (CitationRutgeerts et al 2006).
Small molecules targeting TNF and TNF receptor signalling are being developed although they are at an early stage. These compounds are less selective than monoclonal antibodies and generally have a pleiotropic mechanism of action.
RDP-58 is an oral decapeptide inhibiting TNF production, but the molecule is also an inhibitor of p38 MAP kinase and of IL-12 synthesis. Procter and Gamble initiated a phase II trial in Europe in patients with refractory CD and UC with this molecule. RDP-58 at 3 dose levels once daily for 28 days was not better than placebo at inducing clinical response in patients with CD (CitationTravis et al 2003). Of note, the placebo response in this trial was high as has been reported in other recent CD trials.
Semapimod (CNI-1493) is an oral guanylhydrazone p 38 MAP kinase and JNK kinase signaling inhibitor. These kinases are important intermediates in the signaling of TNF and other inflammatory cytokines such as IL-1 and IFN-gamma. A phase II uncontrolled pilot trial suggested clinical efficacy (CitationHommes et al 2002), but a placebo-controlled trial failed to meet its endpoints. Moreover, safety concerns with peripheral thrombophlebitis and liver abnormalities led to the interruption of further development.
Safety
From experience with clinical trials and commercial use with adalimumab in rheumatology, it appears that the safety profile of this compound is similar to that of infliximab with a decrease in immunogenicity (maximum 3.8% in the CD trials and maximum 17% antibodies in patients without concomitant methotrexate in rheumatoid arthritis) (CitationBartelds et al 2007; CitationColombel et al 2007). The principal specific safety issues are discussed below.
Injection site reactions
These reactions are a common observation with the sc injection of proteins in solution. In the combined safety data from the trials in CD recently reported in abstract form by Colombel et al (CitationColombel et al 2007). Injection-site-related adverse events were noted in 65/279 patients treated with adalimumab 40 mg eow and 49 out of 275 with weekly treatment. Of the placebo-treated patients, 21/279 reported injection related adverse events. This represents 42 and 30 events per patient-year of follow up for the two adalimumab groups respectively, and 21/100 patient-year for placebo. Injection site reactions hardly ever led to discontinuation of the medication.
Serious infections
Anti-TNF agents such as adalimumab and infliximab have immunomodulatory properties. Even if ex vivo experiments have shown that human lymphocyte function is not affected by infliximab (CitationCornillie et al 2001), recent experience has revealed a limited but real risk of serious infections. For anti-TNF agents in general, serious infections with intracellular organisms such as tuberculosis, listeriosis, and histoplasmosis have received most attention and it is considered a class effect of these agents (CitationKeane et al 2001; CitationWallis et al 2005; CitationListing et al 2005). TNF membrane expression signals replication of intracellular pathogens to patroling immune cells. Therefore, inhibiting the cytokine with an anti-TNF agent hinders adequate and early control of an emerging reactivation of these pathogens. Therefore, screening for latent tuberculosis should be performed prior to initiation of adalimumab therapy as for every other anti-TNF agent. The package inserts of adalimumab mention the risk of serious infections as a boxed warning and strongly advocates screening for latent tuberculosis prior to initiating the drug, but awareness of infectious disease in general should be a standard attitude of every clinician prescribing immunomodulatory agents. An update of the safety data with adalimumab in CD clinical trials has been recently released in abstract from. In 1459 patients (1506 patient-years of exposure), serious adverse events were more likely to occur in placebo-treated than in adalimumab-treated patients, both for induction and maintenance (CitationColombel et al 2007). For serious infections no difference was found in blinded maintenance therapy between the placebo and the two (40 mg eow or weekly) adalimumab groups (serious infection rate: placebo 9/100 patient-years, adalimumab 40 mg eow: 5/100 patient-years, adalimumab 40 mg/week: 4/100 patient-years). Of all 1459 adalimumab-exposed patients, a combined 2% reported an opportunistic infections. Candidiasis was most prevalent, but 3 cases of tuberculosis were documented (0.2%). Of note, 66% of these patients had been exposed for at least 1 year when the analysis was performed. As a reference, the cumulative safety data with adalimumab in 10,050 patients with rheumatoid arthritis enrolled in adalimumab clinical trials and representing 12,506 patient-years of exposure, showed that the rate of serious infections was 5.1/100 patient-years, and the incidence rate of tuberculosis was 0.27/100 patient-years (CitationSchiff et al 2006). It should be noted that in rheumatoid arthritis and in CD, adalimumab is often used in combination with other immunosuppressive agents. Although there is no clear signal of an increased infection rate of anti-TNF agents combined with methotrexate or azathioprine, the large (more than 10,000 patient years as of August, 2005) TREAT cohort study, which follows North American patients started either on infliximab or on alternative immunomodulatory treatment, revealed that the risk of serious infections in infliximab-treated patients is mainly linked to concomitant use of systemic steroids (CitationLichtenstein et al 2006).
Immunogenicity
As discussed before, humanization of therapeutic antibodies diminishes but does not abolish the risk of anti-drug antibodies (CitationHwang and Foote 2005). In clinical practice only antibodies that interfere with drug efficacy (neutralizing antibodies) or instigate adverse events really matter. In recently reported IBD trials, humanized antibodies, in which only the hypervariable or complimentarity determining regions are of murine origin, induced 8%–44% of human anti-human antibodies (HAHA). The humanized and pegylated anti-TNF Fab fragment, certolizumab, induced HAHA in 12% of patients but short-term response at 12 weeks was not affected by the antibody status (CitationSchreiber et al 2005). Data on induction of antibodies to adalimumab in CD are available from one trial only with long-term administration of the antibody. The induction trials (CLASSIC I and GAIN) with exposure limited to 4 weeks are less useful to assess the immunogenicity of adalimumab, and in CHARM antibody formation was not evaluated. In the open label induction CLASSIC-II trial 7/269 (2.6%) patients tested positive for antibodies to adalimumab, all in the 185 patient group without concomitant immunosuppression (3.8%) (CitationSandborn et al 2007b). At the present time these data don’t allow to reach final conclusions about the relevance of these antibodies for the clinical efficacy of adalimumab. In the open label trial reported by Youdim et al (CitationYoudim et al 2004) 79% and in the open label arm (CitationSandborn 2007b) of the CLASSIC-2 trial 46% of patients stepped up to once weekly dosing for an increase in symptoms. However, data on the correlation between the need for decrease in dosing interval and AAA or adalimumab serum concentrations are not available at present. Also, in general, determining antibodies to adalimumab in the presence of measurable adalimumab serum concentrations is not reliable. In GAIN none of the 159 adalimumab-treated patients tested positive for antibodies to adalimumab at 4 weeks, but the median adalimumab serum level at that point was 12.2 μg/mL, and most patients had measurable serum adalimumab levels. Again, as a reference, in all rheumatoid arthritis trials combined, the drug induced HAHA in 5% of patients and concomitant methotrexate was clearly protective (CitationWeinblatt et al 2003; CitationAnderson 2005).
Autoimmunity
Anti-TNF agents are known to induce anti-nuclear antibodies (ANAs). These antibodies are associated with adverse events such as arthralgias and dermatological problems. Also, anti-TNF therapy has been associated with drug-induced lupus, almost always associated with the induction of anti-double stranded (ds) DNA antibodies. The chimeric monoclonal antibody, infliximab has been reported to induced ANA in up to 56% of patients, with anti ds DNA in more than half of them (CitationVermeire et al 2003). For adalimumab, to the best of our knowledge, ANAs were only measured in the CLASSIC II trial. Of the 172 patients with measurements at baseline and 56 weeks and no ANAs at baseline, 33 (19%) tested positive at their final visits, and anti dsDNA Ab were observed in all 33 patients at this time point (CitationSandborn et al 2007b). None of the patients developed novel clinical symptoms suggestive of auto-immunity, but cases of drug-induced lupus have been reported in CD and rheumatoid arthritis (RA) trials with adalimumab.
Malignancy
The safety data accumulated with the use of anti-TNF agents in clinical trials and from cases reported in commercial use indicate that there is no clear increase over the background incidence of malignancies in general.
Non-Hodgkin’s lymphoma has been a particular concern in patients treated with immunomodulators. The incidence of lymphoma in IBD patient cohorts has been variable (relative risk 1–31) probably due to the heterogeneity of patient populations. A recent report from a large cohort of Swedish CD and UC patients found a marginally increased risk of lymphoma (standardized incidence ratio [SIR] 1.3) (CitationAskling et al 2005). In patients with UC the risk of myeloid leukemia (SIR 1.8) was increased while the lymphoma risk was identical to the control population. As in previous reports the lymphoma risk in CD was elevated only in the first year after diagnosis and no trend of an increase in more recent sub-populations (with a presumably higher exposure to immunomodulators) was observed. In the cumulative safety experience with adalimumab in CD trials 16/1,459 patients (1.1%) developed a malignancy, including 1 lymphoma (0.07/100 patient-years) (CitationColombel et al 2007). In the rheumatoid arthritis clinical trials 15 lymphomas were seen after 12,506 patient-years of exposure (0.12/100 patient-years) resulting in a standardized incidence rate (SIR) of 3.19, which is comparable to that of a patient population not treated with biological (CitationSchiff et al 2006). A recent report from the US national database for rheumatic diseases in 19,562 patients with RA (55% treated with an anti-TNF agent) representing 89,710 patients-years of follow up indicated that there was no increase in lympoma risk attributable to biological therapy in general or adalimumab specifically (OR 4.5, 95% CI 0.9–23.1) (CitationWolfe and Michaud 2007). Since RA severity is linked to the lymphoma risk and patients with more severe disease are more likely to receive biological therapy, the analysis was corrected for disease severity. On the other hand, a meta-analysis also using statistical modeling of data from clinical trials and reports from large patients cohorts in rheumatoid arthritis has suggested that infliximab and adalimumab at higher doses (>40 mg eow for adalimumab) is associated with an increased malignancy risk (CitationBongartz et al 2006). The total body of evidence warrants careful follow-up of patients treated with biological agents, but in general do not show a clear increased risk for developing lymphoma or other malignancies.
Adalimumab: place in therapy
Both the FDA and the European agency EMEA approved of the use of adalimumab (Humira®) in patients with moderate to severe (US) or severe (Europe) CD failing other standard therapies or who have become intolerant of infliximab. The US label recommends using an induction dose of 160 mg at week 0 followed by 80 mg 2 weeks later and 40 mg eow in maintenance. The European label recommends an induction dose of 80 mg followed by 40 mg after 2 weeks, with the option of giving double the dose (160/80 mg) at these time points if a more rapid effect is needed for the patients. Also the European label demands concomitant steroid therapy when adalimumab is initiated, if steroid use is not contraindicated. None of the biologicals or small molecule agents currently developed to treat CD have been tested in a comparative trial against infliximab or other standard therapies. Although regulatory agencies favor active comparator trials for registration purposes, the complexity of double dummy trials with two IV agents and the recent move towards subcutaneous administration of therapeutic antibodies has hindered this process. Also, most patients entering clinical trials with biologicals are failing standard therapies such as corticosteroids and azathioprine. One open label randomized trial enrolling patients early on in their disease course favored “top down” infliximab + azathioprine over “step up” steroids and later initiation of azathioprine for short-term steroid-free remission and long-term endoscopic mucosal healing (CitationHommes et al 2006). Data on biological first, top down strategies with adalimumab are not available. Given the lack of comparative trials with anti-TNF and other biological agents in CD, deciding between first- and second-line biologicals is tedious. Outcomes of phase II trials with novel anti-TNF therapies such as adalimumab and certolizumab can be hardly compared with historical pivotal infliximab trials due to an unmistakable change in patient populations. Approximately half of the patients with CD currently entering clinical trials have lost their response or have become intolerant to infliximab. This may work both ways to influence the outcome. Those patients may be enriched for the effect of anti-TNF agents or biologcals in general because they were initially responding. However, infliximab refractory patients may also have developed a less inflammatory disease phenotype with symptoms driven by bowel obstruction or by a disturbed motility. The latter hypothesis is supported by the fact that in the GAIN trial after 4 weeks only 21% of patients achieved remission with adalimumab 160/80/40 mg induction therapy (CitationSandborn et al 2007a).
The precise mechanism of action for biologicals in IBD also needs to be elucidated further. The relative role of T-cell apoptosis induction, considered crucial for the efficacy of anti-TNF agents in CD (CitationLugering et al 2001; Citationten Hove et al 2002; Citationvan den Brande et al 2003; CitationDi Sabatino et al 2004), needs to be revisited in view of recent data of efficacy with certolizumab, which does not induce apoptosis (CitationNesbitt et al 2007).
Subcutaneous administration may be preferred by many patients because it allows home-based care and abolishes the need for visits to an infusion unit, which will undoubtedly also affect the total drug cost. However, subcutaneous dosing of novel anti-TNF agents is generally more frequent than the infliximab re-treatment schedule. Also, the regular visits of a patient to the infusion enable closer monitoring of compliance and adverse events, and this monitoring should not be neglected by physicians and patients alike.
Abbreviations
| IBD | = | inflammatory bowel diseases |
| IL | = | interleukin |
| Ig | = | immunoglobulin |
| TNF | = | tumor necrosis factor |
| CD | = | Crohn’s disease |
| UC | = | UC |
| IV | = | intravenous |
| SC | = | subcutaneous |
References
- AndersonPJ2005Tumor necrosis factor inhibitors: clinical implications of their different immunogenicity profilesSemin Arthritis Rheum34S1192215852250
- AsklingJBrandtLLapidusA2005Risk of haematopoietic cancer in patients with inflammatory bowel diseaseGut546172215831904
- BaertFNomanMVermeireS2003Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s diseaseN Engl J Med348601812584368
- BarteldsGMWijbrandtsCANurmohamedMT2007Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritisAnn Rheum Dis66921617301106
- BeaugerieLSeksikPNion-LarmurierI2006Predictors of Crohn’s diseaseGastroenterology130650616530505
- BongartzTSuttonAJSweetingMJ2006Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trialsJAMA29522758516705109
- ColombelJSandbornWJRutgeertsP2007Adalimumab induces and maintains clinical response and remission in patients with active Crohn’s disease: the CHARM trialGastroenterology132526517241859
- ColombelJFSandbornWSReinischW2007Adalimumab safety in Crohn’s disease clinical trialsGastroenterology132A–504
- CornillieFShealyDD’HaensG2001Infliximab induces potent anti-inflammatory and local immunomodulatory activity but no systemic immune suppression in patients with Crohn’s diseaseAliment Pharmacol Ther154637311284774
- Di SabatinoACiccocioppoRCinqueB2004Defective mucosal T cell death is sustainably reverted by infliximab in a caspase dependent pathway in Crohn’s diseaseGut5370714684579
- FarrellRJAlsahliMJeenYT2003Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn’s disease: a randomized controlled trialGastroenterology1249172412671888
- HanauerSLukášMMacIntoshD2006A randomized, double-blind, placebo-controlled trial of the human anti-TNF-a monoclonal antibody adalimumab for the induction of remission in patients with moderate to severely active Crohn’s diseaseGastroenterology1303233316472588
- HanauerSBFeaganBGLichtensteinGR2002Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trialLancet3591541912047962
- HanauerSBWagnerCLBalaM2004Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s diseaseClin Gastroenterol Hepatol25425315224278
- HinojosaJGomollonFGarciaS2007Spanish Scientific Group on Crohn’s Disease and Ulcerative Colitis. Efficacy and safety of short-term adalimumab treatment in patients with active Crohn’s disease who lost response or showed intolerance to infliximab: a prospective, open-label, multicentre trialAliment Pharmacol Ther254091817269996
- HommesDBaertFVan AsscheG2006The ideal management of Crohn’s disease: top down versus step up strategies, a randomized controlled trial [abstract]Gastroenterology130A108
- HommesDvan den BlinkBPlasseT2002Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn’s diseaseGastroenterology12271411781274
- HwangWYKFooteJ2005Immunogenicity of engineered antibodiesMethods3631015848070
- KeaneJGershonSWiseRP2001Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agentN Engl J Med345109810411596589
- LichtensteinGRFeaganBGCohenRD2006Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registryClin Gastroenterol Hepatol46213016678077
- ListingJStrangfeldAKaryS2005Infections in patients with rheumatoid arthritis treated with biologic agentsArthritis Rheum5234031216255017
- LugeringASchmidtMLugeringN2001Infliximab induces apoptosis in monocytes from patients with chronic active Crohn’s disease by using a caspase-dependent pathwayGastroenterology12111455711677207
- MunkholmPLangholzEDavidsenM1994Frequency of glucocorticoid resistance and dependency in Crohn’s diseaseGut3536028150347
- NesbittAFossatiGBerginM2007Mechanism of action of certolizumab pegol (CDP870): In vitro comparison with other anti-tumor necrosis factor alpha agentsInflamm Bowel Dis1332332
- OsbournJGrovesMVaughanT2005From rodent reagents to human therapeutics using antibody guided selectionMethods3661815848075
- Peyrin-BirouletLLaclotteCRoblinX2007Adalimumab induction therapy for ulcerative colitis with intolerance or lost response to infliximab: An open-label studyWorld J Gastroenterol1323283217511032
- PodolskyDK2002Inflammatory bowel diseaseN Engl J Med3474172912167685
- PresentDHRutgeertsPTarganS1999Infliximab for the treatment of fistulas in patients with Crohn’s diseaseN Engl J Med340139840510228190
- RutgeertsPFeaganBGLichtensteinGR2004Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s diseaseGastroenterology1264021314762776
- RutgeertsPSandbornWJFedorakRN2006Onercept for moderate-to-severe Crohn’s disease: a randomized, doubl-blind, placebo-controlled trialClin Gastroenterol Hepatol48889316797249
- SalfieldJAllenDHoogenboomH2000BASF Aktiengesellschaft, assigneeHuman antibodies that bind human TNF alpha US patent 6,090,382
- SandbornWJFeaganBGStoinovS2006Certolizumab pegol administered subcutaneously is effective and well tolerated in patients with active Crohn’s disease: results from a 26 week placebo-controlled phase III study (PRECiSe 1)Gastroenterology130A–107
- SandbornWJHanauerSLoftusEVJr2004An open-label study of the human anti-TNF monoclonal antibody adalimumab in subjects with prior loss of response or intolerance to infliximab for Crohn’s diseaseAm J Gastroenterol991984915447761
- SandbornWJHanauerSBKatzS2001Etanercept for active Crohn’s disease: a randomized, double-blind, placebo-controlled trialGastroenterology12110889411677200
- SandbornWJHanauerSBRutgeertsPJ2007Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II TrialGut561232917299059
- SandbornWJRutgeertsPEnnsR2007Adalimumab induction therapy for Crohn’s disease previously treated with infliximabAnn Int Medicine146I20
- SandbornWJFeaganBGStoinovSPRECISE 1 Study Investigators2007Certolizumab pegol for the treatment of Crohn’s diseaseN Engl J Med3572283817634458
- SandsBEAndersonFHBernsteinCN2004Infliximab maintenance therapy for fistulizing Crohn’s diseaseN Engl J Med3508768514985485
- SchiffMHBurmesterGRKentJD2006Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritisAnn Rheum Dis658899416439435
- SchreiberSKhaliq-KareemiMLawranceIC2007Maintenance therapy with certolizumab pegol for Crohn’s diseaseN Engl J Med3572395017634459
- SchreiberSRutgeertsPFedorakRN2005A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s diseaseGastroenterology1298071816143120
- ShenCVan AsscheGColpaertS2005Adalimumab induces apoptosis of human monocytes: a comparative study with infliximab and etanerceptAliment Pharmacol Ther21251815691299
- ShenCVan AsscheGRutgeertsP2006Caspase activation and apoptosis induction by adalimumab: demonstration in vitro and in vivo in a chimeric mouse modelInflamm Bowel Dis1222816374254
- SiemanowskiBKipKPlevyS2006Adjustment in infliximab dose or dosing interval in Crohn’s disease Experience from the University of PittsburghGastroenterology130A–142
- TarganSRHanauerSBvan DeventerSJ1997A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study GroupN Engl J Med3371029359321530
- ten HoveTvan MontfransCPeppelenboschMP2002Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn’s diseaseGut502061111788561
- TravisSPYapLRDP Investigators’ Study Group2003RDP58-a novel and effective oral therapy for ulcerative colitis (UC) results of parallel prospective, multicentre, blinded placebo-controlled trialsGut52SVIA5
- Van AsscheGPainteaudGD’HaensG2006Continuation of immunomodulators is not required to maintain adequate infliximab efficacy in patients with Crohn’s disease but may improve pharmacokineticsGastroenterology130A–142
- van den BrandeJMBraatHvan den BrinkGR2003Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn’s diseaseGastroenterology12417748512806611
- VermeireSNomanMVan AsscheG2003Autoimmunity associated with anti-tumor necrosis factor alpha treatment in Crohn’s disease: a prospective cohort studyGastroenterology12532912851868
- WallisRSBroderMWongJ2005Reactivation of latent granulomatous infections by infliximabClin Infect Dis41S194815983899
- WeinblattMEKeystoneECFurstDE2003Adalimumab a fully human anti-tumor necrosis factor-α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate. The ARMADA TrialArthritis Rheum48354512528101
- WolfeFMichaudK2007The effect of methotrexate and anti-tumor necrosis factor therapy on the risk of lymphoma in rheumatoid arthritis in 19,562 patients during 89,710 person-years of observationArthritis Rheum561433917469100
- YoudimAVasiliauskasEATarganSR2004A pilot study of adalimumab in infliximab-allergic patientsInflamm Bowel Dis10333815475739