Abstract
Angiogenesis is essential for cancer growth and metastasis. Vascular endothelial growth factor (VEGF) is a key modulator of angiogenesis. In addition, overexpression of VEGF is correlated with advanced disease and poor prognosis. Bevacizumab, a recombinant humanized anti-VEGF monoclonal antibody, is the first anti-angiogenic agent approved by Food and Drug Administration for use in treatment of human solid cancers. Although bevacizumab has received most attention for first-line treatment of advanced colorectal and nonsmall-cell lung cancer, there is a rapidly growing body of evidence for its efficacy in treatment of a number of other solid tumors. We present the current status and potential use of bevacizumab therapy in gastrointestinal cancers.
Development of bevacizumab
Angiogenesis has been known to be a critical process in tumorigenesis and metastases.Citation1 Vascular endothelial growth factor (VEGF), a pro-angiogenic factor, binds to two receptos VEGFR-1 (Flt-1) and VEGFR-2 (Flk-1/KDR), activates receptor tyrosine kinase (RTK) activity and induces angiogenesis.Citation1 VEGF and its receptors are often overexpressed in tumors.Citation2–Citation5 Therefore, targeting angiogenesis was proposed by Folkman et al as a therapeutic strategy in the 1970s. Citation6–Citation10 Approaches to block the VEGF signaling pathway fall into two categories: anti-VEGF mAB (eg, bevacizumab) and RTK inhibitors (RTKi, eg, sunitinib and sorafenib).Citation11–Citation13
Bevacizumab (Avastin®; Genentech/Roche) is a recombinant humanized mAB VEGF (VEGF-A for human variant), which theoretically blocks all isoforms of VEGFs to bind to VEGF receptors.Citation14 Bevacizumab was approved by the Food and Drug Administration (FDA) in 2004 for use in metastatic colorectal cancer (mCRC) when used with fluorouracil-containing regimens.Citation15 Its approval was subsequently extended to nonsquamous nonsmall cell lung cancer and breast cancer in 2006 and 2008.Citation16,Citation17
Clinical studies of bevacizumab are underway in early stage colon cancer, unresectable hepatocellular carcinoma (HCC), pancreatic cancer, neuroendocrine tumors, nonmetastatic breast cancer, renal cell carcinoma, glioblastoma multiforme, ovarian cancer, and castrate resistant prostate cancer.Citation18,Citation19 This review article will focus on the current use and potential role of bevacizumab in gastrointestinal malignancies.
Colorectal cancer (CRC)
Colorectal cancer, the third-leading cause of cancer-related death in the United States, kills approximately 50,000 patients per year.Citation20 The mortality rate has slightly decreased in recent decades. In addition to public awareness and early detection, effective adjuvant therapies are significant players in improving the outcome.
Bevacizumab in first-line therapy for advanced CRC ()
Four cytotoxic chemotherapy regimens are generally accpeted as first line therapies for mCRC: fluouracil and leucovorin (5-FU/LV), 5-FU/LV/oxaliplatin (FOLFOX), 5-FU/LV/irinotecan (FOLFIRI), and capecitabine/oxaliplatin (CapeOX, also named XelOX).Citation21–Citation25
Table 1 Summary of the phase II trial investigating two doses of bevacizumab plus 5-FU/LV
The first phase II trial investigating the safety and efficacy of bevacizumab in the first-line treatment of mCRC was conducted in 2003.Citation26 One hundred and four patients with untreated mCRC randomized to receive one of the three regimens: 5-FU/LV (control arm), 5-FU/LV plus low-dose bevacizumab (5 mg/kg) and 5-FU/LV plus high-dose bevacizumab (10 mg/kg). The addition of bevacizumab to 5-FU/LV increased the response rate (RR), prolonged median time to disease progression (TTP), and median overall survival (OS) (). Surprisingly, the higher dose did not correlate with higher efficacy. Bevacizumab-related toxicities were thrombosis, hypertension, proteinuria, and epistaxis.
Pooled results from several phase II studies have subsequently demonstrated that adding bevacizumab to 5-FU/LV regimens improved OS in untreated mCRC from 14.6 to 17.9 months.Citation26–Citation29
The encouraging phase II result led to randomized large phase III trials with the goal of incorporating bevacizumab into first-line therapy for mCRC. ‘Low-dose’ bevacizumab (5 mg/kg) was used in combination with irinotecan, 5-FU, and LV (IFL) in the pivotal phase III trial.Citation30 Eight hundred and thirteen patients with untreated mCRC received either IFL plus placebo, or IFL plus bevacizumab. This trial includes approximately 20% of all patients with diagnosis of rectal cancer. IFL plus bevacizumab combination was proved to be superior to IFL plus placebo not only in RR (44.8% vs 34.8%, P = 0.004) but also in OS with an absolute benefit of 4.7 months (20.3 vs 15.6 months, P < 0.001), as well as progression-free survival (PFS) with an absolute benefit of 4.4 months (10.6 vs 6.2 months, P < 0.001). Safety data in this trial revealed manageable hypertension related to bevacizumab, but no thromboembolic events or proteinuria.
This was the first time that a large trial confirmed the role of bevacizumab in prolonging OS in mCRC in a clinically meaningful way. This trial led to FDA approval of bevacizumab in first-line setting in combination with fluorouracil-based chemotherapy.Citation15
The efficacy of bevacizumab in irinotecan-containing regimens was also tested in phase III trial. Fuchs and colleagues initially designed a phase III trial (BICC-C) to compare the efficacy of 3-irinotecan containing regimens in the first-line treatment of mCRC: irinotecan plus infusional (FOLFIRI), bolus (mIFL) or oral fluoropyrimidine (CapeIRI).Citation31 430 patients were enrolled into this trial. After FDA approved bevacizumab in 2004, this trial was amended to add 117 patients to receive either FOLFIRI plus bevacizumab (n =57) or mIFL plus bevacizumab (n = 60), no further enrollment was made to CapeIRI arm due to toxicity concerns.Citation31,Citation32 After a median follow-up of 34.4months, the data demonstrated superior activity of FOLFIRI plus bevacizumab over mIFL plus bevacizumab in terms of OS (28 vs 19.2 months; P = 0.037), and 1-year survival rate (87% vs 61%; respectively). However, both regimens achieved similar overall response rate (57.9% for FOLFIRI plus bevacizumab and 53.3% for mIFL plus bevacizumab). This trial proved the efficacy of bevacizumab in combination with irinotecan-containing regimens, more importantly, this trial established the standard of care of infusional 5-FU for the irinotecan-based regimen.
Another large study, NO16966 which originally designed to compare the noninferiority of CapeOX to FOLFOX-4, was modified to test the additional benefit of bevacizumab in first-line therapies after the aforementioned phase III trial demonstrated superiority of adding bevacizumab into first-line cytotoxic chemotherapy. Therefore, this trial included 4 arms in order to compare the efficacy of CapeOX and FOLFOX-4 with or without bevacizumab.Citation33 A total of 1401 patients were enrolled. Bevacizumab arms achieved significantly longer PFS compared with non-bevacizumab arms (9.4 vs 8.0, hazard ratio [HR] = 0.83; 97.5% confidence interval [CI], 0.72 to 0.95; P = 0.0023). However, the addition of bevacizumab did not affect OS (21.3 vs 19.9 months, HR = 0.89; 97.5% CI, 0.76 to 1.03; P = 0.077). The authors argued the lack of OS benefit might be caused by early discontinuation of bevacizumab. Nevertheless, this trial is still considered a clinically meaningful study. The National Comprehensive Cancer Network of the United States (NCCN) recommend bevacizumab be added with front-line oxaliplatin-containing regimens for initial treatment.
Besides FOLFOX-4, bevacizumab was also investigated in combination with other three oxaliplatin-containing regimens in Three Regimens of Eloxatin Evaluation (TREE)-2 study.Citation34 In this study, oxaliplatin with infusional, bolus, and oral fluoropyrimidine with or without bevacizumab were evaluated as first-line treatment for mCRC. The addition of bevacizumab to all three regimens improved overall RR and prolonged OS compared with their comparators. Among these three regimens, oxaliplatin plus infusional 5-FU/LV (FOLFOX) plus bevacizumab appeared to be superior to the other two ().
Table 2 Summary of trials investigating bevacizumab in first-line setting for metastatic colorectal cancer
Bevacizumab in combination with another oxaliplatin-containing regimen, modified (m) FOLFOX-7, was investigated CONcePT (Combined Oxaliplatin Neurotoxicity Prevention Trial) Trial.Citation35 This trial was designed to optimize oxaliplatin dose in order to reduce neurotoxicity. Therefore, the major two arms are alternating mFOLFOX-7 with 5-FU/LV plus bevacizumab, and continuous mFOLFOX-7 plus bevacizumab. The primary endpoint was time to treatment failure. CONcePT also measured the effect of intravenous calcium and magnesium supplement on neurotoxicity prevention. After 140 patients with mCRC were enrolled, the first interim analysis suggested calcium/magnesium supplement might be negatively impacting anti-tumor activity of chemotherapy. Therefore, this trial was closed early in June 2007. However, an independent radiology review demonstrated that response was not affected by calcium/magnesium supplementation. The intermittent oxaliplatin arm showed significant improvement of PFS and time to treatment failure (TTF) when compared with the continuous oxaliplatin arm (12 vs 7.3 months, P = 0.03; and 5.6 vs 4.2 months, P = 0.003; respectively). This trial proved the convincing benefit of intermittent oxaliplatin over continuous oxaliplatin. However, the role of bevacizumab was impossible to be investigated given both arms contain same dose/schedule of bevacizumab.
Bevacizumab in second-line therapy for advanced CRC
Bevacizumab was also tested in mCRC in combination with oxaliplatin-based regimen in second-line setting. In the Eastern Cooperative Oncology Group (ECOG) trial (E3200), 829patients with previously treated CRC but without prior oxaliplatin or bevacizumab exposure were randomized to 3 arms at 1:1:1 ratio: FOLFOX-4 plus bevacizumab, FOLFOX-4, or bevacizumab alone.Citation36 The dose of bevacizumab chosen was 10 mg/kg although a phase II trial concluded that 5 mg/kg dose was more effective than 10mg/kg dose. Citation26 The authors of E3200 trial believed that the effect of bevacizumab was dose-dependent and 10mg/kg should be more active in combination with chemotherapy. FOLFOX-4 plus bevacizumab treatment in irinotecan-refractory mCRC demonstrated not only an improved overall RR but also statistically significant survival benefit compared with FOLFOX-4 or bevacizumab alone ().
Table 3 Summary of the efficacy data of E3200 trial Citation36
Interestingly, prior to the publication of this trial, the study of FOLFOX-4 as first-line therapy for mCRC was just accepted as the standard of care for initial treatment in the United States.Citation25 Therefore, the E3200 trial also proved the anti-tumor effect of FOLFOX-4 with or without bevacizumab in second-line setting after irinotecan failure. More importantly, bevacizumab in combining with cytotoxic chemotherapy provided additional response and survival benefit.
Similarly, bevacizumab was investigated in combination with an irinotecan-based regimen in second-line setting. Bowel Oncology and Cetuximab Antibody (BOND)-1 study conducted by Cunningham et al had established the role of irinotecan and epithelial growth factor receptor (EGFR) monoclonal antibody (mAb)–cetuximab in second-line setting.Citation37 The BOND-2 study was subsequently designed to evaluate any benefit of adding bevacizumab to irinotecan–cetuximab combination therapy.Citation38 Until now, this was the first and remained the only trial showing that concurrent use of dual-mAb therapy did not increase toxicity but improved PFS. Unfortunately, these results were not shown in the large trials Panitumumab Advanced Colorectal Cancer Evaluation (PACCE) or CApecitabine, IRinotecan, and Oxaliplatin in advanced CRC (CAIRO)-2 studies,Citation39–Citation52 both of which showed unacceptable toxicities when 2 monoclonal antibodies were administered in combination with cytotoxic chemotherapy. Dual biologic therapy will be discussed in another section in this review.
There are well-accepted convincing data of incorporating bevacizumab with irinotecan-, oxaliplatin-based regimens as first-line or second-line therapies for mCRC. However, many clinical questions remained unanswered, such as the role of bevacizumab maintenance, or continuation of bevacizumab in second- or third-line settings in patients who had prior exposure to bevacizumab-containing regimens. The DREAM (Double Reintroduction with Erlotinib and Avastin in Metastatic CRC) trial was designed to evaluate the role of bevacizumab with or without erlotinib as maintenance treatment.Citation43 Patients were treated with 6 cycles of FOLFOX-7 plus bevacizumab or 6 cycles of CapeOX-4 plus bevacizumab, then maintenance bevacizumab with or without erlotinib, followed by reinduction of FOLFOX-7 or CapeOX plus bevacizumab on disease progression. The primary endpoints are PFS and OS. We hope the final data will be able to answer the above questions.
Current consensus does not support the continuation of bevacizumab if a patient has already had it in first-line therapy, because of lack of supporting data.
Dual-biologic therapy in mCRC
In theory, targeting two separate pathways such as VEGF and EGF pathways simultaneously potentially produces higher anti-tumor effect. However, in reality, we have seen extremely dangerous toxicities in several trials using 2 mAbs or 1 mAb plus 1 RTKi.Citation39–Citation42
Combination of bevacizumab and EGFR mAb
The first trial combining bevacizumab with another biologic agent was BOND-2 study.Citation38 A regimen of irinotecan, cetuximab plus bevacizumab was investigated. The efficacy data demonstrated that the dual-biologic regimen potentially provided survival benefit compared with mono-biologic regimen (such as irinotecan/cetuximab) and could be considered as a second-line option for irinotecan-refractory mCRC.
However when a similar idea was tested in large trials, an unexpectedly high incidence of severe toxicity emerged. The PACCE trial was designed to investigate the role of combining anti-EGFR and anti-VEGF antibody with chemotherapy as first-line treatment in patients with advanced CRC.Citation39,Citation40 In this study, FOLFOX or FOLFIRI plus bevacizumab with or without panitumumab, an anti-EGFR mAb, were compared. The rationale for this design was based on preclinical studies suggestive of additive anti-tumor effect of targeting on 2 separate pathways. The data from the PACCE trial were certainly surprising. The dual-biologic target therapy did not provide any benefit to the efficacy but significantly increased the incidence of severe gastrointestinal (GI) toxicity such as diarrhea and infections.
CAIRO-2 study conducted by the Dutch Colorectal Cancer Group is another large phase III trial attempting to evaluate the effectiveness of dual biologic therapy.Citation41 This is a well-designed, multicenter clinical trial in which 736 patients with previously untreated mCRC were randomly assigned to receive capecitabine, oxaliplatin, and bevacizumab (CB) with or without cetuximab (CBC) every 3 weeks. Besides the use of bevacizumab, there are 2 differences between CAIRO-2 and PACCE: panitumumab was changed to a more commonly used anti-EGFR antibody, cetuximab; the backbone conventional chemotherapy switched from FOLFOX/FOLFIRI to CapeOX. The final results of CAIRO-2 were recently published ().Citation42 The results were somewhat expected – reduced survival length plus overwhelming toxicities.
Table 4 Toxicity and efficacy profile of CAIRO-2 studyCitation41
The two large trials, PACCE and CAIRO-2, both showed significant toxicity without any clinical benefit when combining 2 monoclonal antibodies with cytotoxic chemotherapy. Until now, there is no clear explanation for the observed negative effect. Therefore, the National Cancer Institute (NCI) suspended another ongoing large trial being conducted by the Cancer and Leukemia Group B (CALGB) and South West Oncology Group (SWOG).Citation44 The study was designed to answer the question of whether 2 antibodies are superior to single antibody when combined with chemotherapy. The current consensus is that combined biologic therapy with anti-EGFR and anti-VEGF antibodies should not be used outside an appropriate clinical trial setting. We have known that neither cetuximab nor panitumumab would provide any response benefit in KRAS mutant CRC. Nevertheless, the negative outcome could not be all attributed to the KRAS status.
Combination of bevacizumab and erlotinib
Bevacizumab and erlotinib were combined with FOLFOX for first-line treatment of mCRC in a phase II study.Citation45 All 35 patients initially enrolled for intention to treat were off study secondary to intolerable toxicity rather than disease progression. Major toxicities included rash, diarrhea, and neuropathy. Interestingly, despite premature termination of this trial because of toxicity, 12 patients achieved objective response, and 1 patient even had complete response. However, interpreting these efficacy data in an early closed trial would be challenging. Overall this double-target therapy combination was believed to be too toxic.
Bevacizumab in CRC adjuvant setting
Should we extrapolate the promising data of bevacizumab in advanced CRC into adjuvant setting? The answer to this question is no for now. Clearly current data from the National Surgical Adjuvant Breast and Bowel Project C-08 trial (NSABP C-08) did not support the use of bevacizumab in the adjuvant setting given lack of survival benefit. Allegra and colleagues published the safety data.Citation46 This randomized phase III trial was designed to compare modified FOLFOX-6 (every 2 weeks for 12 cycles) with bevacizumab vs without bevacizumab. For the nonbevacizumab arm, patients received standard mFOLFOX-6 for a total of 12 cycles, while patients in the bevacizumab arm were offered bevacizumab maintenance after completion of 12 cycles of mFOLFOX-6 plus bevacizumab ().
Figure 1 Schema of NSABP C-08 trial.Citation46,Citation47
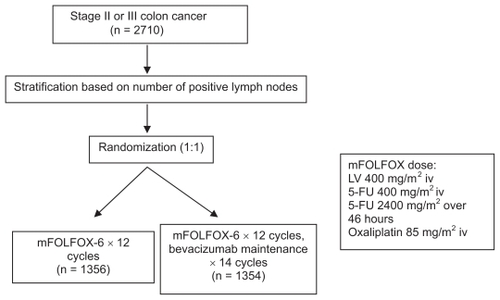
The safety data were obtained from 2647 patients with surgically resected stage II/III colon cancer. The dose of bevacizumab was 5mg/kg every 2 weeks. The primary endpoint is 3-year disease-free survival (DFS). Demographic factors were well balanced. Twenty-five percent of patients had stage II disease in each arm.
The safety data revealed virtually identical incidence of grade 4 toxicities in both arms (15.2% and 15.0%, respectively). Bevacizumab arm did show significantly higher rate of several toxicities; however, those previously concerned toxicities such as GI bleeding, perforation, or ischemic events were not statistically different when compared with the nonbevacizumab arm ().
Table 5 Summary of severe toxicity data of NSABP C-08 trialCitation45
Following the overall acceptable safety data, the final survival data were subsequently presented at the 2009 ASCO annual meeting.Citation47 Surprisingly, no PFS benefit was observed with the addition of bevacizumab regardless of disease stage (). Therefore, use of bevacizumab in the colon adjuvant setting cannot currently be recommended in the absence of efficacy data.
Table 6 Survival data of NSABP C-08Citation47
Pancreatic cancer
Pancreatic cancer is the fourth leading cause of cancer related death in the US.Citation48 The prognosis of pancreatic cancer depends on the resectability of primary tumor; however, more than 80% of patients have locally advanced disease or metastases upon diagnosis. Single agent gemcitabine remains the only universally accepted effective palliative treatment that not only provides clinical benefit but also prolongs OS.Citation49
Since the approval of gemcitabine in 1997 by the FDA, numerous combinations using gemcitabine as a backbone were investigated in many clinical trials. The combination of erlotinib with gemcitabine reached statistical significance in terms of OS, leading to FDA approval of erlotinib in advanced pancreatic cancer (APC) in November 2005.Citation50
Use of bevacizumab in phase II clinical trials
Bevacizumab was tested in several combinations with cytotoxic chemotherapy in phase II trials. Kindler et al published the first phase II trial of bevacizumab in combination with gemcitabine in untreated APC as first-line therapy.Citation51 Gemcitabine (1000 mg/m2) was given on days 1, 8, and 15 every 28 days; bevacizumab (10 mg/kg) was given on days 1 and 15 after gemcitabine. Fifty-two patients with confirmed APC were treated. Eleven patients (21%) had partial responses, and median OS and PFS were 8.8 and 5.4 months, respectively. These results appear encouraging, but the toxicity profile was worrisome. In this 52-patient trial, the incidence of bowel perforation was 8%, which was strikingly high compared to the observations in other colorectal trials.
The North Central Cancer Treatment Group (NCCTG) investigated bevacizumab in combination with gemcitabine and oxaliplatin,Citation52,Citation53 a combination that regained attention after several pooled- and meta-analyses demonstrated a small benefit for selected patients with good performance status.Citation54–Citation57 Objective RR was 11.3% in a total of 80 eligible patients. The 6-month survival was 65.0%; median OS and PFS were 8.1 and 5.7 months, respectively.Citation52,Citation53
Bevacizumab with or without docetaxel was tested by Astsaturov and colleagues,Citation58 although the rationale of this trial was not convincing given the minimal activity of taxanes in pancreatic cancer in general. Bevacizumab was given at 10mg/kg every 2 weeks alone or with docetaxel at 35 mg/m 2 weekly for 3 weeks followed by 1 week off. Fifteen out of 30 patients experienced severe adverse events including 5 deep venous thromboses and 2 bowel perforations. There was no difference in terms of PFS or OS in either arm. The results of this trial only confirmed an already-known fact that taxanes generally do not have an anti-tumor effect in APC. In addition, single-agent bevacizumab was also proved to lack efficacy in this aggressive disease.
Overall, all the above-mentioned phase II trials except the combination of bevacizumab and gemcitabine failed to demonstrate any convincing benefit compared with historical gemcitabine monotherapy.Citation51–Citation53,Citation58,Citation59
Use of bevacizumab in phase III clinical trials
The high response rate in phase II results for bevacizumab plus gemcitabine provided the rationale for a phase III CALGB 80303 study, in which gemcitabine with or without bevacizumab were compared.Citation60 Six hundred and two patients with APC were enrolled into this large placebo-controlled trial, randomized for patients to receive gemcitabine plus bevacizumab (GB) versus gemcitabine plus placebo (GP). The primary endpoint was OS, secondary endpoints were RR, duration of response, PFS, and toxicity. Median OS and PFS of GB/GP were 5.8/6.1 and 4.9/4.7 months, respectively. RR and 1-year survival rate failed to reach statistical difference.
The comparison of phase II and phase III data revealed that difference in performance status, adjuvant treatment, and/or previous thrombotic events might be able to explain the negative outcome. Toxicity profile was not substantially different from any other large bevacizumab trials, including hypertension (8%), GI perforation (0.4%), GI bleeding (3%), proteinuria (4%), and venous thrombosis (9%). Nevertheless, the addition of bevacizumab to gemcitabine did not offer any meaningful clinical benefit.
Another phase III trial of bevacizumab was the Tarceva ± Avastin in APC (AViTA) trial, which was unfortunately another negative trial.Citation61 AViTA aimed to explore the superiority of gemcitabine/erlotinib plus bevacizumab over gemcitabine/erlotinib plus placebo in APC. The biologic rationale for such a design came from retrospective studies demonstrating an adverse correlation between overexpression of EGFR and VEGF in pancreatic cancer with survival.Citation62,Citation63
OS was the primary objective, PFS, disease control rate, and toxicity were secondary objectives. Six hundred and seven patients with metastatic pancreatic cancer were randomized to receive gemcitabine/erlotinib with or without bevacizumab as initial therapy ().
Figure 2 Schema of AViTA trial. Citation61
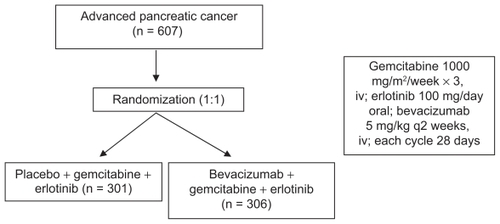
Although DFS (from 3.6 to 4.6 months) met statistical significance (HR 0.73, P = 0.0002), no significant prolongation but a trend toward OS benefit was seen in the bevacizumab arm (7.1 vs 6.0 months, HR 0.89, P = 0.2087). The authors believe this 1-month disease-free survival benefit suggests that a subset of metastatic patients might derive more benefit from anti-angiogenic therapy than others. However, how to identify these subgroup patients remains unclear.
Hepatocellular carcinoma (HCC)
HCC is a highly vascularized tumor with poor prognosis. Its incidence is rising in Europe and US as a result of the high prevalence of hepatitis C.Citation48 Surgical resection and liver transplantation are the effective curative interventions only for local resectable disease; however, the majority of cases present with advanced stage or metastatic stage. Sorafenib is the only standard of care for advanced HCC, modest benefit at the sacrifice of toxicity made the development of novel agents urgent.Citation64 VEGF is found to be aberrantly expressed in HCC and plays a critical role in tumorigenesis as well as disease progression.Citation5 The use of anti-angiogenic agents appears to be a promising approach in view of the highly vascular nature of this tumor. Several early phase trials have investigated the role of bevacizumab in the treatment of HCC.
Finn et al established a mouse model to assess the anti-angiogenic effect of bevacizumab. Hep 3B cells, a human HCC cell line, were inoculated into the livers of severe combined immunodeficient (SCID) mice under anesthesia.Citation65 Bevacizumab at 5 mg/kg was administered intraperitoneally twice weekly into half of the mice. Serum alpha-fetoprotein (AFP) and VEGF level were measured as surrogate markers for response, while tumors were harvested for vascular study such as immunohistochemical staining of the endothelial marker CD31. Bevacizumab significantly decreased microvessel density in tumors and decreased serum AFP level. These preclinical data led to bevacizumab being tested in human subjects.
Forty-six patients with unresectable nonmetastatic HCC were enrolled into a phase II study.Citation66 12 patients received bevacizumab at 5 mg/kg, while the other patients received a higher dose at 10mg/kg every 2 weeks until disease progression or treatment-limiting toxicity. Overall objective RR was 13%. Median PFS was 6.9 months (95% CI 6.5 to 9.1 months). More than 50% of patients survived more than 1 year, more than 25% survived beyond 2 years. The efficacy results were encouraging, but hypertension, bleeding, and thrombotic events remain the major safety concern for bevacizumab treatment.
Zhu et al conducted a phase II trial to examine the efficacy of gemcitabine and oxaliplatin plus bevacizumab (GEMOX-B) in unresectable or metastatic HCC.Citation67 The schedule was bevacizumab 10mg/kg administered every 14days, fixed dose rate gemcitabine 1000 mg/m 2 every 14days, and oxaliplatin 85 mg/m 2 on the following dayafter gemcitabine. Thirty patients were eligible for efficacy analysis. Six patients achieved objective response on imaging studies according to RECIST; median PFS and OS were 5.3and 9.6 months, respectively. Severe toxicities related to treatment were bone marrow suppression, abnormal liver function, hypertension, and fatigue. The benefit derived from this regimen appears to be comparable with that of sorafenib, but a larger trial is warranted for further investigation.
The effect of bevacizumab on angiogenesis was examined by the same research group utilizing computed tomography perfusion scan.Citation68 Bevacizumab induced a significant decrease in tumor blood flow, blood volume, and permeability in HCC.
Bevacizumab and erlotinib combination was also investigated in advanced or metastatic HCC at phase II level.Citation69 This regimen consists of bevacizumab 10mg/kg every 14days and erlotinib 150 mg oral daily, for each 28-day cycle. The primary endpoint of this phase II study was PFS at 16weeks. Of 40 patients, 62.5% survived beyond 16 weeks without evidence of progression. Ten patients achieved a partial response, while median PFS and OS were 9.1 and 15.9 months, respectively. The dual-biologic agent strategy showed very promising anti-tumor activity in HCC. More importantly, toxicity profile of this combination regimen was acceptable. More recently, an updated report was presented at the 2009 ASCO annual meeting.Citation70 With a total of 57 patients, overall RR was 28%, and median PFS and OS were 7.9 months and 12.8 months, respectively. PFS at 16weeks was 73%. Currently, a multi-institution randomized phase II trial is ongoing to further characterize the clinical benefit of the combination.
Among other regimens, the combinations of bevacizumab and mammalian target of rapamycin (mTOR) inhibitors appear encouraging. Bevacizumab and rapamycin combination offered promising anti-tumor activity in an orthotopic intrahepatic xenograft mouse model.Citation71 Bevacizumab and everolimus combination is also being tested in human subjects.Citation72
Cholangiocarcinoma and gallbladder cancer
Unlike other GI malignancies, there is very limited experience with the use of bevacizumab in cholangiocarcinoma or gallbladder cancer. A Taiwan oncology group reported an interesting case in 2006. An elderly patient with cholangiocarcinoma achieved quick response in liver metastases after being treated with cisplatin, 5-FU, leucovorin plus bevacizumab.Citation73 However, this is just a single case observation outside a clinical trial setting. In addition, the effect of tumor shrinking can hardly be attributed to bevacizumab anti-angiogenic effect alone. More prospective studies should address whether this combination can be an appropriate option for advanced biliary duct cancer.
Given the popularity of the double pathway blocking approach, especially the combination of bevacizumab and erlotinib, Holen and colleagues also investigated this combination in previously untreated advanced gallbladder cancer or cholangiocarcinoma. The data were presented at the 2008 ASCO annual meeting.Citation74 Twenty-nine patients were eligible for enrollment; 3 out of 17 (17.6%) evaluable patients achieved partial response (PR). The projected accrual is not completed yet for this trial, and final efficacy data are therefore pending. The data are currently being validated in other larger trials (). Several other trials are ongoing to investigate the efficacy of bevacizumab with cytotoxic agents, and/or radiation in biliary duct and gallbladder cancer.
Table 7 Ongoing clinical trials investigating bevacizumab for biliary duct carcinoma and gallbladder cancer
Gastric and esophageal cancer
Gastric cancer is one of the leading causes of cancer-related death in the US. Median OS for metastatic or unresectable disease is usually less than 10 months. Despite response rates of 30% to 60% to combination chemotherapy, response duration is usually 4 to 6 months and 24-month survival is 5% to 10%. VEGF overexpression was also found in gastric cancer and was associated with poor prognosis.Citation75,Citation76 This suggests that anti-VEGF therapy might have also have an effect on gastric cancer. In a mouse model with gastric peritoneal metastases, retarded tumor growth and development of malignant ascites were demonstrated after being treated with bevacizumab.Citation77
Shah and colleagues conducted a phase II trial to evaluate the efficacy and safety of adding bevacizumab to irinotecan/cisplatin combination in metastatic or unresectable gastric and gastroesophageal (GE) junction adenocarcinoma.Citation78 This is a multicenter trial that enrolled 47 patients. The dose and schedule were: bevacizumab 15 mg/kg on day 1, irinotecan 65 mg/m2, and cisplatin 30 mg/m2 on days 1 and 8 every 21days. With a median follow-up of 12.2 months, this combination regimen achieved overall RR of 65% (95% CI 46% to 80%), median TTP of 8.3 months (95% CI 5.5 to 9.9), and median OS of 12.3 months (95% CI 11.3 to 17.2), suggesting a 75% improvement compared with historical control. Toxicity profile was not different from other bevacizumab-containing regimens, including hypertension, GI perforation, GI bleeding, and thromboembolic events. Further optimization of the use of bevacizumab in gastric and GE junction cancers is warranted.
Two abstracts presented at the 2009 ASCO annual meeting are worth discussing.Citation79,Citation80 Based on the data obtained in the Shah phase II study, Kelsen and colleagues investigated modified docetaxel, cisplatin, fluorouracil (mDCF), and bevacizumab in metastatic GE junction adenocarcinoma as first-line therapy.Citation79 The primary objective was to improve 6-month PFS from 43% (historical DCF control) to 63% with the addition of bevacizumab. Thirty-nine of 44 eligible patients had measurable disease. Overall RR was 67% (95% CI 50% to 81%), 6-month PFS was 79% (95% CI 68% to 93%), median PFS was 12 months (95% CI 8.8 to 16), and median OS was 16.2 months (95% CI 11.4 to infinitiy) after a median follow-up of 12.3 months. This bevacizumab-containing regimen provided a durable survival benefit without significant toxicities.
A similar regimen with docetaxel and oxaliplatin in combination with bevacizumab was investigated by El-Raves and colleagues.Citation80 The primary endpoint was TTP. A total of 23 patients with locally advanced or metastatic gastric/GE junction cancer were enrolled. PR was confirmed in 10(59%) patients. However, in contrast to the safety report of the other mDCF plus bevacizumab trial, 3 GI perforations was reported in this trial. This alerted the investigators not to use bevacizumab outside clinical trials until more safety data are obtained.
Neuroendocrine tumors (NET)
Neuroendocrine tumors, also named gastroenteropancreatic NET (GEP-NET), are cancers arising from endocrine (hormonal) system and the nervous system. There are essentially two distinct categories: functional or secretory, and nonfunctional tumors. Secretory NETs are usually treated with somatostatin analog-based therapy such as octreotide for symptomatic control. In metastatic stage, chemotherapy generally has low response rate with short duration.
Chemotherapeutic agents tested in locoregionally advanced or metastatic NETs include doxorubicin, capecitabine, oxaliplatin, streptozocin, 5-FU, temozolomide, and dacarbazine as single agents or in combination. Early data suggest that single-agent bevacizumab for advanced carcinoid tumors inhibits tumor blood flow and increases PFS.Citation81 At the 2008 ASCO annual meeting, Kunz and colleagues presented the interim analysis of a phase II trial investigating a combination of capecitabine, oxaliplatin, and bevacizumab for metastatic/unresectable NETs.Citation82 This is a relatively well tolerated regimen for CRC. The primary endpoints were to determine median TTP and assess the toxicities associated with this regimen. Among 13 patients of a planned 37, 4 achieved a PR with durations of 33, 27+, 27+, 27+ weeks, respectively. Toxicities were expected to be related to chemotherapeutic agents.
The Dana-Farber cancer institute also conducted a phase II trial to investigate the efficacy of bevacizumab in combination with temozolomide in advanced NET.Citation83 This was a nonrandomized, open label, single-arm study. The primary endpoint was to assess the response to bevacizumab and temozolomide in metastatic NET. Secondary endpoints were assess TTP, PFS, and safety of this combination. The schedule of temozolomide was once daily for 1 week followed by 1 week off. Bevacizumab was given intravenously every 2 weeks. Restaging CT scan was performed every 8 weeks to assess response. Among 29 evaluable patients for efficacy, 4 achieved PR, which made the response rate of 24%. Bevacizumab-related toxicities were not severe except one patient developed hypertension.
The question of whether octreotide has an anti-tumor effect in addition to symptomatic control was answered at the 2009 GI ASCO meeting.Citation84 PROMID (Placebo-controlled prospective Randomized study on the antiproliferative efficacy of Octreotide LAR in patients with metastatic neuroendocrine MIDgut tumors) was the first randomized, double-blind, placebo-controlled, multicenter, phase IIIb study of long-acting release (LAR) octreotide in patients with metastatic NETs.Citation84 Eighty-five eligible patients randomly received either octreotide LAR or placebo. Octreotide LAR significantly lengthened median TTP from 6 months in the placebo groups to 14.3 months (HR 0.34; 95% CI 0.20 to 0.59; P = 0.000072). Therefore, a trial to test bevacizumab and octreotide combination in metastatic NETs would be the next step.
Future directions
Bevacizumab has provided the proof of concept that angiogenesis is an important target for cancer therapy and is the first anti-VEGF monoclonal antibody to receive FDA approval. Bevacizumab has shown clinical efficacy in a variety of advanced malignancies including CRC, nonsmall cell lung cancer, breast cancer, kidney cancer, ovarian epithelial cancer, and hepatocellular carcinoma.
Although bevacizumab has an established role in the treatment of metastatic colon, breast, and lung cancer, but many questions remain about its use in other disease types and demographic groups. Future studies are designed to evaluate. Important questions in treatment of advanced cancers are the optimal dose and duration of treatment with bevacizumab. One of the main aims of the ongoing CAIRO3 trial is to investigate the possible benefit of maintenance therapy with bevacizumab and low-dose capecitabine after initial therapy with chemotherapy and bevacizumab in mCRC. Resolution of the question abut optimal dose, however, has become increasingly challenging, and it is the opinion of the authors that a determination of optimal duration is likely to have the greater effect on outcomes.
Biomarker studies are crucial in identifying patients who would most benefit from bevacizumab therapy. The ECOG 5202 trial is a unique ongoing phase III prospective study aimed at determining the role of chemotherapy in stage II colon cancer patients by assigning treatment base risk as determined by molecular profiling. Patients will be stratified to ‘high risk’ or ‘low risk’ based on microsatellite instability and loss of heterozygosity at 18q. Patients deemed to be ‘high risk’ will be randomized to 5-FU/LV/oxaliplatin versus 5-FU/LV/oxaliplatin plus bevacizumab. Those patients deemed to be ‘low risk’ will be registered for observation only. Thus, identifying criteria for individualized treatment with bevacizumab may not only improve efficacy but, through better patient selection, could also limit the use of expensive therapies in populations not likely to benefit from them.
Disclosures
The authors declare no conflicts of interest.
References
- CoultasLChawengsaksophakKRossantJEndothelial cells and VEGF in vascular developmentNature200543893794516355211
- WertherKChristensenIJBrünnerNNielsenHJSoluble vascular endothelial growth factor levels in patients with primary colorectal carcinoma. The Danish RANX05 Colorectal Cancer Study GroupEur J Surg Oncol20002665766211078612
- SeoYBabaHFukudaTHigh expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinomaCancer2000882239224510820344
- IkedaNAdachiMTakiTPrognostic significance of angiogenesis in human pancreatic cancerBr J Cancer1999791553156310188906
- ClaudioPPRussoGKumarCApRb2/p130, vascular endothelial growth factor, p27(KIP1), and proliferating cell nuclear antigen expression in hepatocellular carcinoma: their clinical significanceClin Cancer Res2004103509351715161709
- FolkmanJTumor angiogenesis: therapeutic implicationsN Engl J Med1971285118211864938153
- FolkmanJKlagsbrunMAngiogenic factorsScience19872354424472432664
- FolkmanJWhat is the evidence that tumors are angiogenesis dependent?J Natl Cancer Inst199082461688381
- AugustinHGTranslating angiogenesis research into the clinic: the challenges aheadBr J Radiol2003761S3S1015456709
- RheeJHoffPMAngiogenesis inhibitors in the treatment of cancerExpert Opin Pharmacother200561701171116086656
- FerraraNAlitaloKClinical applications of angiogenic growth factors and their inhibitorsNat Med199951359136410581076
- IgnoffoRJOverview of bevacizumab: a new cancer therapeutic strategy targeting vascular endothelial growth factorAm J Health Syst Pharm20046121 Suppl 5S21S2615552623
- ChaseJLClinical use of anti-vascular endothelial growth factor monoclonal antibodies in metastatic colorectal cancerPharmacotherapy20082811 Pt 223S30S18980549
- KimKJLiBHouckKThe vascular endothelial growth factor proteins: identification of biologically relevant regions by neutralizing monoclonal antibodiesGrowth Factors1992753641380254
- http://www.fda.gov/bbs/topics/NEWS/2004/NEW01027.html.
- http://www.fda.gov/bbs/topics/NEWS/2006/NEW01488.html.
- http://www.fda.gov/cder/Offices/OODP/whatsnew/bevacizumab200802.html.
- de GramontAVan CutsemEInvestigating the potential of bevacizumab in other indications: metastatic renal cell, non-small cell lung, pancreatic and breast cancerOncology200569Suppl 3465616301835
- BurrisH3rdRocha-LimaCNew therapeutic directions for advanced pancreatic cancer: targeting the epidermal growth factor and vascular endothelial growth factor pathwaysOncologist20081328929818378539
- JemalASiegelRWardECancer statisticsCA Cancer J Clin200858719618287387
- GoldbergRMSargentDJMortonRFA randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancerJ Clin Oncol200422233014665611
- CassidyJTaberneroJTwelvesCXELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancerJ Clin Oncol2004222084209115169795
- SaltzLBCoxJVBlankeCIrinotecan plus fluorouracil and leucovorin for metastatic colorectal cancerN Engl J Med200034390591411006366
- TournigandCAndreTAchilleEFOLFIRI followed by FOLFOX or the recer sequence in advanced colorectal cancer: a randomized GERCOR studyJ Clin Oncol20042222923714657227
- ColucciGGebbiaVPaolettiGPhase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Grouppo Oncologico Dell’Italia MeridionaleJ Clin Oncol2005234866487515939922
- KabbinavarFHurwitzHIFehrenbacherLPhase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancerJ Clin Oncol200321606512506171
- HurwitzHFehrenbacherLHainsworthJDBevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancerJ Clin Oncol2005233502350815908660
- KabbinavarFFSchulzJMcCleodMAddition of bevacizumab to bolus fluorouracil and leucovorin in first line metastatic colorectal cancer: results of a randomized phase II trialJ Clin Oncol2005233697370515738537
- KabbinavarFFHambletonJMassRDCombination analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancerJ Clin Oncol20052337053712
- HurwitzHFehrenbacherLNovotnyWBevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancerN Engl J Med2004350232335234215175435
- FuchsCSMarshallJMitchellERandomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C StudyJ Clin Oncol2007254779478617947725
- FuchsCSMarshallJBarruecoJRandomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C studyJ Clin Oncol20082668969018235136
- SaltzLBClarkeSDíaz-RubioEBevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III studyJ Clin Oncol2008262013201918421054
- HochsterHHartLLRamanathanRKSafety and efficacy of oxaliplatin/fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer (mCRC): final analysis of the TREE studyJ Clin Oncol20082635233529 Erratum in: J Clin Oncol. 2008;26:469718640933
- HochsterHSGrotheyAShpilskyAEffect of intravenous (IV) calcium and magnesium (Ca/Mg) versus placebo on response to FOLFOX+bevacizumab (BEV) in the CONcePT trial2008 Gastrointestinal Cancers Symposium Abstract 280
- GiantonioBJCatalanoPJMeropolNJBevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200J Clin Oncol2007251539154417442997
- CunninghamDHumbletYSienaSCetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancerN Engl J Med200435133734515269313
- SaltzLBLenzHJKindlerHLRandomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 studyJ Clin Oncol2007254557456117876013
- HechtJRMitchellEChidiacTAn updated analysis of the safety and efficacy of oxaliplatin (Ox)/bevacizumab (bev) ± panitumumab (pmab) for firstline treatment (tx) of metastatic colorectal cancer (mCRC) from a randomized, controlled trial (PACCE). [abstract]Program and abstracts of the 2008 Gastrointestinal Cancers SymposiumOrlando, FLJanuary 25–27, 2008
- HechtJRMitchellEChidiacTInterim results from PACCE: Irinotecan (iri)/bevacizumab (bev) ± panitumumab as first-line treatment for metastatic colorectal cancer. [abstract]Proceedings and abstracts of the 2008 Gastrointestinal Cancers SymposiumOrlando, FLJanuary 25–27, 2008
- TolJKoopmanMRodenburgCJA randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicityAnn Oncol20081973473818272912
- TolJKoopmanMCatsAChemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancerN Engl J Med200936056357219196673
- Combination chemotherapy and bevacizumab with or without erlotinib in treating patients with metastatic colorectal cancer that cannot be removed by surgery www.clinicaltrials.gov. ClinicalTrials.gov identifier: NCT00265824
- Irinotecan and Cetuximab with or without bevacizumab in treating patients with metastatic colorectal cancer that progressed during first-line therapy http://clinicaltrials.gov/ct2/show/NCT00499369
- MeyerhardtJAStuartKFuchsCSPhase II study of FOLFOX, bevacizumab and erlotinib as first-line therapy for patients with metastatic colorectal cancerAnn Oncol2007181185118917483115
- AllegraCJYothersGO’ConnellMJInitial safety report of NSABP C-08, a randomized phase III study of modified 5-fluorouracil (5-FU)/leucovorin (LCV) and oxaliplatin (OX) (mFOLFOX6) with or without bevacizumab (bev) in the adjuvant treatment of patients with stage II/III colon cancer. 2008 ASCO annual meetingJ Clin Oncol200826May 20 Suppl; abstr 4006
- WolmarkNYothersGO’ConnellMJA phase III trial comparing mFOLFOX6 to mFOLFOX6 plus bevacizumab in stage II or III carcinoma of the colon: Results of NSABP Protocol C-08J Clin Oncol20092718s(suppl; abstr LBA4)
- JemalASiegelRWardECancer statistics, 2009CA Cancer J Clin20095922524919474385
- BurrisHA3rdMooreMJAndersenJImprovements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trialJ Clin Oncol199715240324139196156
- MooreMJGoldsteinDHammJErlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials GroupJ Clin Oncol2007251960196617452677
- KindlerHLFribergGSinghDAPhase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancerJ Clin Oncol2005238033804016258101
- KimGPObergAKabatBNCCTG phase II trial of bevacizumab, gemcitabine, oxaliplatin in patients with metastatic pancreatic adenocarcinoma. [abstract]J Clin Oncol2006 ASCO Annual Meeting Proceedings Part I. Vol 24, No. 18S (June 20 Suppl):4113
- KimGPObergALFosterNRPhase II trial of bevacizumab, gemcitabine, oxaliplatin in patients with metastatic pancreatic adenocarcinoma. [abstract]J Clin Oncol2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (June 20 Suppl):4553
- LouvetCLabiancaRHammelPGemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trialJ Clin Oncol2005233509351615908661
- HeinemannVQuietzschDGieselerFRandomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancerJ Clin Oncol2006243946395216921047
- HeinemannVLabiancaRHinkeALouvetCIncreased survival using platinum analog combined with gemcitabine as compared to single-agent gemcitabine in advanced pancreatic cancer: pooled analysis of two randomized trials, the GERCOR/GISCAD intergroup study and a German multicenter studyAnn Oncol2007181652165917660491
- HeinemannVBoeckSHinkeALabiancaRLouvetCMeta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancerBMC Cancer200888218373843
- AstsaturovNJMeropolNJAlpaughRKA randomized phase II and coagulation study of bevacizumab alone or with docetaxel in patients with previously treated metastatic pancreatic adenocarcinoma. [abstract]J Clin Oncol2007 ASCO Annual Meeting Proceedings. Part I. Vol. 25, No. 18S (June 20 Suppl):4556
- KindlerHLGangadharTKarrisonTFinal analysis of a randomized phase II study of bevacizumab (B) and gemcitabine (G) plus cetuximab (C) or erlotinib (E) in patients (pts) with advanced pancreatic cancer (PC). [abstract]J Clin Oncol2008 ASCO Annual Meeting Proceedings:Abstract 4502
- KindlerHLNiedzwieckiDHollisDA double-blind, placebo-controlled, randomized phase III trial of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus placebo (P) in patients (pts) with advanced pancreatic cancer (PC): A preliminary analysis of Cancer and Leukemia Group B (CALGB)J Clin Oncol20072518s(Suppl) Abstract 4508
- Van CutsemEVervenneWLBennounaJPhase III Trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancerJ Clin Oncol2009272231223719307500
- YamanakaYFriessHKobrinMSCoexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressivenessAnticancer Res1993135655698317885
- LongoRCacciamaniFNasoGPancreatic cancer: From molecular signature to target therapyCrit Rev Oncol Hematol20086819721118436450
- LlovetJMRicciSMazzaferroVSorafenib in advanced hepatocellular carcinomaN Engl J Med200835937839018650514
- FinnRSBentleyGBrittenCDAmadoRBusuttilRWTargeting vascular endothelial growth factor with the monoclonal antibody bevacizumab inhibits human hepatocellular carcinoma cells growing in an orthotopic mouse modelLiver Int20092928429018482274
- SiegelABCohenEIOceanAPhase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinomaJ Clin Oncol2008262992299818565886
- ZhuAXBlaszkowskyLSRyanDPPhase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinomaJ Clin Oncol2006241898190316622265
- ZhuAXHolalkereNSMuzikanskyAHorganKSahaniDVEarly antiangiogenic activity of bevacizumab evaluated by computed tomography perfusion scan in patients with advanced hepatocellular carcinomaOncologist20081312012518305056
- ThomasMBMorrisJSChadhaRPhase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinomaJ Clin Oncol20092784385019139433
- KasebAOIwasakiMJavleMBiological activity of bevacizumab and erlotinib in patients with advanced hepatocellular carcinoma (HCC)J Clin Oncol20092715s Suppl Abstract 4522
- OngLCSongICJinYEffective inhibition of xenografts of hepatocellular carcinoma (HepG2) by rapamycin and bevacizumab in an intrahepatic modelMol Imaging Biol20091133434219330383
- Antiangiogenic treatment of HCC with bevacizumab and Rad001 http://www.clinicaltrials.gov/ct2/show/NCT00775073
- TaiCJChiouHYWuCHPanSLiuJDRapid resolution of liver metastasis from cholangiocarcinoma after bevacizumab with cisplatin and high-dose fluorouracil plus leucovorinOnkologie20062917918016601375
- HolenKDMahoneyMRLoConteNKEfficacy report of a multicenter phase II trial testing a biologic-only combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer (BC): A Phase II Consortium (P2C) studyJ Clin Oncol200826Meeting Abstracts Abstract 4522
- ErogluADemirciSAyyildizASerum concentrations of vascular endothelial growth factor and nitrite as an estimate of in vivo nitric oxide in patients with gastric cancerBr J Cancer1999801630163410408410
- KarayiannakisASyrigosKNPolychronidisACirculating VEGF levels in the serum of gastric cancer patientsAnn Surg2002236374212131083
- NinomiyaSInomataMTajimaMEffect of Bevacizumab, a Humanized Monoclonal Antibody to Vascular Endothelial Growth Factor, on Peritoneal Metastasis of MNK-45P Human Gastric Cancer in MiceJ Surg Res200915419620219329124
- ShahMARamanathanRKIlsonDHMulticenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinomaJ Clin Oncol2006245201520617114652
- KelsenDJhawerMIlsonDAnalysis of survival with modified docetaxel, cisplatin, fluorouracil (mDCF), and bevacizumab (BEV) in patients with metastatic gastroesophageal (GE) adenocarcinoma: Results of a phase II clinical trialJ Clin Oncol20092715s Suppl Abstract 4512
- El-RayesBFPatelBZalupskiMA phase II study of bevacizumab, docetaxel, and oxaliplatin in gastric and GEJ cancerJ Clin Oncol20092715s Suppl Abstract 4563
- YaoJCNgCHoffPMImproved progression free survival (PFS), and rapid, sustained decrease in tumor perfusion among patients with advanced carcinoid treated with bevacizumabJ Clin Oncol2005 ASCO Annual Meeting Proceedings. Vol 23, No. 16S, Part I of II (June 1 Suppl):Abstract 4007
- KunzPLKuoTKaiserHLA phase II study of capecitabine, oxaliplatin, and bevacizumab for metastatic or unresectable neuroendocrine tumors: Preliminary resultsJ Clin Oncol200826May 20 Suppl Abstract 15502
- KulkeMHStuartKEarleCCA phase II study of temozolomide and bevacizumab in patients with advanced neuroendocrine tumorsJ Clin Oncol2006 ASCO Annual Meeting Proceedings Part I. Vol 24, No. 18S (June 20 Suppl):Abstract 4044
- ArnoldRMüllerHSchade-BrittingerCPlacebo-controlled, double-blind, prospective, randomized study of the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID study groupJ Clin Oncol20092715s Suppl Abstract 4508