Abstract
This review evaluates methods, success and limitations of transgenes delivery in central nervous system (CNS). Both viral and nonviral (such as liposome mediated) methods, expression and stability of transgenes have been discussed. The controlled expression and delivery techniques of transgene at the injured or diseased sites have also been discussed. Mifepristone (RU486) and tetracycline-based switch system for controlled expression could be a very useful tool for clinical purposes. Here we emphasized the importance and consequences of viral- and nonviral-mediated transgenes transfer and therapeutic ability along with advantages of controlled expressions.
Introduction
Damaged neuronal cells in the central nervous system (CNS) could not be repaired/regenerated, leading to a partial disability or complete paralysis due to disruption of communication between brain and body. However, new findings and developments in the gene therapy techniques related to CNS have improved the prospects for recovery to some extent. While research in this field is still in the early stage, this work could lead to the clinical applications that can help to restore lost functions in the wake of brain and spinal cord injury. Development of new viral and nonviral vectors with cell type specific, physiologically-relevant and long term transgene expression at specific site is under progress. Nonviral vector such as cationicCitation1,Citation2 and anionic liposomeCitation3 shows no-immune response or toxicity to host. Genetically engineered cells and direct DNA transferCitation4 have also shown potential in certain experimental paradigms. This review provides an update and recent advancement in the gene therapy techniques related to CNS diseases and injuries.
Potentials of therapeutic genes in CNS injury and disorders
Many therapeutics genes, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), B-cell lymphoma-2 (BCL-2), heat shock protein (HSP), etc, could be used to prevent and cure CNS related injuries or disorders ().
Table 1 Therapeutic applications of various genes in central nervous system diseases and their current status
NGF gene for therapeutic applications
Nerve growth factor (NGF) is the prototypical neurotrophic factor having ability to protect peripheral sensory and sympathetic neurons from programmed cell death (apoptosis).Citation5 Studies done so far have shown that direct CNS administration of recombinant growth factors including NGF can rescue damaged neurons and promote regeneration.Citation2,Citation6 In addition, NGF is able to protect adult sensory and sympathetic neurons against a variety of insults that include axotomy, ionophore treatment, exposure to hydrogen peroxide and excitatory amino acids.Citation7,Citation8 Localized diffusion of gene products into targeted region of CNS parenchyma could secrete proteins only within CNS regions relevant to neuropathological states, thus limiting the peripheral side effects.Citation9 The intracerebroventricular injection of NGF gene increases mNGF (messenger RNA of NGF) levels in the hippocampus that causes increased cholinergic neurotransmitter synthetic enzyme choline acetyltransferase (ChAT) activity within the brain.Citation9 Chronic intraventricular injection of rhNGF (recombinant human Nerve growth factor) via cannulae for 21 days increased synaptosomal high affinity choline uptake, choline acetyltransferase activity, and [3H] acetylcholine synthesis by 50%–90% compared to lesion control values.Citation10 Cholinergic neurotransmission deficits after traumatic brain injury (TBI) might be the result of presynaptic alterations in the storage and release of acetylcholine (Ach) or due to conformational changes in the receptors for Ach.Citation11
BDNF gene for therapeutic applications
Brain-derived neurotrophic factor (BDNF) is well documented for their therapeutic role in the development and survival of injured CNS. BDNF is known to stimulate axon outgrowth, branching, proliferation, differentiation, and can work as neurotransmitter.Citation12 BDNF have also been implicated in synaptic plasticity,Citation13 long-term memory,Citation14 and expression of NMDA receptors.Citation15 Loss of NMDA receptors is a cause of memory impairmentCitation16,Citation17 after injury.Citation18,Citation19 BDNF is found in soluble form and induces differentiation and survival of neurons by binding to its receptor known as trkB. TrkB receptors are present in three isoforms, namely full length isoform TK+, and two truncated isoforms, TKT1 and TKT2, in the cellular membrane of mammalian CNS. In fact, trkB is a part of tyrosin kinase receptor groupCitation20 capable of adding a phosphate group at tyrosin/serine/theronine residues on target proteins after interaction with BDNF. While trkB kinase is activated by BDNF, NT-3, and NT-4, but the other subtypes trkA and trkC are activated by NGF and NT-3, respectively. The cationic lipid-mediated BDNF gene transfection in primary hippocampal cell cultures enhances recovery of neurofilament loss produced by CNS injury.Citation1 BDNF gene transfection could increases phosphoinositide 3-kinases (PI3-kinases) activity in CNS cells. PI-3 kinases are family of related enzymes capable of phosphorylating the 3-position hydroxyl group of the inositol ring of phosphatidylinositol. PI-3 kinases play important role in a variety of cellular responses such as mitogenesis, membrane trafficking and preventor of apoptosis.Citation21 Moreover, BDNF has been shown to induce anti-apoptotic mechanisms after stroke that reduces infarct size and secondary neuronal cell death. BDNF is also a potent stimulator of adult neurogenesis.Citation22 Apart from that BDNF is able to protect the brain from inflammatory brain injury in bacterial meningitis.Citation23 Increasing the level of BDNF is an effective way to decrease mortality and to improve sequela upon bacterial meningitis.
BCL-2 gene for therapeutic applications
BCL-2 gene is known for the synthesis of anti-apoptotic protein. The name BCL-2 has been derived from B-cell lymphoma 2, and its anti-apoptotic group includes BCL-2 proper, BCL-xl, and BCL-w. Herpes simplex virus (HSV)-mediated delivery of BCL-2 gene into hippocampus and striatum in vivo can attenuate the damaging effects of ischemic brain.Citation24 Over-expression of BCL-2 gene prevents the release of apoptosis-inducing factor.Citation25 The post-ischemic injection of adeno-associated virus (AAV)-containing BCL-2 gene has a neuroprotective effect that inhibits ischemic neuronal cell death.Citation26 BCL-2 gene may also delay disease progression in chronic degenerative disorder such as Parkinson’s disease.Citation27 6-Hydroxydopamine (6-OHDA) is a neurotoxin to dopaminergic neurons. BCL-2 produced by the vector prevented 6-OHDA-induced degeneration of neurons and increased the surviving capabilities of TH (tyrosine hydroxylase) immunoreactive neurons in the Substantia Niagra two weeks after the lesioning.Citation28 Administration and expression of BCL-2 gene in adult rat CNS neurons prevent retrograde cell death and minimizes atrophy.Citation29 In vivo neuroprotection of injured CNS neurons occurs by an injection of a DNA plasmid encoding the BCL-2 gene.Citation30 Overexpression of BCL-2 gene in primary cultured neurons protects an insult in cAMP receptor dependent manner, whereas protection is not seen against severe traumatic insults.Citation31 These informations will provide a new insight into the molecular therapeutics for neurodegenerative conditions in future.
HSP72 genes for therapeutic applications
Heat shock protein 72 (HSP72) protein is expressed into the brain after stroke and seizures and is able to remove denatured proteins from a cell to assist new protein synthesis. Gene transfer therapy with defective HSV-vector over expressing HSP72 improves neuronal survival against focal cerebral ischemia and systemic kainic acid administration.Citation32 Overexpression of HSP72 gene into rat brain can improve striatal and hippocampal dentate gyrus neuron survival after systemic kainic acid administration. The transgenic mice over-expresing HSP72 could attenuated hippocampal injury after focal cerebral ischemia.Citation33
Interleukin-1 receptor antagonist genes for therapeutic applications
Interleukin-1 (IL-1) acts as a cofactor and is responsible for inflammatory reaction after transient ischemia and local brain injury. Central and systemic administration of an IL-1 receptor antagonist (IL-1ra) reduces ischemic brain injury in short-term. IL-1ra is usually produced by the normal brain cells that produce IL-1. The adenovirus vector mediated over expression of human IL-1ra gene can attenuate ischemic inflammatory response in the mouse brain and inflammation based neuronal diseases.Citation34 The mechanism involves binding of IL-1ra to the receptor of IL-1 preventing inflammatory reaction in ischemic cortex, striatum and corpus callosum regions. It is still unknown whether IL-1 is responsible for neuronal cell death directly or exacerbates other forms of damage or both.
Ex vivo transfer mediated therapeutic applications for CNS injury and disorders
Ex vivo gene transfer is a potential means of treating chronic neurological disorders and injury related neural degeneration. In this approach cells are modified genetically in vitro and then transplanted to the injured site of CNS. Injured cell replacement therapy is not suitable due to the blood–brain barrier (BBB). To circumvent the BBB, ex vivo gene therapy is most acceptable and is able to traverse the BBB or other membranes of the CNS. Fibroblast, peripheral nerves, astrocytes, and myoblasts cells could be used for the ex vivo gene therapy in the CNS.
Fibroblast cells modified with NGF genes have been transplanted into the brain and spinal cord to provide neurotrophic factors and substrates for axonal growth and elongation. NGF secreting fibroblast cells transplant have been shown to prevent degeneration of cholinergic neurons in the basal forebrain of primates. Transplant induces sprouting of sensory, motor, and noradrenergic neurites after spinal cord injury. The controlled and targeted expression of tetracycline-regulated ex vivo delivery of NGF is possible at transplanted sites.Citation34 Genetically transduced Schwann cells grafted to spinal cord injury sites increase axonal growth by the over expression of NGF.Citation35,Citation36 When fibroblasts cells, genetically modified to secrete NGF, BDNF, NT-3, and basic fibroblast growth factor (bFGF), transplanted into the central gray matter of the spinal cord in the adult rats, sensory neurites of dorsal root origin extensively penetrated NGF-, NT-3-, and bFGF-secreting grafts, whereas no growth has been found in BDNF-secreting grafts.Citation37
Injured CNS tissues and damaged neurons are unable to regenerate their axons spontaneously. Genetically modified peripheral nerves can be implanted ex vivo, in transected sciatic nerve, avulsed ventral root, hemisected spinal cord, and intact brain to overexpress the transgene encoding growth promoting NT-3 proteins that improves the permissive properties of the nerves.Citation15 The rat fibroblasts, genetically modified to produce NT-3, grafted to acute spinal cord dorsal hemisection lesion cavities showed significant partial functional recovery in corticospinal axon growth at distal to the injury site.Citation38
Astrocytes originated from CNS have efficient secretory mechanisms to play an important role in neuronal growth. Human adult astrocytes modified with specific transgene could be used for ex vivo gene therapy. Ex vivo cell transplantation decreases the chances of immunological rejection at minimum level and thus obviating the side effects of immunosuppressors.Citation39
Myoblast cellsCitation40 and astrocytesCitation41 could be genetically modified to express tyrosine hydroxylase (TH) and dopamine in culture. These modified myoblasts, not showing immuno-rejection property, might be used as gene carriers for ex vivo gene therapy in the CNS. Thus, ex vivo gene therapy in the CNS could be an efficient and convenient tool for the future.Citation42 Deficiency of beta-glucuronidase (GUSB) causes multisystem progressive degenerative syndrome, mucopolysaccharidosis (MPS) type VII (Sly disease), in adult brain that could be cured by transplanting engineered GUSB-secreting cells to super-secrete the normal enzyme for export to surrounding neural tissues.Citation43
Controlled expression of therapeutic genes in CNS
There is a need to control the transgene expression to prevent adverse effects of overexpression. The concept of molecular switches is based on the use of tissue-specific promoters, which confers restricted expression of transgene appropriately within the tissue. Appropriate regulation means the capability of the system to turn the transgene on and off in response to symptoms (expression) of the targeted disease. Many gene switch systems are available to control transgene expression but in CNS, mifepristone (RU486), and tetracycline (tet)-based switch systems are important.
Transgene regulation by inducible promoter (mifepristone)
Transgene expression can be controlled by using a specific promoter whose activity can be controlled by mifepristone,Citation44 a progesterone hormone antagonist. Mifepristone is a 19-nonsteroid which has a specific high affinity binding to the progesterone and glucocorticoid receptor. Mifepristone-responsive gene switch system has become most attractive for an application in traumatized CNS. The synthetic progesterone antagonist readily crosses the BBB when administered systemically.Citation45 In this system the transgene to be regulated is placed under transcriptional control of a promoter, which in turn is activated by a specific transactivator, consisting of a fused tripartite protein. The tripartite proteins are Gal4 (Yeast DNA-binding domain), HBD (mutated progesterone receptor that binds specifically to mifepristone), and VB 16 (activation factor derived from HSV). The vector used for gene therapy encodes the fusion protein, and either a cytomegalovirus (CMV) or tissue-specific promoter, which drives its expression after delivery. In its native state, the transactivator does not induce transgene transcription but binding of mifepristone to the transactivator enable the administered transactivator to immediately initiate the transgene expression. This switch system has been used to regulate genes systemically when transferred with either plasmid DNACitation46 or adenoviral vectors,Citation47 or in conjunction with HSV vector-mediated gene transfer.Citation48 With inclusion of a mifepristone-responsive gene switch into gene delivery vector, transgene expression could be regulated according to therapeutic need.
Transgene regulation by tetracycline antibiotic-based gene switch
Tetracycline-based switch system is based on the use of inducible elements and factors along with transgene, regulated by the administration of a second-step drug or by the end product. Based on the above principle, highly controlled gene expression of recombinant Ad and AAV vectors using combinations of a tissue-specific promoter and a tetracycline transcription factor have been constructed.Citation49 Thus, it is possible to transfer a putative therapeutic gene to specific tissue in a completely dormant state. Expression of the dormant gene can be induced by the oral administration of a second-step drug (rapamycin or mifepristone) that directs the formation of an active transcription factor complex on the silent promoter of the transferred gene. Despite the above information, no literature is available to show a switch based promoter in AAV vector for the transfer of therapeutic gene in brain injury. In contrast, a switch based promoter with stable expression of a constitutive AAV-erythropoietin vector in non-CNS tissues has been successfully demonstrated. Moreover, the regulation of tet-promoter is simple because gene induction or repression is being controlled by only one protein. Also, this switch system can be packaged into a single vector due to smaller size of tet-transactivator and tet-regulatable promoter. The minimal CMV promoter is fused to the tet-operator sequence to stimulate transcription of tet-transactivator in the absence of tetracycline. Tet-regulatable gene expression system can release NGF-GFP (green fluorescent protein) in a controlled manner from primary rat fibroblasts in a dose-dependent manner by the exposure to the tet analog doxycyline.Citation50
Recent developments in CNS gene delivery vectors/carriers
Many techniques (such as viral and nonviral vectors, chemical carriers, and physical forces) could be used for targeted delivery of transgenes at the diseased or injured CNS site ().
Figure 1 Vehicles for gene delivery in central nervous system to regenerate the damaged cells.
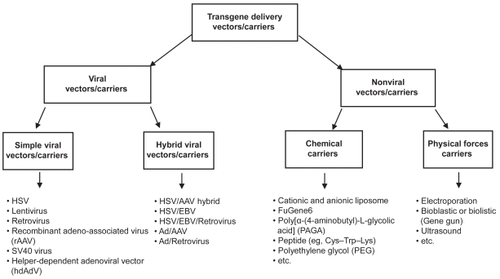
Viral vectors for CNS therapeutic gene delivery
Viral vectors have become important tool for the gene delivery at particular site in the brain. Different strategies are used to deliver genes to CNS and enhance its distribution. One way is to inject the viral vectors directly into the cerebral lateral ventricles (LVs). By this way, virus will be delivered throughout the CNS.Citation51 Another way is to inject at multiple sites to cover a large volume. Some agents such as MannitolCitation52 and heparinCitation53 are used to increase the distribution of vectors. Some important viral vectors have been discussed below.
Simple viral vectors
Herpes simplex virus (HSV) vectors
HSV is a neurotrophic virus having ability to establish a life-long latent state in neurons. It is an enveloped (envelope contains at least 10 glycoproteins) double-stranded DNA encoding more than 80 identified genes bearing 152 kb viral genome. HSVs infect neurons by rapid retrograde axonal transport mechanism, thus providing a means of gene transfer to targeted cells that are not easy to reach directly.Citation54 Two types of HSV-vectors were constructed for gene transfer: recombinant virus (RV) and amplicon vectors. Recombinant replication-conditional viruses contain one or more mutated genes (such as r34.5 or ICP6) in the full genome to reduce overall toxic effects. Replication defective RV vectors or multiplication-defective genomic HSV vectors were constructed by deleting all immediate-early (IE) genes such as ICP0,Citation55 ICP4, ICP22, ICP27, and ICP47Citation56,Citation57 that encode transactivating factors. Such deletion eliminated expression of other viral genesCitation56,Citation58 that may be toxic to cells. These multiple gene-deleted vectors have many advantages: (a) can propagate to high titers in the complementing cell lines, and (b) long-term expression of latency associated transcripts (LATs) in genome does not depend on IE-genes. This provided a chance to construct a highly defective HSV-vector that can readily persist in a latent state in neurons, and transgenes could be expressed using the LAT promoter. Basically HSV amplicon based vectors are plasmid-based DNA constructs. A typical HSV amplicon construct contains, in addition to the gene of interest, a copy of HSV replication origin (oris) and packaging signal (pac). When these vectors were introduced into cells together with a helper HSV, the amplicon plasmid DNA efficiently amplified and packaged into viral particles. Helper virus free amplicon stock could be generated by co-transfecting amplicon set of cosmids or BAC plasmids containing fragmented or modified HSV genome.Citation59
The HSV amplicon mediated gene transfer has many advantages over recombinant HSV vectors. These advantages include nontoxic and nonimmunogenic responses to the target cells, since amplicon based vectors do not encode any viral gene products. Amplicon is a multiple gene delivery system because each amplicon contains 10–15 copies of the inserted gene due to its concatemeric form. This gives much higher expression of transgene in comparison to recombinant virus containing similar type of gene cassette.Citation60 Moreover, it is easy to insert gene of choice into amplicon vectors that has long-term transgene expression in different areas of CNS.Citation61 In order to improve the proportion of amplicons generated, a selection system for amplicon production is developed in which the HSV thymidine kinase (TK) gene is inserted into an amplicon plasmid and an HSV mutant with both TK and glycoprotein H (gH) genes deleted is used as a helper.Citation60 HSV virus has been designed in which the prokaryotic Cre–loxP site-specific recombination system is employed. In this system, gH−helper virus is engineered in such a way that loxP sites flank both copies of its packaging signals and thus generated stocks with high amplicon titer and much improved amplicon over helper virus ratio. The injection and expression of HSV vectors containing β-NGF gene under the transcriptional control of either human cytomegalovirus immediate early promoter (HCMV Iep) element or HSP-latency active promoter (HSV-LAP2) produced biologically active NGF in transfected PC-12 cells. HSV virus-mediated NGF synthesis induces expression of superoxide dismutase and catalase, and is effective in protecting cells from apoptosis induced by hydrogen peroxide.Citation62 Replication defective genomic HSV vector mediated transfer of β-NGF, under the control of either the LAP2 or HCMV Iep promoter, into the knee joint of animals has been effective for treatment of peripheral neuropathesis.Citation57 Amplicon HSV has a lot of advantages but it still has some limitations because it is usually difficult to generate a stock with a high amplicon titer and high ratio of amplicon to helper virus.
Lentivirus vectors
Lentivirus vectors are derived either from the HIV-1 (human immunodeficiency virus type-1) vector or FIV (feline immunodeficiency virus) vector after genetic manipulationCitation63,Citation64 and able to carry 8 kb of sequence to any neuronal cell type with sustained expression in which normal cellular functions are not compromised either in vitro or in vivo.Citation65 HIV-1-derived vectors have ability to integrate into the host genome of dividing and nondividing cells, and hence can be utilized for the transfer of genes with stable expression even in post-mitotic neurons. Lentiviral vector-encoded beta-galactosidase transgene showed very efficient transfer, integration, and sustained long-term expression without showing any pathology in adult rat brains. In vivo gene transfer using lentiviral vector depends on a functional integrase protein.Citation66 A recent report suggests that lentiviral vectors surpass retroviral vectors in efficient long-term and stable gene transfer in adult neural stem cells.Citation67 On the other hand, the HIV-1 has a broad host range and can infect brain, liver, and muscle cells. The targeted transduction of transgene in the CNS was achieved using specific envelope glycoproteins to pseudotype lentivrial vectors. The use of Ebola-pseudotyped virus, Mokola-pseudotyped, and murine leukemia virus (MuLV)-pseudotyped lentiviral vectors are more efficient and stable alternatives to vesicular stomatitis virus glycoprotein (VSV-G)-pseudotyped vector gene for the transduction of transgene in mouse CNS.Citation68 Despite of these developments the clinical application of both the HIV-1 and FIV vectors for CNS has yet to be confirmed experimentally.
Retrovirus vectors
The higher and unequal efficiency of retroviral vectors to integrate their genome into host cell chromosomal DNA has made it the first choice for many gene therapy applications. In many clinical trials so far no single case has been reported which attributes adverse events of insertional mutagenesis, caused by retrovirus vector application. The vectors have 8.5 kb of transgenes flanked by retroviral long terminal repeat (LTR) regions, a virion packaging signal, and a primer binding site for reverse transcription. After delivery into cells, double-stranded DNA sequences can be reversely transcribed which can then get integrated randomly into host cell genome. This vector has limited use for gene delivery to CNS because of their ability to transfer genes only to dividing cells, yet have been well suited for on-site delivery to neural precursors for lineage studies.Citation69
Recombinant adeno-associated viral (rAAV) vectors
There are 11 AAV serotypes have been reported so far.Citation70–Citation72 They infect cells from several diverse tissue types. Capsid serotype is the main determinant of tissue specificity and pseudotyping of AAV vectors to alter their tropism range for their use in gene therapy. Different serotypes can bind to different cellular receptors. Among these, serotype 2, 4, 5 (AAV2, 4, 5) have been studied most extensivelyCitation73,Citation74 and are found efficient for transduction in the mammalian brain.Citation73 rAAV vector is the vector of choice for gene delivery to neurons due to several advantages: (a) easy to manipulate genetically, (b) ability to transduce most tissues including terminally differentiated cells, (c) purification to high titers, and are (d) relatively safe. The replication defective recombinant adenoviruses are commonly used as gene transfer vectors because of their less immunogenicity and ability to transduce both neurons and glial cells efficiently.Citation75 rAAV-mediated in vivo gene transfer has demonstrated efficient long-term transduction (from three months to more than 1.5 years), lack of cytotoxicity and cellular immune response in target tissues, especially in the CNS.Citation76
The contamination risk could be eliminated in rAAV production by (i) substituting adenovirus with a plasmid-bearing E2a, E4orf6, and VA helper function, and (ii) growing on HEK 293 cells which express Ad E1a and E1b.Citation77,Citation78 There are two types of toxicity due to (a) aggregation of rAAV with cell lysate proteins, and (b) residual hyperosmotic CsCl2 during purification step.Citation79 Both types of toxicity could be removed by substitution of density centrifugation with iso-osmotic and inert medium iodixanol. Affinity chromatography is being used for high level of purification of rAAV.Citation80,Citation81 The heparin sulfate proteoglycan (a cellular receptor for attachment and infectivity of AAV-2Citation81) and virion-specific monoclonal antibodiesCitation82 could be utilized as core facility for the purification and production of rAAV using ligand specific matrix chromatography. Moreover, rAAVs have been developed having capability to express human protooncogene BCL-236 that confers an ability to block neuronal death after transient ischemia. rAAVs have capability to package 6.6 kb vector sequence that could be used for gene therapy for hemophilia A and other diseases with large cDNA such as muscular dystrophy and cystic fibrosis.Citation83
Aromatic l-amino acid decarboxylase (AADC) converts l-dopa to dopamine. The AADC gene encoded in rAAV vectors has been used for therapy purposes to treat Parkinson’s disease.Citation84 Prevention of dopaminergic neuron death by AAV vector-mediated glial cell derived neurotrophic factor (GDNF) gene transfer in rat mesencephalic cells has also been reported.Citation85 The behavioral recovery in 5-hydroxydopamine-lesioned rats by co-transduction of striatum with tyrosine hydroxylase and AADC genes using two separate AAV vectors is possible.Citation85 Although, rAAV has shown its potential in gene therapy, further improvements are needed to consider for clinical uses.
Simian virus 40 (SV40) vectors
SV40 virus is a member of the nonenveloped particle of polyoma family of viruses with double stranded circular DNA genome of 5.25 kb. SV40-derived vectors can express both in vitro or in vivo with long term transgene expression either into caudate-putamen or lateral ventricle after injection.Citation86 For transgene expression in CNS, rAAV utility is measured in comparison to SV40. SV40 can package genomes up to 5.7 kb without difficulty after deleting structural genes. rSV40s (recombinant SV40) do not elicit detectable neutralizing antibody responses.Citation87
Helper-dependent adenoviral vectors
Third generation adenovirus vectors (AdV), called gutless or helper dependent adenoviral vector (hdAdV), have been developed that retain only the sequences necessary for packaging and replication of viral genome and lack all structural genesCitation75 thus extending the cloning capacity of the vector (up to 37 kb). This novel construct has capacity to propagate to high titers without contaminating helper Ad virus using a Cre-lox-based recombinase system. Gene transfer by hdAdV demonstrated persistent gene expression with negligible toxicity in peripheral organs such as liver.Citation88,Citation89 hdAdV has been used to transduce genes to CNS for stable gene transfer that significantly prolonged transgene expression (up to 183 days).Citation89 The number of macrophages and T lymphocytes infiltrating the brain could be greatly reduced in hdAdV-treated host in comparison to first generation adenovirus (fgAd)-treated host.Citation6 The hdAdV provides equally efficient or higher infectivity but significantly reduced toxicity than fgAd vectors.Citation90 Low toxicity is extremely important for the clinical applications of hdAdV as a future tool for gene delivery to CNS. Studies delivering α–antitrypsin and leptin gene using hdAdVs in vitro or in vivo have shown that these fully deleted Ad vectors can provide high-level, long-term gene expression with improved tolerance due to absence of the viral genome.Citation88,Citation89 However, hdAdV infection causes moderate but significant changes in cell function and viability at excessive viral titers in primary neuronal cultures.Citation91
The expected improvements for clinical use of hdAdVs are: (1) to remove all helper viruses from the hdAdV preparation to avoid even trace amount of contamination, (2) development of targeted hdAdVs to localize gene transfer to specific cell types, (3) production of high-titer vector without the cost of contamination by wild-type AAV, and (4) use of molecular switches for controlled expression of transgene by the vectors. Moreover, it is possible that some of improvements can be achieved through the construction of hybrid viral vectors utilizing two different viruses.
Hybrid viral vectors
These vectors are also known as chimeric or hybrid viral vectors and constructed or developed to achieve reproducible and stable gene delivery to the CNS or other parts of the body. Such kind of hybrid vectors has been constructed to incorporate different viral elements with particular features to stabilize the transgenes at transfected site. Various types of vectors have been constructed by utilizing the combination of two or more viral elements or gene sources. Some important hybrid viral vectors have been discussed below.
HSV/AAV hybrid amplicon vectors
The hybrid amplicon vectors contain oris and pac signals of HSV-1 and ITR sequences of AAV to flank the transgene. It was produced either with or without the AAV rep gene to evaluate its importance in producing sustained transgene expression in human glioma cells.Citation92 The hybrid amplicon vector extended transgene expression in dividing human glioma cells well beyond the capacity of HSV amplicons. Higher transduction efficiency in primary neuronal cultures and longer expression of the transgene in neurons were also noted in hybrid amplicon-mediated gene transfer in comparison to AAV and AdV.Citation93,Citation94
HSV/EBV and HSV/EBV/retrovirus hybrid amplicon vectors
Amplicon elements of HSV, latent origin of DNA replication (ori-P), and Epstein–Barr nuclear antigen-1 gene (EBNA-1) elements of Epstein–Barr virus (EBV) are used to construct HSV/EBV hybrid vector. Inclusion of ori-P and EBNA-1 increased the stability of transgene during replication in the dividing cells.Citation95 A hybrid vector having the character of HSV and retrovirus has also been constructed.Citation96,Citation97 This hybrid HSV/retrovirus vector confers ability of retrovirus vector to transduce into both dividing and nondividing cells in a single step of infection. Since this new construct has ability to transduce into nondividing cells it can be efficiently utilized for the transfer of gene in CNS.
Ad/AAV hybrid vectors
This hybrid vector encodes the AAV Rep78 protein and an ITR-flanked transgene.Citation98 Another type of Ad/AAV vector consist of an AAV ITR-flanked transgene in which the AAV Rep isoforms are conjugated to the Ad-virion via poly-lysine bridge to site specific delivery of the transgene.
Ad/retrovirus hybrid vectors
This chimeric vector is constructed to increase the transfection capability and to successful expression of transgene in the neighboring cells. When any cell is transfected by the hybrid (Ad/retrovirus) vector, transfected cells produce two types of functional progeny: a retroviral packaging functions and retroviral vector/transgene sequences. The progeny after release can infect neighboring cells leading to the incorporation of transgene.Citation91
Nonviral vectors for CNS therapeutic gene delivery
Problems associated with viral vector gene delivery systems (eg, unwanted deleterious immune response or changes in the properties of delivered virus due to endogenous recombination and mutagenic behavior leading to oncogenesis) lead to the development of nonviral vector delivery systems. This contains use of chemical carriers and naked gene delivery using electro-poration, gene gun (bio-ballistic or biolistic), ultrasound and hydrodynamics (high pressure).
Chemical carriers mediated CNS gene delivery
Chemical carriers are designed to protect the delivered DNA from nuclease activity. Nonviral vector such as cationicCitation1 and anionic liposomeCitation3 with no immune response or toxicity have been reported. Although, the cationic liposome mediated gene transfer to different cell types is successful, this method is limited in use due to its lower transfection efficiency in compared to viral systems. A novel compound, FuGene6,Citation99 has also been tested to transfer gene of choice using reporter plasmid pEF-beta galactosidase. This compound has less toxicity in comparison to the Lipofectamine, as shown by Trypane blue staining.
The novel compound FuGene6, a commercially-available cationic lipid, has a very high potential to transfer DNA into cells of glial origin, and might be an interesting candidate for ex vivo and in vivo gene therapeutic approaches.Citation99 The FuGene6-mediated gene transfer is useful to transfer the reporter gene β-galactosidase into C6 glioma cells, primary glia, and primary neurons.Citation100 The cationic liposome DNA complexes (CLDCs) produces significant levels of expression of both reporter genes and biologically relevant genes in non-parenchymal cells lining CNS.Citation9 The intracerebroventricular or intrathecal injection of either CLDCs containing the β-galactosidase (β-Gal) gene produced patchy and widely scattered areas of β-Gal expression. The chloramphenicol acetyl transferase (CAT) reporter gene product is present at significant levels after single intracerebroventricular injection. To improve efficiency, the fusion proteins derived from the Sendai virus is incorporated into cationic liposomes to avoid degradation by endocytosis.Citation101 Nonhistone chromatin proteins, a high mobility group protein, have been incorporated into the liposome for the transfer of transgene at specific site.Citation102 Many cationic polypeptides eg, polylysine, spermidine, etc having capacity to bind the negatively charged DNA are used to target the gene transfer to the cell linked with specific ligands. Although liposome mediated gene transfer into the brain has nontoxic and nonimmunogenic effect, it has low efficiency of transgene expression in compared to the viral mediated transgene expression. The ori-P and EBNA-1 gene elements from the EBV have been used in the liposome-associated DNA to prolong the stability of the transgene in the dividing cells.Citation103 Biodegradable polymer, poly[α-(4-aminobutyl)-l-glycolic acid] (PAGA) a derivative of poly-l-lysine is under trial for delivery of transgene. Other than PAGA, PEG (polyethylene glycol) and peptide mediated (eg, Cys–Trp–Lys) are also under trial.
Physical forces-mediated CNS transgenes delivery
Successful transfer of naked DNA into the adult mouse brain has been reported. Microprocessor-controlled injector, an important tool for nonviral gene transfer technique, has successfully been used to deliver the gene into the CNS.Citation40 Reports are also available to show the use of electroporation technique to deliver naked transgene in the skin at particular site.Citation104,Citation105 Few reports show the success of in vivo electroporation technique in the nervous system of embryonic miceCitation106 that can be used to repair the injured or diseased CNS. In mice brain organotypic slice cultures, both biolistics and electroporation techniques provide better transfer rate and transgene expression than lipotransfection techniqueCitation107 providing the use of nonviral techniques for therapeutic purposes in clinical studies.
Nanotechnology could also be used for the targeted gene delivery.Citation108 Research is in progress to use this technology to transfer the gene or drug at particular site which is otherwise impossible in clinical studies. Studies are in progress to use ultrasound as a physical force to introduce transgenes in CNS.Citation109
Conclusion
Genes with good expression ability of therapeutic molecules have significant potential in CNS injury. Many viral vectors are available to deliver therapeutic genes at target site. However, CNS gene delivery remains a challenge. A smart viral delivery vehicle with optimal gene titer could solve the problems associated with CNS therapeutic gene delivery. In vivo studies suggest that recombinant virus along with lentivirus is a better and more promising vehicle for long-term efficient transgene expression in CNS tissues. Other option perhaps is a nonviral, targeted transgenes delivery with CNS compatible biomaterial. This emerges as a viable option for CNS gene delivery and therapeutic expression for use in many neuronal diseases. In the place of in vivo gene delivery, ex vivo gene delivery techniques looks very exciting for the repair of lost tissues after TBI or necrosis in CNS.
Acknowledgments
Authors acknowledge financial support from the Canadian Institute of Health Research (CIHR) grant to Prakash and University Grants Commission (UGC), India to AK, TDS, and SKS. We would also like to thank technical support from Jasmine Bhathena.
References
- YangKCliftonGLHayesRLGene therapy for central nervous system injury: the use of cationic liposomes: an invited reviewJ Neurotrauma19971452812979199395
- ZouLLHuangLHayesRLLiposome-mediated NGF gene transfection following neuronal injury: potential therapeutic applicationsGene Ther199966994100510455401
- HagiharaYSaitohYKanedaYKohmuraEYoshimineTWidespread gene transfection into the central nervous system of primatesGene Ther20007975976310822302
- BrooksAIHaltermanMWChadwickCAReproducible and efficient murine CNS gene delivery using a microprocessor-controlled injectorJ Neurosci Methods19988021371479667386
- SatohTEnokidoYKuboTYamadaMHatanakaHOxygen toxicity induces apoptosis in neuronal cellsCell Mol Neurobiol19981866496669876872
- ZouLKumarAYuanXHannayJShineDYangKImprovement of cognitive function and cholinergic neuron transmission by adenovirus-mediated nerve growth factor gene transfer in aged rat brainMol Ther20013S125S126
- HoltzmanDMSheldonRAJaffeWChengYFerrieroDMNerve growth factor protects the neonatal brain against hypoxic-ischemic injuryAnn Neurol19963911141228572656
- MattsonMPScheffSWEndogenous neuroprotection factors and traumatic brain injury: mechanisms of action and implications for therapyJ Neurotrauma19941113338201625
- Meuli-SimmenCLiuYYeoTTGene expression along the cerebral-spinal axis after regional gene deliveryHum Gene Ther199910162689270010566897
- LapchakPAHeftiFBDNF and NGF treatment in lesioned rats: effects on cholinergic function and weight gainNeuroreport1992354054081633277
- ShaoLCiallellaJRYanHQDifferential effects of traumatic brain injury on vesicular acetylcholine transporter and M2 muscarinic receptor mRNA and protein in ratJ Neurotrauma199916755556610447068
- GuillinODemilyCThibautFBrain-derived neurotrophic factor in schizophrenia and its relation with dopamineInt Rev Neurobiol20077837739517349867
- SinghTDMizunoKKohnoTNakamuraSBDNF and trkB mRNA expression in neurons of the neonatal mouse barrel field cortex: normal development and plasticity after cauterizing facial vibrissaeNeurochem Res19972277917979232630
- MonfilsMHCowansageKKLeDouxJEBrain-derived neurotrophic factor: linking fear learning to memory consolidationMol Pharmacol200772223523717522182
- CaldeiraMVMeloCVPereiraDBCarvalhoRFCarvalhoALDuarteCBBDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neuronsMol Cell Neurosci200735220821917428676
- SinghTDBashamMENordeenEJNordeenKWEarly sensory and hormonal experience modulate age-related changes in NR2B mRNA within a forebrain region controlling avian vocal learningJ Neurobiol2000441829410880134
- SinghTDNordeenEJNordeenKWSong tutoring triggers CaMKII phosphorylation within a specialized portion of the avian basal gangliaJ Neurobiol200565217919116114029
- GizaCCMariaNSHovdaDAN-methyl-D-aspartate receptor subunit changes after traumatic injury to the developing brainJ Neurotrauma200623695096116774479
- KumarAZouLLYuanXQLongYYangKN-methyl-D-aspartate receptors: Transient loss of NR1/NR2A/NR2B subunits after traumatic brain injury in a rodent modelJ Neurosci Res200267678178611891792
- QiuYHZhaoXHayesRLActivation of phosphatidylinositol 3-kinase by brain-derived neurotrophic factor gene transfection in septo-hippocampal culturesJ Neurosci Res19985221922009579409
- KapellerRCantleyLCPhosphatidylinositol 3-kinaseBioessays19941685655768086005
- SchabitzWRSteiglederTCooper-KuhnCMIntravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesisStroke20073872165217217510456
- LiLShuiQXLiangKRenHBrain-derived neurotrophic factor rescues neurons from bacterial meningitisPediatr Neurol200736532432917509465
- XuDGCrockerSJDoucetJPElevation of neuronal expression of NAIP reduces ischemic damage in the rat hippocampusNat Med19973999710049288726
- SusinSALorenzoHKZamzamiNMitochondrial release of caspase-2 and -9 during the apoptotic processJ Exp Med199918923813949892620
- ShimazakiKUrabeMMonahanJOzawaKKawaiNAdeno-associated virus vector-mediated bcl-2 gene transfer into post-ischemic gerbil brain in vivo: prospects for gene therapy of ischemia-induced neuronal deathGene Ther20007141244124910918494
- DunnettSBBjorklundAProspects for new restorative and neuroprotective treatments in Parkinson’s diseaseNature19993996738A32A3910392578
- YamadaMOliginoTMataMGossJRGloriosoJCFinkDJHerpes simplex virus vector-mediated expression of Bcl-2 prevents 6-hydroxydopamine-induced degeneration of neurons in the substantia nigra in vivoProc Natl Acad Sci U S A19999674078408310097166
- TakahashiKSchwarzELjubeticCMurrayMTesslerASaavedraRADNA plasmid that codes for human Bcl-2 gene preserves axotomized Clarke’s nucleus neurons and reduces atrophy after spinal cord hemisection in adult ratsJ Comp Neurol199940421591719934991
- SaavedraRAMurrayMdeLSTesslerAIn vivo neuroprotection of injured CNS neurons by a single injection of a DNA plasmid encoding the Bcl-2 geneProg Brain Res200012836537211105694
- CheungNSBeartPMPascoeCJJohnCABernardOHuman Bcl-2 protects against AMPA receptor-mediated apoptosisJ Neurochem20007441613162010737619
- YenariMAFinkSLSunGHGene therapy with HSP72 is neuroprotective in rat models of stroke and epilepsyAnn Neurol19984445845919778256
- PlumierJCKruegerAMCurrieRWKontoyiannisDKolliasGPagoulatosGNTransgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injuryCell Stress Chaperones1997231621679314603
- YangGYZhaoYJDavidsonBLBetzALOverexpression of interleukin-1 receptor antagonist in the mouse brain reduces ischemic brain injuryBrain Res199775121811889099804
- TuszynskiMHWeidnerNMcCormackMMillerIPowellHConnerJGrafts of genetically modified Schwann cells to the spinal cord: survival, axon growth, and myelinationCell Transplant1998721871969588600
- WeidnerNBleschAGrillRJTuszynskiMHNerve growth factor-hypersecreting Schwann cell grafts augment and guide spinal cord axonal growth and remyelinate central nervous system axons in a phenotypically appropriate manner that correlates with expression of L1J Comp Neurol1999413449550610495438
- NakaharaYGageFHTuszynskiMHGrafts of fibroblasts genetically modified to secrete NGF, BDNF, NT-3, or basic FGF elicit differential responses in the adult spinal cordCell Transplantation1996521912048689031
- GrillRMuraiKBleschAGageFHTuszynskiMHCellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injuryJ Neurosci19971714556055729204937
- RidetJLCortiOPencaletPToward autologous ex vivo gene therapy for the central nervous system with human adult astrocytesHum Gene Ther199910227128010022551
- CaoLZhaoYCJiangZHLong-term phenotypic correction of rodent hemiparkinsonism by gene therapy using genetically modified myoblastsGene Ther20007544544910694827
- SegoviaJVergaraPBrennerMAstrocyte-specific expression of tyrosine hydroxylase after intracerebral gene transfer induces behavioral recovery in experimental parkinsonismGene Ther19985121650165510023444
- LiuYHimesBTTryonBIntraspinal grafting of fibroblasts genetically modified by recombinant adenovirusesNeuroreport199896107510799601670
- TaylorRMWolfeJHDecreased lysosomal storage in the adult MPS VII mouse brain in the vicinity of grafts of retroviral vector-corrected fibroblasts secreting high levels of beta-glucuronidaseNat Med1997377717749212105
- WangYLOmalleyBWTsaiSYOmalleyBWA regulatory system for use in gene transferProc Natl Acad Sci U S A19949117818081848058776
- HeikinheimoOHaukkamaaMLahteenmakiPDistribution of RU 486 and its demethylated metabolites in humansJ Clin Endocrinol Metab19896822702752918046
- AbruzzeseRVGodinDBurcinMLigand-dependent regulation of plasmid-based transgene expression in vivoHum Gene Ther19991091499150710395375
- BurcinMMSchiednerGKochanekSTsaiSYO’MalleyBWAdenovirus-mediated regulable target gene expression in vivoProc Natl Acad Sci U S A19999623553609892637
- OliginoTGhivizzaniSWolfeDIntra-articular delivery of a herpes simplex virus IL-1Ra gene vector reduces inflammation in a rabbit model of arthritisGene Ther19996101713172010516720
- GhersaPGobertRPSattonnet-RochePRichardsCAPichEMvan HuijsduijnenRHHighly controlled gene expression using combinations of a tissue-specific promoter recombinant adenovirus and a tetracycline-regulatable transcription factorGene Ther199859121312209930322
- BleschAUyHSDiergardtNTuszynskiMHNeurite outgrowth can be modulated in vitro using a tetracycline-repressible gene therapy vector expressing human nerve growth factorJ Neurosci Res200059340240910679776
- PassiniMAWolfeJHWidespread gene delivery and structure-specific patterns of expression in the brain after intraventricular injections of neonatal mice with an adeno-associated virus vectorJ Virol20017524123821239211711628
- BurgerCNguyenFNDengJMandelRJSystemic mannitol-induced hyperosmolality amplifies rAAV2-mediated striatal transduction to a greater extent than local co-infusionMol Ther200511232733115668145
- NguyenJBSanchez-PernauteRCunninghamJBankiewiczKSConvection-enhanced delivery of AAV-2 combined with heparin increases TK gene transfer in the rat brainNeuroreport20011291961196411435930
- BurtonEAFinkDJGloriosoJCReplication-defective genomic HSV gene therapy vectors: Design, production and CNS applicationsCurr Opin Mol Ther20057432633616121698
- SamaniegoLANeiderhiserLDeLucaNAPersistence and expression of the herpes simplex virus genome in the absence of immediate-early proteinsJ Virol1998724330733209525658
- KriskyDMMarconiPCOliginoTJDevelopment of herpes simplex virus replication-defective multigene vectors for combination gene therapy applicationsGene Ther1998511151715309930305
- WolfeDGoinsWFYamadaMEngineering herpes simplex virus vectors for CNS applicationsExp Neurol19991591344610486173
- WuPPhillipsMIBuiJTerwilligerEFAdeno-associated virus vector-mediated transgene integration into neurons and other nondividing cell targetsJ Virol1998727591959269621054
- StavropoulosTAStrathdeeCAAn enhanced packaging system for helper-dependent herpes simplex virus vectorsJ Virol1998729713771439696807
- ZhangXO’SheaHEntwisleCBoursnellMEfstathiouSInglisSAn efficient selection system for packaging herpes simplex virus ampliconsJ Gen Virol199879Pt 11251319460933
- WangYYuLGellerAIDiverse stabilities of expression in the rat brain from different cellular promoters in a helper virus-free herpes simplex virus type 1 vector systemHum Gene Ther199910111763177110446916
- GoinsWFLeeKACavalcoliJDHerpes simplex virus type 1 vector-mediated expression of nerve growth factor protects dorsal root ganglion neurons from peroxide toxicityJ Virol19997315195329847358
- JohnstonJCGasmiMLimLEMinimum requirements for efficient transduction of dividing and nondividing cells by feline immunodeficiency virus vectorsJ Virol19997364991500010233961
- KimVNMitrophanousKKingsmanSMKingsmanAJMinimal requirement for a lentivirus vector based on human immunodeficiency virus type 1J Virol19987218118169420292
- WongLFGoodheadLPratCMitrophanousKAKingsmanSMMazarakisNDLentivirus-mediated gene transfer to the central nervous system: therapeutic and research applicationsHum Gene Ther20061711916409120
- NaldiniLBlomerUGageFHTronoDVermaIMEfficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vectorProc Natl Acad Sci U S A1996932111382113888876144
- GeraertsMEggermontKHernandez-AcostaPGarcia-VerdugoJMBaekelandtVDebyserZLentiviral vectors mediate efficient and stable gene transfer in adult neural stem cells in vivoHum Gene Ther200617663565016776572
- WatsonDJKobingerGPPassiniMAWilsonJMWolfeJHTargeted transduction patterns in the mouse brain by lentivirus vectors pseudo-typed with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteinsMol Ther200255 Pt 152853711991743
- CepkoCLRyderEAustinCGoldenJFields-BerrySLinJLineage analysis using retroviral vectorsMethods19981443934069608510
- DavidsonBLSteinCSHethJARecombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous systemProc Natl Acad Sci U S A20009773428343210688913
- BurgerCGorbatyukOSVelardoMJRecombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous systemMol Ther200410230231715294177
- MoriSWangLTakeuchiTKandaTTwo novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid proteinVirology2004330237538315567432
- BartlettJSSamulskiRJMcCownTJSelective and rapid uptake of adeno-associated virus type 2 in brainHum Gene Ther199898118111869625257
- FuHMuenzerJSamulskiRJSelf-complementary adeno-associated virus serotype 2 vector: global distribution and broad dispersion of AAV-mediated transgene expression in mouse brainMol Ther20038691191714664793
- HermensWTJMGigerRJHoltmaatAJGDDijkhuizenPAHouwelingDAVerhaagenJTransient gene transfer to neurons and glia: Analysis of adenoviral vector performance in the CNS and PNSJ Neurosci Methods199771185989125378
- XiaoXLiJMcCownTJSamulskiRJGene transfer by adeno-associated virus vectors into the central nervous systemExp Neurol199714411131249126160
- XiaoXLiJSamulskiRJProduction of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirusJ Virol1998723222422329499080
- GrimmDKernARittnerKKleinschmidtJANovel tools for production and purification of recombinant adenoassociated virus vectorsHum Gene Ther1998918274527609874273
- ZolotukhinSByrneBJMasonERecombinant adeno-associated virus purification using novel methods improves infectious titer and yieldGene Ther19996697398510455399
- ClarkKRLiuXLMcGrathJPJohnsonPRHighly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type virusesHum Gene Ther19991061031103910223736
- SummerfordCBartlettJSSamulskiRJAlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infectionNat Med19995178829883843
- GrimmDKernAPawlitaMFerrariFKSamulskiRJKleinschmidtJATitration of AAV-2 particles via a novel capsid ELISA: packaging of genomes can limit production of recombinant AAV-2Gene Ther1999671322133010455443
- LuHChenLWangJComplete correction of hemophilia A with adeno-associated viral vectors containing a full-size expression cassetteHum Gene Ther200819664865418500941
- MuramatsuS[Gene therapy for Parkinson’s disease]Brain Nerve200759442543017447529
- FanDSOgawaMFujimotoKBehavioral recovery in 6-hydroxydopamine-lesioned rats by cotransduction of striatum with tyrosine hydroxylase and aromatic L-amino acid decarboxylase genes using two separate adeno-associated virus vectorsHum Gene Ther1998917252725359853519
- LouboutinJPReyesBASAgrawalLVan BockstaeleEStrayerDSStrategies for CNS-directed gene delivery: in vivo gene transfer to the brain using SV40-derived vectorsGene Ther2007141293994917443215
- StrayerDSAgrawalLCordelierPLong-term gene expression in dividing and nondividing cells using SV40-derived vectorsMol Biotechnol200634225727017172671
- MorralNParksRJZhouHHigh doses of a helper-dependent adenoviral vector yield supraphysiological levels of alpha1-antitrypsin with negligible toxicityHum Gene Ther1998918270927169874269
- MorsyMAGuMMotzelSAn adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgeneProc Natl Acad Sci U S A19989514786678719653106
- CreganSPMacLaurinJGendronTFHelper-dependent adeno-virus vectors: their use as a gene delivery system to neuronsGene Ther20007141200120910918488
- FengMZJacksonWHGoldmanCKStable in vivo gene transduction via a novel adenoviral/retroviral chimeric vectorNature Biotechnol19971598668709306401
- JohnstonKMJacobyDPechanPAHSV/AAV hybrid amplicon vectors extend transgene expression in human glioma cellsHum Gene Ther1997833593709048203
- CostantiniLCJacobyDRWangSFraefelCBreakefieldXOIsacsonOGene transfer to the nigrostriatal system by hybrid herpes simplex virus/adeno-associated virus amplicon vectorsHum Gene Ther199910152481249410543613
- FraefelCJacobyDRLageCGene transfer into hepatocytes mediated by helper virus-free HSV/AAV hybrid vectorsMol Med19973128138259440115
- Sena-EstevesMSaekiYCampSMChioccaEABreakefieldXOSingle-step conversion of cells to retrovirus vector producers with herpes simplex virus-Epstein-Barr virus hybrid ampliconsJ Virol19997312104261043910559361
- TanBTWuLBerkAJAn adenovirus-Epstein-Barr virus hybrid vector that stably transforms cultured cells with high efficiencyJ Virol19997397582758910438848
- OehmigAFraefelCBreakefieldXOAckermannMHerpes simplex virus type 1 amplicons and their hybrid virus partners, EBV, AAV, and retrovirusCurr Gene Ther20044438540815578989
- RecchiaAParksRJLamartinaSSite-specific integration mediated by a hybrid adenovirus/adeno-associated virus vectorProc Natl Acad Sci U S A19999662615262010077559
- WiesenhoferBKaufmannWAHumpelCImproved lipid-mediated gene transfer in C6 glioma cells and primary glial cells using FuGeneJ Neurosci Methods1999921–214515210595712
- WiesenhoferBHumpelCLipid-mediated gene transfer into primary neurons using FuGene: comparison to C6 glioma cells and primary gliaExp Neurol20001641384410877913
- SaekiYMatsumotoNNakanoYMoriMAwaiKKanedaYDevelopment and characterization of cationic liposomes conjugated with HVJ (Sendai virus): reciprocal effect of cationic lipid for in vitro and in vivo gene transferHum Gene Ther1997817213321419414261
- NamikiYTakahashiTOhnoTGene transduction for disseminated intraperitoneal tumor using cationic liposomes containing non-histone chromatin proteins: cationic liposomal gene therapy of carcinomatosaGene Ther1998522402469578844
- KanedaYSaekiYMorishitaRGene therapy using HVJ-liposomes: the best of both worlds?Mol Med Today19995729830310377521
- DrabickJJGlasspool-MaloneJSomiariSKingAMaloneRWCutaneous transfection and immune responses to intradermal nucleic acid vaccination are significantly enhanced by in vivo electroperme-abilizationMol Ther20013224925511237682
- MaruyamaHMiyazakiJGejyoFEpidermis-targeted gene transfer using in vivo electroporationMethods Mol Biol200528943143615502204
- SwartzMEberhartJMastickGSKrullCESparking new frontiers: using in vivo electroporation for genetic manipulationsDev Biol20012331132111319854
- MurphyRCMesserAGene transfer methods for CNS organotypic cultures: a comparison of three nonviral methodsMol Ther20013111312111162318
- JainKKNanobiotechnology – Based drug delivery to the central nervous systemNeurodegener Dis20074428729117627131
- HynynenKUltrasound for drug and gene delivery to the brainAdv Drug Deliv Rev200860101209121718486271
- BishopKMHoferEKMehtaATherapeutic potential of CERE-110 (AAV2-NGF): targeted, stable, and sustained NGF delivery and trophic activity on rodent basal forebrain cholinergic neuronsExp Neurol2008211257458418439998
- BennettGDWlodarczykBCalvinJACraigJCFinnellRHValproic acid-induced alterations in growth and neurotrophic factor gene expression in murine embryosReprod Toxicol200014111110689198
- CernaianuGBrandmaierPScholzGAll-trans retinoic acid arrests neuroblastoma cells in a dormant state. Subsequent nerve growth factor/brain-derived neurotrophic factor treatment adds modest benefitJ Pediatr Surg20084371284129418639684
- KordowerJHIsacsonOEmerichDFCellular delivery of trophic factors for the treatment of Huntington’s disease: Is neuroprotection possible?Exper Neurol1999159142010486171
- PodlesniyPKichevAPedrazaCPro-NGF from Alzheimer’s disease and normal human brain displays distinctive abilities to induce processing and nuclear translocation of intracellular domain of p75NTR and apoptosisAm J Pathol2006169111913116816366
- SalehiADelcroixJDSwaabDFAlzheimer’s disease and NGF signalingJ Neural Transm2004111332334514991458
- XiromerisiouGHadjigeorgiouGMEerolaJBDNF tagging polymorphisms and haplotype analysis in sporadic Parkinson’s disease in diverse ethnic groupsNeurosci Lett20074151596317229524
- KanthasamyAGKitazawMKaulSProteolytic activation of proapoptotic kinase PKC delta is regulated by overexpression of bcl-2 implications for oxidative stress and environmental factors in Parkinson’s diseaseAnn N Y Acad Sci2003101068368615033812
- VolobouevaLADuanMOuyangYEmeryJFStoyCGiffardRGOverexpression of mitochondrial Hsp70/Hsp75 protects astrocytes against ischemic injury in vitroJ Cereb Blood Flow Metab20082851009101618091755
- AndreRLerouetDKimberIPinteauxERothwellNJRegulation of expression of the novel IL-1 receptor family members in the mouse brainJ Neurochem200595232433016086690