Abstract
Chemotherapy alone has limited ability to significantly improve survival in non-small lung cancer (NSCLC) beyond what has already been achieved. The epidermal growth factor (EGF) pathway plays a vital role in the pathogenesis and progression of NSCLC. Two classes of drugs inhibit the EGF receptor (EGFR) pathway: small molecules that inhibit the intracellular tyrosine kinase activity of the receptor, and monoclonal antibodies that target the extracellular domain in the ligand-binding region. Cetuximab is a human – mouse chimeric immunoglobulin G1 class monoclonal antibody directed against EGFR. Preclinical studies with cetuximab suggested that there was inhibition of growth of human NSCLC cell lines. Cetuximab is currently the focus of intense investigation in various patient populations with NSCLC. This review focuses on clinical trials of cetuximab in NSCLC and identifies future directions with this agent.
Introduction
Lung cancer is the most common cause of cancer death in both men and women in the United States and around the world.Citation1,Citation2 Approximately 85% of lung cancers are non-small cell lung cancers (NSCLC), and approximately 75% of these are metastatic at diagnosis.Citation3,Citation4 While therapy has evolved over the last few decades and has repeatedly been shown to improve survival, the five-year overall survival of patients with NSCLC is a dismal 15%.Citation2 The significant toxicity associated with cytotoxic chemotherapy also excludes a number of patients based on age, co-morbidities, or poor performance status.Citation5
Platinum-based doublets have been established therapy for metastatic NSCLC since the mid-1990s.Citation6 Numerous studies have shown that, among cytotoxic chemotherapeutics, two-agent therapy is more effective than single-agent therapy.Citation7–Citation9 Using three or more cytotoxic therapies does not improve efficacy and increases toxicity.Citation10,Citation11 Commonly used chemotherapy doublets incorporate cisplatin or carboplatin with agents that include paclitaxel, docetaxel, vinorelbine, gemcitabine, or more recently pemetrexed. Many regimens given in the first-line setting result in a median survival time of eight to 10 months, with one- and two-year survival rates of 35% to 45%, and 10% to 20%, respectively.Citation12
While studies with new chemotherapeutic agents are ongoing, evidence now available suggests that chemotherapy alone has limited ability to significantly improve survival beyond what has already been achieved.Citation13 Furthermore, toxicities associated with these medicines not only exclude some patients based on poor performance status but also preclude a number of patients from completing chemotherapy courses as scheduled at the full therapeutic dose.Citation5 As the knowledge of tumor biology has improved, biologic agents that specifically target molecules thought to be critical to tumorigenesis have emerged. Theoretically, the greater specificity against malignant cells with targeted agents should result in simultaneously greater efficacy and less toxicity, thereby allowing usage in patients previously excluded from consideration of systemic therapy.
Biologic therapies targeting the vascular endothelial growth factor receptor (VEGFR) and the endothelial growth factor receptor (EGFR) are undergoing evaluation to determine their potential role in NSCLC.Citation13 Bevacizumab, a monoclonal antibody against VEGFR,Citation14 and erlotinib, a small molecule inhibitor of the intracellular tyrosine kinase of EGFR,Citation15 currently are indicated for use in NSCLC. Cetuximab, a monoclonal antibody against EGFR, is currently being studied extensively. Here we review the role of cetuximab in the treatment of NSCLC.
EGFR in lung cancer
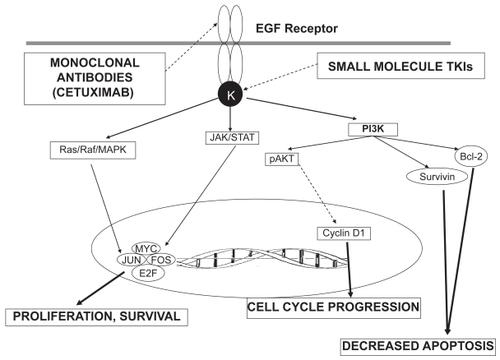
EGFR, whose ligands include epidermal growth factor (EGF) and transforming growth factor-alpha (TGF-α), is a member of the ErbB1 (HER-1) family of receptors.Citation16,Citation17 Binding of the extracellular domain by EGF or TGF-α induces dimerization of EGFR, leading directly to activation of kinase activity in the intracellular domain. Receptor tyrosine kinases transfer phosphate groups from bound ATP to tyrosine residues on the carboxy (C) terminal portion of the receptor.Citation18,Citation19 Multiple intracellular cascades are activated when adaptor molecules recognize phosphotyrosines on the C-terminal of EGFR: 1. KRAS/RAF/MEK/MAP kinase pathway via binding of Grb2/SOS; 2. PI3-K pathway and the 3. STAT3/5 pathwayCitation20 (). The complexity (ie, pathway overlap and crosstalk) of intracellular signaling via tyrosine kinases is vastly oversimplified by a cursory discussion, but it is evident that activation of these pathways serves to increase transcription and cellular proliferation, and inhibit apoptosis.Citation20 In malignant cells, they also contribute to angiogenesis and metastasis.Citation21,Citation22
Figure 1 EGFR signaling pathway. The figure depicts the downstream pathways following activation of the EGF receptor. Binding of a ligand to the receptor activates the tyrosine kinase (K) which then activates the downstream signaling pathways that eventually lead to increased proliferation, cell cycle progression and decreased apoptosis. Solid arrows indicate stimulation, while dashed arrows indicate suppression. Sites of action of the tyrosine kinase inhibitors (TKIs) and monoclonal antibodies are also shown.
The EGF pathway plays a vital role in the pathogenesis and progression of NSCLC. Approximately 50%–80% of NSCLC demonstrate overexpression of EGFR activity.Citation23–Citation27 Although the majority of NSCLC overexpress EGFR at diagnosis, expression appears to vary with histology (65% in adenocarcinomas to 84% in squamous carcinomas).Citation28 EGFR expression also seems to correlate with stage at presentation. EGFR levels are higher in pathological stage IV NSCLC than in stage I and II disease and higher in cases with mediastinal involvement than in cases without it.Citation29
Although EGFR expression is important in the development and progression of malignancy, its prognostic significance is unclear. Some studies observed a correlation between EGFR expression and tumor invasiveness,Citation30 and poorer survival,Citation31–Citation33 while there was no relation seen in another study.Citation25 EGFR amplification in a subset of patients from the INTACT trialsCitation34,Citation35 appeared to correlate with a response to gefitinib (Iressa®; AstraZeneca, Wilmington, DE); but no effect on overall survival was seen.Citation36 In a meta-analysis of 2792 patients enrolled in 18 different studies, Nakamura and colleagues found that EGFR overexpression had no impact on survival in patients with NSCLC (hazard ratio [HR], 1.14; 95% confidence interval [CI]: 0.97–1.34; p = 0.1). Citation37 Other studies however found that not only is EGFR expression increased in NSCLC, but its presence is associated with poor prognosis.Citation30–Citation32,Citation38
Various mechanisms for upregulation of receptor activity in NSCLC have been discovered:
Increased production of EGFR with or without increased EGFR gene copy numberCitation39
EGFR mutations resulting in constitutive activation of the receptor, regardless of ligand bindingCitation39,Citation40
Increased production of ligands, TGF-α, or EGF or related proteins.Citation24
The most common clinically relevant assays for EGFR expression in NSCLC include assays for gene amplification,Citation41 immunohistochemical (IHC) stains for protein overexpression,Citation42 and detection of specific mutations by DNA sequencing.Citation43 EGFR copy number can be measured using fluorescence in situ hybridization (FISH),Citation44 while EGFR mutations can be detected using sequencing analyses of DNA.Citation45
Targeted agents against EGFR
Two classes of drugs inhibiting the EGFR pathway are currently in clinical usage: small molecules that inhibit the intracellular tyrosine kinase activity of the receptor, and monoclonal antibodies that target the extracellular domain in the ligand-binding region. Gefitinib (Iressa®; AstraZeneca) and Erlotinib (Tarceva®; OSI Pharmaceuticals, Inc. Melville, NY) are the two most studied drugs in the EGFR-tyrosine kinase inhibitor class. Canertinib (C11033), lapatinib (GW572016, Tykerb®; GlaxoSmithKline, Research Triangle Park, NC), PKI166, and EKB569 are examples of other drugs in this class currently in development. The most well known EGFR monoclonal antibody is cetuximab (Erbitux®; Bristol-Myers Squibb Company, Princeton, NJ). Panitumumab (AMG706, Vectibix®; Amgen Inc., Thousand Oaks, CA), matuzumab (EMD7000), and nimotuzumab (h-R3) are EGFR monoclonal antibodies currently being developed.
Clinical trials of tyrosine kinase inhibitors
Gefitinib
Gefitinib was the first EGFR-TKI tested in clinical trials. Two phase II trials (IDEAL-1 and IDEAL-2) showed that gefitinib produced a response rate of 9%–18% and overall disease control rate of 43%–50% in patients with relapsed NSCLC.Citation46,Citation47 However the phase III ISEL trial that randomized nearly 1700 patients with advanced NSCLC to gefitinib or placebo, failed to reveal an overall survival benefit.Citation48 A recent phase III trial (INTEREST) showed that gefitinib was noninferior to docetaxel in terms of overall survival (OS) in patients with relapsed NSCLC; it was also better tolerated and correlated with better quality of life.Citation49 Similarly, another randomized phase II study comparing gefitinib with vinorelbine in chemo-naïve elderly patients with advanced non-small-cell lung cancer found that gefitinib had similar response rates and progression-free (PFS) and overall survival to vinorelbine.Citation50
Two trials (INTACT-1 and INTACT-2) trials that combined gefitinib with chemotherapy in the first-line setting,Citation34,Citation35 did not show any survival benefit from the addition of gefitinib. On the other hand, although the IPASS (Iressa Pan-Asia Study) comparing gefitinib with carboplatin/paclitaxel for previously untreated Asian never- or light-smokers with advanced adenocarcinoma found no difference in overall survival,Citation51 patients who had EGFR gene mutations had a greater response rate (71.2% vs 41.3%) and improved overall survival (HR, 0.48; 95% CI: 0.36–0.64; p < 0.0001) with gefitinib.
Erlotinib
Unlike gefitinib, erlotinib has shown clinical efficacy that has resulted in its unrestricted US Food and Drug Administration (FDA) approval in NSCLC. BR21, a randomized phase III trial of 731 patients with stage IIIB or IV NSCLC showed that patients randomized to erlotinib had a statistically significant increase in overall survival, PFS, and overall response rate.Citation15 However, similar to the results seen with gefitinib, phase III trials evaluating the combination of a platinum-based doublet alone or with erlotinib, gemcitabine – cisplatin (TALENT)Citation52 and carboplatin – paclitaxel (TRIBUTE)Citation53 did not show any advantage to the addition of erlotinib.
Cetuximab
Introduction
Cetuximab is a human-mouse chimeric immunoglobulin G1 (IgG1) class monoclonal antibody directed against the EGFR with proven second- or third-line efficacy in colorectalCitation54 and head and neck cancers.Citation55,Citation56 This monoclonal antibody binds to the extracellular ligand-binding domain with affinity five times greater than natural ligands like TGF-α and EGF.Citation57 Binding of cetuximab prevents dimerization and subsequent activation by auto-phosphorylation of the receptor in the intracellular kinase domain.Citation58 The receptor-antibody complex is internalized and degraded, thereby decreasing EGFR availability.Citation59 In vitro studies show the antibody also mediates antibody-dependent cellular cytotoxicity (ADCC) against the receptor.Citation60
Preclinical studies with cetuximab suggested that there was inhibition of growth of human NSCLC cell lines and other EGFR-expressing cell lines in vitroCitation61–Citation63 and in athymic nude mice,Citation61,Citation63 and combinations with cytotoxic agents produced synergistic greater delay of tumor growth.Citation63,Citation64 In addition, cetuximab had a potentiating effect on the growth delay produced by radiotherapy.Citation65
Doody and colleagues studied the activity of cetuximab with NSCLC lines bearing both wild-type EGFR and those with activating mutations in the intracellular kinase domain: not only those known to confer sensitivity to gefitinib and erlotinib (L858R and delL747-T753insS) but also the TKI-resistant mutation T790M.Citation66 They found that ligand-independent phosphorylation of the T790M lines was unaffected by cetuximab (as measured by assays for phosphorylated EGFR, and downstream phosphorylated molecules Akt and MAPK) and cellular proliferation was inhibited. Previous studies had shown that binding of EGFR antibodies to wild-type EGFR resulted in increased internalization and degradation of the receptor-antibody complex without stimulating phosphorylation of the receptor.Citation67 Similarly, Doody and colleagues demonstrated that mutant EGFR (including T790M) was internalized and degraded at a higher rate than wild-type EGFR.Citation66
Clinical trials
Given the promising results of these preclinical experiments, cetuximab was introduced into clinical trials. While cetuximab was generally well tolerated, the common side effects seen in phase I testing included: skin toxicity, nausea, fever/chills, asthenia, and elevation of transaminases; grade 3 or 4 toxicities included dyspnea, aseptic meningitis, anaphylactoid reaction, diarrhea, and epiglottitis.Citation68
First-line therapy
A phase I/II trial combining cetuximab with paclitaxel/carboplatin produced 65% overall disease control rate (partial response [PR], 26%; stable disease, 39%).Citation69 Median PFS, OS, and one-year survival were 5 months, 11 months, and 40%, respectively. Similar results were seen when cetuximab was combined with gemcitabine and carboplatin (PR 28%; stable disease, 60%; median PFS, 5.3 months; median OS, 10.3 months; one-year survival, 45%).Citation70 A randomized phase II study comparing vinorelbine/cisplatin with the same chemotherapy plus cetuximabCitation71 suggested that addition of cetuximab led to better outcomes (odds ratio [OR], 35% vs 28%; median PFS, 5.0 vs 4.6 months; median OS, 8.3 vs 7.3). Similar results were seen in another randomized phase II study of gemcitabine plus cisplatin or carboplatin with or without cetuximab. Although the study design was non-comparative, addition of cetuximab seemed to improve outcomes in terms of PR (27.7% vs 18.2%), median PFS (5.1 vs 4.2 months), and median OS (11.99 vs 9.26 months).Citation72
The results of these trials led to the conduct of the phase III FLEX trial,Citation73 which randomized 1125 patients with EGFR overexpressing advanced NSCLC to cisplatin/vinorelbine with or without cetuximab. The basis for patient selection in this trial was presence of the EGFR staining by IHC, which the investigators defined as the presence of at least one EGFR-positive cell. This is the first study that has used IHC to determine eligibility for EGFR-targeted therapy.
Addition of cetuximab led to a significant improvement in overall survival (11.3 vs 10.1 months; HR, 0.871; P = 0.0441). One-year survival also was higher in the cetuximab group (47% vs 42%). Interestingly however there was no difference in PFS between the two groups. Though the benefit with the addition of cetuximab was modest, the results of this trial led to cetuximab being incorporated into the National Comprehensive Cancer Network (USA) guidelines for use in the first-line setting in combination with cisplatin and vinorelbine (see http://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf).
Preliminary results of pre-specified subgroup analyses suggest a greater benefit with the addition of cetuximab in Caucasians. Caucasian patients who received cetuximab had a median survival of 10.5 months as opposed to 9.1 months in those who did not receive cetuximab (HR, 0.8; P = 0.0025). However although Asians had a better prognosis in general, there was no additional benefit to the addition of cetuximab. Median survival in the cetuximab group among Asians was 17.6 months as compared to 20.4 months in the placebo group (HR, 1.179; P = 0.4992).
Second-line therapy
Single agent cetuximab seems to result in outcomes similar to docetaxel, pemetrexed or erlotinib in patients with recurrent or progressive NSCLC,Citation74 all of which are currently approved for the treatment of patients with NSCLC, who have failed first-line therapy. In this study by Hanna and colleagues, although the response rate was only 4.5% (3/66), 30.3% of patients had stable disease. Moreover, median time to progression and median OS were 2.3 and 8.9 months, respectively, and one-year survival was 43.9%, values numerically similar to those seen in studies with erlotinib, pemetrexed, and docetaxel. In the second-line setting, cetuximab was combined with docetaxelCitation75 and demonstrated a response rate of 25% among 20 patients with minimal toxicities.
Combination with chemoradiotherapy
Positive results for the radio-sensitizing effects of cetuximab in head and neck cancerCitation55 led investigators to test cetuximab in combination with synchronous radiotherapy following induction chemotherapy in chemotherapy-naïve stage III NSCLC patients.Citation76 The toxicity results published thus far suggest that this is a safe regimen. The Cancer and Leukemia Group B (CALGB) conducted a randomized phase II study of thoracic radiation (70 Gy) along with carboplatin and pemetrexed for four cycles (arm A) or the same chemotherapy regimen along with cetuximab for additional six weeks (arm B). Subsequently all patients received four additional cycles of pemetrexed as consolidation therapy. The main adverse effects were hematologic with grades 3/4 neutropenia seen in 36% and 37% patients in arms A and B respectively and grades 3/4 thrombocytopenia in 30% and 34% of patients. Esophagitis (35% and 22%), fatigue (22% and 18%) and skin rash (3% and 22%) were the most common nonhematologic toxicities. These initial results suggest acceptable tolerability. Efficacy results of this study are awaited.
Currently, multiple studies are currently underway evaluating the role of cetuximab in different patient populations with NSCLC (). The results of these studies should help us understand the exact role of cetuximab in the treatment of NSCLC.
Table 1 Current or planned trials of cetuximab in non-small cell lung cancer
Targeting EGFR inhibitors
One of the major issues in the use of EGFR-targeted therapy in NSCLC has been the identification of patients who would actually benefit from these agents. Identifying predictive markers for a response to the EGFR-targeted therapy has been quite challenging. Most of the available information focuses on response to EGFR TKIs rather than cetuximab, since these agents have been in use for a longer period of time.
Clinical factors that can help predict response to EGFR TKIs have been investigated. A subset analysis of the BR21 trial showed that women, nonsmokers, patients with adenocarcinoma and patients with Asian ethnicity had better outcomes with erlotinib.Citation15 A subset analysis of the IDEAL-1 trialCitation47 revealed that patients with adenocarcinoma had superior outcomes, despite the observation that high EGFR expression is more common in squamous cell carcinomas than in adenocarcinomas.Citation77 Mutation studies of the EGFR gene showed that somatic mutations in the tyrosine kinase domain of the EGFR gene predicted for response to gefitinib and were associated with an improved outcome.Citation36,Citation78–Citation80
Correlative studies from the ISEL trial showed that a high EGFR gene copy number was a predictor of clinical benefit from gefitinib.Citation81 Patients whose tumors expressed the EGFR protein and who were treated with gefitinib had a slightly greater survival advantage (HR, 0.77; 95% CI: 0.56–1.08; P = 0.126). Also, patients with EGFR mutations had higher response rates than patients without EGFR mutations (37.5% vs 2.6%). In another analysis of 204 patients treated with gefitinib, Hirsch and colleagues found that although increased EGFR and HER2 gene copy number, EGFR protein overexpression, EGFR mutations, and pAKT overexpression were all associated with significantly higher response rates only increased EGFR gene copy number and EGFR protein overexpression correlated with improved survival.Citation82 In similar studies on samples from patients treated with erlotinib, there was no association between the presence of EGFR mutations and responsiveness to erlotinib. However similar to the findings of Hirsch and colleagues, in this analysis, expression of EGFR and an increased number of copies of EGFR were associated with responsiveness to erlotinib but not with increased survival.Citation83
Three retrospective studies from Japan and Korea, where the somatic EGFR mutations are detected approximately thrice as common than in the United States and Europe have shown that response rates following treatment with gefitinib in patients with EGFR mutations ranged from 65%–83% as compared to 10%–15% for those without the mutations.Citation84–Citation86 This translated into an overall survival advantage for treatment with gefitinib in patients with the mutations.
Hirsch and colleagues analyzed tissue samples from patients enrolled onto a phase II trial of paclitaxel/carboplatin with either sequential (cetuximab weekly for one year following the completion of chemotherapy) or concurrent (weekly cetuximab during and for one year following chemotherapy) cetuximab therapy for EGFR status.Citation87 Progression free survival and disease control rate (DCR) were statistically significantly better in FISH (+) patients, and there was a nonsignificant trend toward higher objective response rate (ORR) as compared to FISH (−) patients in both arms. The authors concluded that EGFR FISH status is a predictive factor for selection of NSCLC patients for cetuximab plus chemotherapy. Critics point out many potential flaws in attempting to draw conclusions based on these results and urge caution in interpreting them.Citation88
Although EGFR-targeted therapies have been approved for use without molecular testing, immunohistochemistry to detect EGFR protein overexpression, fluorescence in situ hybridization to detect EGFR gene amplification, and mutational analyses of the EGFR gene have all been proposed as candidates to help predict benefit from EGFR-directed therapy in NSCLC. In fact the FLEX trial was the first to incorporate the presence of EGFR positivity as an inclusion criterion for the study.Citation73 However even in this trial, a rather loose definition of EGFR positivity was employed; patients who had even one cell staining for EGFR by IHC were considered EGFR positive.
As is evident from the disparity in the results from the studies discussed above, there is an urgent need for standardization of these assays. Without such standardization, routine utilization of these technologies to guide clinical decision making in a given patient will be difficult. In order to address this issue the Molecular Assays in NSCLC Working Group was convened under the sponsorship of Genentech Inc, Roche Pharmaceuticals, and OSI Pharmaceuticals, Inc, to evaluate the available molecular assays for use in the clinical trial setting and provide recommendations for application and interpretation of these tests.Citation89 The recommendations from this group included the following:
At least three representative areas should be assessed per tumor section.
The minimum cell number to be evaluated should be: FISH: > 100 assessable tumor cell nuclei, IHC/mutation: 2,000 cells, Direct sequencing −50%–70% tumor cells.
Standardization of molecular assays:
Kit-based antibodies preferred for immunohistochemistry, with a simple standard scoring system needed.
FISH: Two-color FISH with CEP® control, using the Colorado scoring system.Citation90
Mutational analysis: Direct sequencing; replicate PCR and sequencing reactions to establish mutation status of each sample amplicon.
The tumor biology of NSCLC prevents easy answers. Unlike CML that relies almost exclusively on the tyrosine kinase activated by the fusion Bcr-Abl,Citation91 or a closer analogy, the overexpression of HER-2 in 25%–30% of breast cancers,Citation92 carcinogenesis of NSCLC is not so heavily dependant on EGFR status (by any measure of EGFR). The absence of this “oncogene addiction”Citation93 in NSCLC illustrates why patients with NSCLC will continue to require multimodal therapy for the foreseeable future.
Future directions
It is likely that the lessons on how to use cetuximab wisely may come from experience in patients with colon cancer. RAS is one of the downstream signaling molecules stimulated by active EGFR. Mutations that cause constitutive activation of the K-RAS oncogene predict resistance to cetuximab in colorectal cancer (CRC) cell lines.Citation94 This study emerged in the wake of retrospective observations that patients who failed to respond to cetuximab were more likely to have mutated, constitutively activated K-RAS,Citation95,Citation96 although some studies observe good clinical response in patients with mutated K-RAS.Citation97 A recently published study noted a mutation in the extracellular domain (R521K of exon 13) of EGFR that predicted response to cetuximab.Citation97
However these observations must be made with the caveat in mind that tumor biology of colon cancer is different from NSCLC. Somatic mutations in EGFR are almost never seen in CRC cell lines.Citation98 Also cetuximab activity in colorectal cancer does not correlate with EGFR protein levels as measured by IHCCitation54,Citation99 but does correlate with gene copy number measured by FISH.Citation100 Despite this, multiple trials in NSCLC have shown that mutant K-RAS predicts resistance to erlotinib.Citation101,Citation102
Future investigations should stratify patients with respect to EGFR status and other potential predictive factors like K-RAS mutations. Future studies should address the predictive markers for cetuximab activity, be it gene amplification by FISH or protein overexpression by IHC or mutation analysis. Also studies should be conducted to determine if cetuximab is useful in patients who have already failed an EGFR-TKI.
Disclosure
The authors report no conflicts of interest in this work.
References
- ParkinDMGlobal cancer statistics in the year 2000Lancet Oncol20012953354311905707
- JemalASiegelRWardECancer statistics, 2008CA Cancer J Clin2008582719618287387
- YangPAllenMSAubryMCClinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003Chest2005128145246216002972
- GovindanRPageNMorgenszternDChanging epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results databaseJ Clin Oncol200624284539454417008692
- AveryEJKessingerAGantiAKTherapeutic options for elderly patients with advanced non-small cell lung cancerCancer Treat Rev2009Jan 18 [Epub ahead of print]
- SouquetPJChauvinFBoisselJPBernardJPMeta-analysis of randomised trials of systemic chemotherapy versus supportive treatment in non-resectable non-small cell lung cancerLung Cancer199512Suppl 1S147S1547551923
- SandlerABNemunaitisJDenhamCPhase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancerJ Clin Oncol200018112213010623702
- WozniakAJCrowleyJJBalcerzakSPRandomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer: a Southwest Oncology Group studyJ Clin Oncol1998167245924659667264
- GatzemeierUvon PawelJGottfriedMPhase III comparative study of high-dose cisplatin versus a combination of paclitaxel and cisplatin in patients with advanced non-small-cell lung cancerJ Clin Oncol200018193390339911013280
- AzimHAJrElattarILoberizaFRJrThird generation triplet cytotoxic chemotherapy in advanced non-small cell lung cancer: A systematic overviewLung Cancer200064219419818809226
- DelbaldoCMichielsSSyzNBenefits of adding a drug to a single-agent or a 2-agent chemotherapy regimen in advanced non-small-cell lung cancer: a meta-analysisJAMA2004292447048415280345
- SchillerJHHarringtonDBelaniCPComparison of four chemotherapy regimens for advanced non-small-cell lung cancerN Engl J Med20023462929811784875
- AzimHAJrGantiAKTargeted therapy in advanced non-small cell lung cancer (NSCLC): where do we stand?Cancer Treat Rev200632863063617034953
- SandlerAGrayRPerryMCPaclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancerN Engl J Med2006355242542255017167137
- ShepherdFARodrigues PereiraJCiuleanuTErlotinib in previously treated non-small-cell lung cancerN Engl J Med2005353212313216014882
- CarpenterGCohenSEpidermal growth factorJ Biol Chem199026514770977122186024
- WellsAEGF receptorInt J Biochem Cell Biol199931663764310404636
- KlapperLNKirschbaumMHSelaMYardenYBiochemical and clinical implications of the ErbB/HER signaling network of growth factor receptorsAdv Cancer Res200077257910549355
- HoneggerAMKrisRMUllrichASchlessingerJEvidence that autophosphorylation of solubilized receptors for epidermal growth factor is mediated by intermolecular cross-phosphorylationProc Natl Acad Sci U S A19898639259292915986
- JorissenRNWalkerFPouliotNEpidermal growth factor receptor: mechanisms of activation and signallingExp Cell Res20032841315312648464
- HuangSMHarariPMEpidermal growth factor receptor inhibition in cancer therapy: biology, rationale and preliminary clinical resultsInvest New Drugs199917325926910665478
- BaselgaJAlbanellJTargeting epidermal growth factor receptor in lung cancerCurr Oncol Rep20024431732412044241
- SobolREAstaritaRWHofeditzCEpidermal growth factor receptor expression in human lung carcinomas defined by a monoclonal antibodyJ Natl Cancer Inst19877934034073476783
- SalomonDSBrandtRCiardielloFNormannoNEpidermal growth factor-related peptides and their receptors in human malignanciesCrit Rev Oncol Hematol19951931832327612182
- RuschVKlimstraDVenkatramanEOverexpression of the epidermal growth factor receptor and its ligand transforming growth factor alpha is frequent in resectable non-small cell lung cancer but does not predict tumor progressionClin Cancer Res1997345155229815714
- RitterCAArteagaCLThe epidermal growth factor receptor-tyrosine kinase: a promising therapeutic target in solid tumorsSemin Oncol2003301 Suppl 131112644979
- FontaniniGDe LaurentiisMVignatiSEvaluation of epidermal growth factor-related growth factors and receptors and of neoangiogenesis in completely resected stage I-IIIA non-small-cell lung cancer: amphiregulin and microvessel count are independent prognostic indicators of survivalClin Cancer Res1998412412499516978
- BunnPAJrFranklinWEpidermal growth factor receptor expression, signal pathway, and inhibitors in non-small cell lung cancerSemin Oncol2002295 Suppl 14384412422312
- FujinoSEnokiboriTTezukaNA comparison of epidermal growth factor receptor levels and other prognostic parameters in non-small cell lung cancerEur J Cancer199632A12207020749014747
- PavelicKBanjacZPavelicJSpaventiSEvidence for a role of EGF receptor in the progression of human lung carcinomaAnticancer Res1993134113311378394672
- VealeDKerrNGibsonGJKellyPJHarrisALThe relationship of quantitative epidermal growth factor receptor expression in non-small cell lung cancer to long term survivalBr J Cancer19936811621658391303
- VolmMRittgenWDringsPPrognostic value of ERBB-1, VEGF, cyclin A, FOS, JUN and MYC in patients with squamous cell lung carcinomasBr J Cancer19987746636699484827
- BrabenderJDanenbergKDMetzgerREpidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer Is correlated with survivalClin Cancer Res2001771850185511448895
- HerbstRSGiacconeGSchillerJHGefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 2J Clin Oncol200422578579414990633
- GiacconeGHerbstRSManegoldCGefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 1J Clin Oncol200422577778414990632
- BellDWLynchTJHaserlatSMEpidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trialsJ Clin Oncol200523318081809216204011
- NakamuraHKawasakiNTaguchiMKabasawaKSurvival impact of epidermal growth factor receptor overexpression in patients with non-small cell lung cancer: a meta-analysisThorax200661214014516284218
- RuschVBaselgaJCordon-CardoCDifferential expression of the epidermal growth factor receptor and its ligands in primary non-small cell lung cancers and adjacent benign lungCancer Res19935310 Suppl237923857683573
- HirschFRVarella-GarciaMBunnPAJrEpidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosisJ Clin Oncol200321203798380712953099
- MoscatelloDKHolgado-MadrugaMGodwinAKFrequent expression of a mutant epidermal growth factor receptor in multiple human tumorsCancer Res19955523553655397585629
- HirschFRVarella-GarciaMBunnPAJrEpidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosisJ Clin Oncol200321203798380712953099
- SelvaggiGNovelloSTorriVEpidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancerAnn Oncol2004151283214679115
- PaoWMillerVZakowskiMEGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinibProc Natl Acad Sci U S A200410136133061331115329413
- CappuzzoFHirschFRRossiEEpidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancerJ Natl Cancer Inst200597964365515870435
- von EybenFEEpidermal growth factor receptor inhibition and non-small cell lung cancerCrit Rev Clin Lab Sci200643429132316769595
- FukuokaMYanoSGiacconeGMulti-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]J Clin Oncol200321122237224612748244
- KrisMGNataleRBHerbstRSEfficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trialJAMA2003290162149215814570950
- ThatcherNChangAParikhPGefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer)Lancet200536694961527153716257339
- DouillardJYKimEHirshVGefitinib (IRESSA) versus docetaxel in patients with locally advanced or metastatic non-small-cell lung cancer pre-treated with platinum-based chemotherapy: a randomized, open-label Phase III study (INTEREST)J Thoracic Oncol200728 Suppl 4S305S306
- CrinoLCappuzzoFZatloukalPGefitinib versus vinorelbine in chemotherapy-naive elderly patients with advanced non-small-cell lung cancer (INVITE): a randomized, phase II studyJ Clin Oncol200826264253426018779612
- MokTWuY-LThongprasertSPhase III, randomised, open-label, first-line study of gefitinib (G) vs carboplatin/paclitaxel (C/P) in clinically selected patients (pts) with advanced non-small-cell lung cancer (NSCLC) (IPASS)Ann Oncol200819Suppl 8viii1viii4
- GatzemeierUPluzanskaASzczesnaAPhase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation TrialJ Clin Oncol200725121545155217442998
- HerbstRSPragerDHermannRTRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancerJ Clin Oncol200523255892589916043829
- CunninghamDHumbletYSienaSCetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancerN Engl J Med2004351433734515269313
- BonnerJAHarariPMGiraltJRadiotherapy plus cetuximab for squamous-cell carcinoma of the head and neckN Engl J Med2006354656757816467544
- VermorkenJBTrigoJHittROpen-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapyJ Clin Oncol200725162171217717538161
- GoldsteinNIPrewettMZuklysKRockwellPMendelsohnJBiological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft modelClin Cancer Res1995111131113189815926
- LiSSchmitzKRJeffreyPDStructural basis for inhibition of the epidermal growth factor receptor by cetuximabCancer Cell20057430131115837620
- WaksalHWRole of an anti-epidermal growth factor receptor in treating cancerCancer Metastasis Rev199918442743610855786
- YardenYUllrichAGrowth factor receptor tyrosine kinasesAnnu Rev Biochem1988574434783052279
- MasuiHMoroyamaTMendelsohnJMechanism of antitumor activity in mice for anti-epidermal growth factor receptor monoclonal antibodies with different isotypesCancer Res19864611559255983756906
- MendelsohnJThe epidermal growth factor receptor as a target for cancer therapyEndocr Relat Cancer2001813911350723
- YangXDJiaXCCorvalanJREradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant chemotherapyCancer Res19995961236124310096554
- FanZBaselgaJMasuiHMendelsohnJAntitumor effect of antiepidermal growth factor receptor monoclonal antibodies plus cisdiamminedichloroplatinum on well established A431 cell xenograftsCancer Res19935319463746428402640
- MilasLMasonKHunterNIn vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibodyClin Cancer Res20006270170810690556
- DoodyJFWangYPatelSNInhibitory activity of cetuximab on epidermal growth factor receptor mutations in non small cell lung cancersMol Cancer Ther20076102642265117913857
- SunadaHMagunBEMendelsohnJMacLeodCLMonoclonal antibody against epidermal growth factor receptor is internalized without stimulating receptor phosphorylationProc Natl Acad Sci U S A19868311382538292424012
- BaselgaJPfisterDCooperMRPhase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatinJ Clin Oncol200018490491410673534
- ThieneltCDBunnPAJrHannaNMulticenter phase I/II study of cetuximab with paclitaxel and carboplatin in untreated patients with stage IV non-small-cell lung cancerJ Clin Oncol200523348786879316246975
- RobertFBlumenscheinGHerbstRSPhase I/IIa study of cetuximab with gemcitabine plus carboplatin in patients with chemotherapy-naive advanced non-small-cell lung cancerJ Clin Oncol200523369089909616301597
- RosellRRobinetGSzczesnaARandomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancerAnn Oncol200819236236917947225
- ButtsCABodkinDMiddlemanELRandomized phase II study of gemcitabine plus cisplatin or carboplatin [corrected], with or without cetuximab, as first-line therapy for patients with advanced or metastatic non small-cell lung cancerJ Clin Oncol200725365777578418089875
- PirkerRSzczesnaAvon PawelJFLEX: A randomized, multicenter, phase III study of cetuximab in combination with cisplatin/vinorelbine (CV) versus CV alone in the first-line treatment of patients with advanced non-small cell lung cancer (NSCLC)J Clin Oncol (Meeting Abstracts)20082615 suppl3
- HannaNLilenbaumRAnsariRPhase II trial of cetuximab in patients with previously treated non-small-cell lung cancerJ Clin Oncol200624335253525817114658
- KimESMauerAMFossellaFVA phase II study of Erbitux (IMC-C225), an epidermal growth factor receptor (EGFR) blocking antibody, in combination with docetaxel in chemotherapy refractory/resistant patients with advanced non-small cell lung cancer (NSCLC)Proc Am Soc Clin Oncol200221293a
- HughesSLiongJMiahAA brief report on the safety study of induction chemotherapy followed by synchronous radiotherapy and cetuximab in stage III non-small cell lung cancer (NSCLC): SCRATCH studyJ Thorac Oncol20083664865118520806
- FranklinWAVeveRHirschFRHelfrichBABunnPAJrEpidermal growth factor receptor family in lung cancer and premalignancySemin Oncol2002291 Suppl 431411894009
- LynchTJBellDWSordellaRActivating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinibN Engl J Med2004350212129213915118073
- KuboANakagawaKKashiiTCorrelative study of EGFR mutations or protein expressions of EGFR, phosphorylated EGFR, HER2, phosphorylated HER2 and IGFR-1 with gefitinib sensitivity in patients with non-small cell lung cancer: Results of West Japan Thoracic Oncology Group trial (WJTOG0203A)J Thoracic Oncol200728 Suppl 4S323
- ToyookaSTakanoTKosakaTThe impact of EGFR mutation and smoking status on non-smallcell lung cancer patients treated with geftinibJ Thorac Oncol200728 Suppl 4S324
- HirschFRVarella-GarciaMBunnPAJrMolecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancerJ Clin Oncol200624315034504217075123
- HirschFRVarella-GarciaMCappuzzoFCombination of EGFR gene copy number and protein expression predicts outcome for advanced non-small-cell lung cancer patients treated with gefitinibAnn Oncol200718475276017317677
- TsaoMSSakuradaACutzJCErlotinib in lung cancer – molecular and clinical predictors of outcomeN Engl J Med2005353213314416014883
- HanSWKimTYHwangPGPredictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinibJ Clin Oncol200523112493250115710947
- MitsudomiTKosakaTEndohHMutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrenceJ Clin Oncol200523112513252015738541
- TakanoTOheYSakamotoHEpidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancerJ Clin Oncol200523286829683715998907
- HirschFRHerbstRSOlsenCIncreased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapyJ Clin Oncol200826203351335718612151
- TakanoTOtaSHoriASekiNEguchiKCan epidermal growth factor receptor-fluorescent in situ hybridization predict clinical benefit from cetuximab treatment in patients with non-small-cell lung cancer?J Clin Oncol2009273464465 author reply 465–46719064956
- EberhardDAGiacconeGJohnsonBEBiomarkers of response to epidermal growth factor receptor inhibitors in Non-Small-Cell Lung Cancer Working Group: standardization for use in the clinical trial settingJ Clin Oncol200826698399418281673
- Varella-GarciaMStratification of non-small cell lung cancer patients for therapy with epidermal growth factor receptor inhibitors: the EGFR fluorescence in situ hybridization assayDiagn Pathol200611916911776
- GoldmanJMMeloJVChronic myeloid leukemia – advances in biology and new approaches to treatmentN Engl J Med2003349151451146414534339
- SlamonDJGodolphinWJonesLAStudies of the HER-2/neu proto-oncogene in human breast and ovarian cancerScience198924449057077122470152
- WeinsteinIBCancer. Addiction to oncogenes – the Achilles heal of cancerScience20022975578636412098689
- BenvenutiSSartore-BianchiADi NicolantonioFOncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapiesCancer Res20076762643264817363584
- LievreABachetJBLe CorreDKRAS mutation status is predictive of response to cetuximab therapy in colorectal cancerCancer Res20066683992399516618717
- Di FioreFBlanchardFCharbonnierFClinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapyBr J Cancer20079681166116917375050
- GoncalvesAEsteyriesSTaylor-SmedraBA polymorphism of EGFR extracellular domain is associated with progression free-survival in metastatic colorectal cancer patients receiving cetuximab-based treatmentBMC Cancer2008816918544172
- BarberTDVogelsteinBKinzlerKWVelculescuVESomatic mutations of EGFR in colorectal cancers and glioblastomasN Engl J Med200435127288315625347
- ChungKYShiaJKemenyNECetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistryJ Clin Oncol20052391803181015677699
- MoroniMVeroneseSBenvenutiSGene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort studyLancet Oncol20056527928615863375
- EberhardDAJohnsonBEAmlerLCMutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinibJ Clin Oncol200523255900590916043828
- ZhuCQda Cunha SantosGDingKRole of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21J Clin Oncol200826264268427518626007