Abstract
The role of granulocyte-macrophage-colony-stimulating factor (GM-CSF) in the supportive care of cancer patients has been evaluated with promising results. More recently, GM-CSF has been added to regimens for the mobilization of hematopoietic progenitor cells. An expanding role for GM-CSF in regulating immune responses has been recognized based upon its activity on the development and maturation of antigen presenting cells and its capability for skewing the immune system toward Th1-type responses. GM-CSF has been shown to preferentially enhance both the numbers and activity of type 1 dendritic cells (DC1), the subsets of dendritic cells responsible for initiating cytotoxic immune responses. The increase in DC1 content and activity following local and systemic GM-CSF administration support a role for GM-CSF as an immune stimulant and vaccine adjuvant in cancer patients. GM-CSF has shown clinical activity as an immune stimulant in tumor cell and dendritic cell vaccines, and may increase antibody-dependent cellular cytotoxicity. The successful use of myeloid acting cytokines to enhance anti-tumor responses will likely require the utilization of GM-CSF in combination with cytotoxic or other targeted therapies.
Keywords:
Introduction/background
Granulocyte-macrophage-colony-stimulating factor (GM-CSF) and granulocyte-colony-stimulating factor (G-CSF) belong to the family of hematopoietic cytokines. Their activities include stimulating the proliferation of granulocyte and/or macrophage progenitor cells, influencing differentiation, inducing maturation, and stimulating the functional activity of mature hematopoietic cells (CitationInaba et al 1992; CitationMetcalf and Nicola 1995; CitationMetcalf 1998). Colony stimulating factors (CSF) are not only important as mediators of the cellular response to immunologic or infectious insults but are also essential for maintaining basal hematopoiesis (CitationDranoff et al 1994; CitationFantuzzi 2003). G-CSF-deficient mice manifest a chronic neutropenia and an impaired response to infectious challenge leading to premature death (CitationLieschke et al 1994). GM-CSF-deficient mice, on the other hand, have normal levels of steady-state blood cell production, but exhibit defective phagocytosis and decreased oxygen radical production by granulocytes and macrophages; responses which are essential for the anti-bacterial defense (CitationStanley et al 1994; CitationZhan et al 1998; CitationLeVine et al 1999). These mice also exhibit decreased tumor necrosis factor-alpha and leukotriene secretion, abnormal para-bronchial accumulations of B and T lymphocytes, and decreased catabolism of alveolar surfactant lipids and proteins leading to a syndrome reminiscent of pulmonary alveolar proteinosis (CitationStanley et al 1994; CitationPaine et al 2001). Double G-CSF and GM-CSF knockout mice exhibited a greater degree of neutropenia, and had an increased mortality rate in the early post-natal period compared to mice deficient in G-CSF alone (CitationSeymour et al 1997). CitationEnzler et al (2003) found that mice deficient in GM-CSF and gamma interferon (IFN-γ) acquired lymphoproliferative disorders and solid tumors in a background of chronic inflammation, supporting the relationship between inflammation and carcinogenesis.
Recombinant GM-CSF has made significant contributions in the supportive care of cancer patients, owing to enhanced myeloid recovery after cytotoxic chemotherapy. Recently GM-CSF has been successfully included in mobilization regimens for hematopoietic progenitor cell transplantation (CitationCashen et al 2004; CitationLonial et al 2004). Recent data on the effects of GM-CSF on dendritic cells has led to growing interest in its use as primary immunotherapy. The ability of GM-CSF to generate of type 1 dendritic cells (DC1), which can skew T-cells toward a Th-1 phenotype has been demonstrated and is an attractive approach toward generating anti-tumor effects (CitationFerlazzo et al 2000). Peripheral blood mononuclear cells (PBMC), T-cells and antigen presenting cells (APC) cultured with GM-CSF exhibited increased production of type 1 cytokines (interleukin-12, interferon-alpha, tumor necrosis factor-alpha) and decreased production of type 2 cytokines (interleukin-4 and interleukin-10) compared to cells treated with control media or G-CSF (CitationEksioglu et al 2007). In addition, APC treated with GM-CSF induced higher proliferation of allogeneic T-cells compared to APC treated with G-CSF or control media (CitationEksioglu et al 2007). The capacity of GM-CSF to skew the immune system toward Th1 effects in vitro suggests a role for GM-CSF in cell-mediated immune therapy and is currently being tested in-vivo. Dendritic cells (DC) have come to be recognized as the sensors of tissue injury, infection, or malignant transformation and as the agents responsible for the initial activation of the immune response (CitationMatzinger 1994). DC are the antigen presenting cells of the immune system, have the capacity to express both HLA class I and HLA class II restricted peptides, and express the co-stimulatory molecules needed for T-cell activation (CitationBanchereau et al 2000). A number of reports have shown that administration of GM-CSF can induce anti-tumor immune responses and tumor regressions. These immune activities are attributed to the action of GM-CSF on DC (CitationDranoff et al 1993; CitationWos et al 1996; CitationDavidson et al 1998). The limitations of GM-CSF as an immune adjuvant, and its modest clinical activity, have been attributed to discordance between the observed immune response measured in the laboratory and the clinical correlates of anti-tumor activity. The need to generate the co-stimulatory signals required to break immune tolerance, the proper dosing and timing of cytokines, the state of disease at time of treatment, and the role of concomitant chemotherapy are the topics of current investigation. In this review, we will discuss the role of GM-CSF as primary treatment and as an immune adjunct to the therapy of cancer.
Relationship between cancer and inflammation
The connection between inflammation and malignant transformation has been recognized for over a century. In 1863, Dr. Rudolf Virchow recognized a possible relationship between chronic inflammation and the development of cancer, based on observations of inflammatory infiltrates and spontaneous regression of malignant tumors (CitationBalkwill and Mantovani 2001; CitationSchreiber 2003). In the late 1890s, William Coley, a surgeon in New York, observed tumor regressions in patients with cancer who recovered from acute skin infections. He then developed a vaccine composed of extracts of inactivated bacteria, which he administered to cancer patients with variable results (CitationNauts et al 1953). He observed that tumor regressions were more common among patients who developed both a local and a systemic inflammatory response (CitationHoption Cann et al 2002). The premise was that non-specific activation of the immune system could lead to (specific) cytokine-mediated anti-tumor effects. While cytokines are credited with potent anti-tumor effects, a counteracting effect of tumor-secreted cytokines, and tumor-associated tolerizing T-cells, or immature DC has also been documented (CitationPerrot et al 2007; CitationWang and Wang 2007). Some solid tumors, for example, are capable of inducing immune tolerance via down-regulation of antigen-specific T-cell responses by tolerigenic APC (CitationCuenca et al 2003). Immature tumor-infiltrating DC are capable of compromising the tumor-specific immune response in draining lymph nodes (CitationPerrot et al 2007). The premise that the immune system can be manipulated in vivo supports a role for the manipulation of the cytokine/co-stimulatory signal milieu in the treatment of cancer using recombinant cytokines, such as GM-CSF.
Approaches will need to account for the level of maturation of tumor-associated DC, and the number of tumor-associated regulatory T-cells
GM-CSF may enhance tumor-specific antigen presentation leading to better recognition of tumors by the immune system via effects on DC. However, its benefit has been limited to patients with minimal residual disease, and dose-escalation has been limited by significant systemic toxicities. The optimal use of cytokines may be directly in the tumor micro-environment. Furthermore, better quantitative measures of antigen-specific immune responses are needed (CitationKeilholz et al 2002).
Role of GM-CSF in supportive therapy
Numerous clinical trials have established the role of CSF in the prevention and treatment of febrile neutropenia (CitationGarcia-Carbonero et al 2001; CitationMizutani et al 2003; CitationRepetto et al 2003). The majority of clinical trials investigated the role of G-CSF in the supportive care of cancer patients; therefore, the current recommendations do not address the specific use of GM-CSF in this setting. The American Society of Clinical Oncology (ASCO) has provided guidelines for the use of CSF (either G-CSF or GM-CSF) in patients receiving chemotherapy for solid and hematological malignancies (CitationSmith et al 2006). No recommendation was made regarding the equivalency of G-CSF and GM-CSF. Rowe et al reported a significant reduction of infectious complications in patients with acute myeloid leukemia (AML) who received GM-CSF after induction chemotherapy, compared to placebo (CitationRowe et al 1995). However, Zittoun et al (2006) reported a decreased complete response (CR) rate in patients with AML who received GM-CSF with induction chemotherapy, indicating that the routine use of cytokines for acceleration of hematopoietic recovery may not always be indicated; and the use of CSF for priming of leukemic cells is not recommended.
Dendritic cells as regulators of immune responses
DC play a central role in the initiation of innate and adaptive immune responses. Pattern recognition receptors, known as toll-like receptors (TLR), on the surface of DC are bound by proteins, lipids, and nucleic acids resulting in DC activation (CitationKadowaki et al 2001; CitationDillon et al 2004). Antigen-specific T-cell immune responses are initiated by DC when these bound antigens are internalized, degraded, and presented as processed peptides on the surface of HLA molecules (CitationHart 1997; CitationBancereau and Steinman 1998; CitationBanchereau et al 2000). Two main categories of peripheral blood and bone marrow derived DC have been described in humans, type 1, myeloid dendritic cells (DC1), and type 2 plasmacytoid dendritic cells (DC2) (CitationGrouard et al 1997). DC1 and DC2 differ in the type of cell differentiation markers and TLR expressed on their surface, their cytokine milieu, and their effect on polarizing T-cell immune responses (CitationRissoan et al 1999; CitationAmsen et al 2004; CitationDillon et al 2004). The maturation status of DC is an important determinant of the type of immune response generated upon DC activation. For example, antigen presentation by immature DC leads to generation of anergic CD4+ T-cells (CitationKuwana et al 2001), and immuno-suppressive CD8+ T-cells with antigen-specificity (CitationGilliet and Liu 2002). Immature DC2 progenitors play a crucial role in the response to viral infection by releasing large amounts of alpha and beta interferon (CitationSiegal et al 1999; CitationFonteneau et al 2003; CitationLarsson et al 2003; CitationCoccia et al 2004). The targeting of DC by synthetic TLR ligands is a topic of current clinical and pre-clinical research.
Generation of dendritic cells by GM-CSF
Colony-stimulating factors can differentiate hematopoietic progenitor cells into specific DC lineages (CitationSantiago-Schwarz et al 1992; CitationGrouard et al 1997; CitationOlweus et al 1997; CitationRissoan et al 1999; CitationSiegal et al 1999; CitationBerthier et al 2000; CitationFerlazzo et al 2000). Hematopoietic stem cells cultured in GM-CSF and Flt3 can differentiate along a myelomonocytic lineage into DC1 (CitationFerlazzo et al 2000). CD14+ progenitor cells cultured in GM-CSF and IL-4 can also differentiate into immature DC1 (CitationFerlazzo et al 2000; CitationBasak et al 2002). In contrast, treatment of hematopoietic progenitors with G-CSF and IL-3 can mobilize large numbers of (plasmacytoid) DC2 (CitationArpinati et al 2000). Thus, while GM-CSF and G-CSF have similar effects on the mobilization of neutrophils, they have significantly different effects on the mobilization and differentiation of DC1 and DC2, with culture in GM-CSF leading to differentiation of progenitors into DC1, and culture in G-CSF leading to differentiation of progenitors into DC2 (CitationArpinati et al 2000). Due to their effects on DC1 and DC2, these 2 cytokines are optimal agents for cellular immune therapy.
Cytokines and peripheral blood hematopoietic progenitor cell transplantation
Peripheral blood as a source of stem cells for clinical stem cell transplantation was introduced by Korbling et al and Kessinger et al in the 1980s (CitationKorbling et al 1981; CitationKessinger et al 1986). CSF are now widely used for the mobilization of hematopoietic progenitor cells into the peripheral circulation, allowing collection of CD34+ cells for autologous and allogeneic hematopoietic progenitor cell transplantation. Essential differences have been noted between bone marrow (BMT) and peripheral blood grafts, owing to differences in the ratio of early pluripotent, self-renewing stem cells to lineage-committed, late progenitor cells, and the accessory cells in the grafts (CitationKorbling and Anderlini 2001). These differences may account for the observed differences in clinical outcomes after transplantation. A study by the European working group for blood and marrow transplant reported similar rates of overall survival, leukemia-free survival, and similar incidence of graft versus host disease (GVHD) when comparing bone marrow with cytokine-mobilized peripheral blood grafts. They did, however, observe improved platelet recovery with cytokine-mobilized peripheral graft compared to BMT (CitationSchmitz et al 1998). A randomized, multi-center trial of cytokine-mobilized peripheral blood progenitor cell grafts versus BMT reported by CitationSchmitz et al (2002), found an increased risk of acute and chronic GVHD among recipients of cytokine-mobilized peripheral blood grafts, but no difference in survival compared to recipients of bone marrow transplants. In contrast, in another randomized trial comparing peripheral blood hematopoietic progenitor cell and bone marrow transplantation, Bensinger et al found more rapid neutrophil and platelet recovery and similar rates of acute and chronic GVHD. In that trial, the duration of chronic GVDH was longer among recipients of blood progenitor cell grafts. Furthermore, recipients of blood progenitor cell grafts had a higher estimated probability of overall survival and 2-year disease-free survival (CitationBensinger et al 2001). Interestingly, Urbini and colleagues found a higher number of CD34+ cells in peripheral blood grafts mobilized with G-CSF compared to bone marrow, and the dose of CD34+ cells infused correlated with the number of DC1 in peripheral blood grafts and DC2 in bone marrow allografts. In addition, among recipients of cytokine mobilized grafts, a significantly shorter overall survival and a trend toward lower disease free survival was noted among recipients of larger numbers of CD34+ cells (CitationUrbini et al 2003).
The fact that the incidence of acute GVHD in recipients of peripheral blood progenitor cell grafts was similar to, or only slightly higher than in BMT recipients, despite the higher content of T cells in the peripheral blood grafts, suggests that there may be quantitative differences in the other immune cellular components of peripheral blood grafts; such as, the presence of increased numbers of immunosuppressive DC2 in G-CSF mobilized peripheral blood grafts compared to bone marrow grafts (CitationArpinati et al 2000).
Clinical outcomes after hematopoietic progenitor cell transplantation: contribution of dendritic cells
Recent growing interest in the role of accessory cells in hematopoietic progenitor cell grafts has led to further studies of the immune-modulatory effects of CSF on the constituents of the peripheral blood progenitor cell graft. Flt3 and GM-CSF administration led to mobilization of increased numbers of DC1 in the cellular apheresis product (CitationGasparetto et al 2002). In contrast, administration of G-CSF lead to mobilization of increased numbers of DC2 cells in the grafts (CitationArpinati et al 2000). The clinical consequences of mobilizing more DC1 with GM-CSF and more DC2 with G-CSF remain unknown, and are the subject of current investigation.
Waller et al tested the hypothesis that the cellular constituents of the graft could affect clinical outcomes after bone marrow transplantation (CitationWaller et al 2001). They performed a retrospective study of 113 patients with hematological malignancies who received non-T cell-depleted bone marrow grafts from HLA-matched siblings. After evaluating patient and disease-specific characteristics, characteristics of the graft constituents, and clinical outcomes, they reported that patients who received larger numbers of donor DC2 had significantly worse clinical outcomes, with lower event-free survival, less chronic GVHD, and an increased incidence of relapse than their counterparts who received fewer numbers of DC2 cells (CitationWaller et al 2001).
The significance of absolute numbers of DC in post-transplant outcomes was also evaluated in a clinical study by Reddy and colleagues (CitationReddy et al 2004). Fifty patients undergoing allogeneic transplantation for hematological disorders were evaluated. After evaluating the constituents of the grafts, as well as the type and number of DC in the peripheral blood early after engraftment, they noted that patients with lower absolute numbers of DC (<4.97 cells/μL) at engraftment had worse clinical outcomes compared to patients with higher numbers of DC at engraftment, with lower overall survival (p = 0.002), increased incidence of relapse (p = 0.002), and a higher incidence of acute GVHD (p = 0.0005). Multivariable analysis confirmed that low DC count was independently associated with death (hazard ratio [HR], 3.8; p = 0.02), time to relapse (HR, 11.6; p = 0.001), and acute GVHD (HR, 3.3; p = 0.04). The effect was similar when DC1 were analyzed separately. However, when DC2 were analyzed separately, the effect was only significant for increased incidence of acute GVHD among patients with lower numbers of DC2 at engraftment. The independent effect of DC1 and DC2 was not confirmed in the multivariate analysis (CitationReddy et al 2004).
Subsequently, Lonial and colleagues hypothesized that the combination of G-CSF and GM-CSF administered for the mobilization of stem cells after chemotherapy would reduce the content of DC2 in the autologous blood hematopoietic progenitor cell grafts compared with administration of G-CSF alone after chemotherapy (CitationLonial et al 2004). They randomized 35 patients with lymphoma and multiple myeloma to receive either G-CSF or the combination of G-CSF plus GM-CSF after chemotherapy. Blood hematopoietic progenitor cell grafts were collected by large volume apheresis. They found a similar incidence of cytokine-related adverse events, and similar numbers of stem cells mobilized between the 2 treatment groups. There were minor differences with respect to the content of T cells in the apheresis products. However, grafts mobilized with the combination of GM-CSF plus G-CSF had significantly fewer DC2 and similar numbers of DC1 compared with grafts mobilized with G-CSF alone. A third cohort of patients received G-CSF and the sequential administration of GM-CSF 6 days later. Grafts from these patients had a markedly decreased DC2 content compared with grafts mobilized from patients treated with G-CSF alone or with the simultaneous administration of both cytokines. This preliminary trial formed the foundation for a randomized clinical trial where normal volunteer donors were randomized to receive either G-CSF or G-CSF plus GM-CSF in order to evaluate the impact of these cytokines on DC content, T-cell polarization, and immune function after allogeneic transplantation. Fifty patients were enrolled in the trial with 25 in the GM+G-CSF and 25 in the G-CSF alone arm. All patients were successfully mobilized. Among donors mobilized with G-CSF alone, the mean number of collections was 1.48 compared with 1.08 in the group receiving the combination of GM+G-CSF (p = 0.01). There was a trend toward a higher total cell dose in the G-CSF arm (p = 0.09). Two of the 25 donors in the G/GM group required more than 1 apheresis, and 8 of 25 donors in the G-CSF alone group required more than 1 collection to achieve an adequate number of CD34+ cells. Analysis of the T-cell and T-cell subset data revealed that in the group receiving G-CSF alone, there was a significantly higher percent and total T-cell, CD4+ and CD8+ cell content of the grafts when compared with the group receiving the GM+G-CSF combination. Among dendritic cell subsets in the grafts, there was a significantly lower percentage and fewer absolute numbers of DC2, as well as a lower delivered DC2 dose/kg for the group randomized to receive GM-CSF plus G-CSF compared with the group randomized to receive G-CSF alone (p < 0.001). There was no significant difference in the DC1 content or the content of CD34+ cells between the 2 treatment arms. Proliferation of the graft in response to T and B-cell mitogens was measured on the graft itself. Cells were exposed to mitogens or control for 72 hours, and then thymidine incorporation was measured. So far, there are available data on mitogen stimulation for 32 patients, showing a trend toward more IL-12 secretion for G+GM-CSF mobilized grafts, and more IL-2 secretion for G-CSF mobilized grafts. There have been no differences in the incidence of GvHD, relapse or survival between the 2 cytokine arms to date (CitationLonial et al 2004; CitationLonial et al 2006).
These data, and data indicating that cross-presentation of antigen by DC2 may induce antigen-specific tolerance by T cells, suggest that the addition of GM-CSF to regimens during mobilization of peripheral blood progenitor cell grafts may be a clinically applicable strategy to enhance innate and acquired immunity after peripheral blood progenitor cell transplantation (CitationKuwana et al 2001; CitationGilliet and Liu 2002). Larger clinical trials are needed to determine the exact consequences of altering the DC1 and DC2 content of peripheral blood hematopoietic progenitor cell grafts. Those effects may include: incidence of acute and chronic GVHD, engraftment, graft rejection, graft versus leukemia effect and response to infection.
Role of GM-CSF in post transplant immune reconstitution
Recent data on the differential effects of GM-CSF and G-CSF on the DC subsets in the graft, has inspired clinical studies to investigate whether the administration of these cytokines following autologous hematopoietic stem cell transplantation may influence the post-transplant reconstitution of cellular immunity. Fattorossi and colleagues conducted a randomized, prospective clinical trial to test for differences in immune recovery among 39 patients with ovarian and breast cancer who received either G-CSF or GM-CSF after high dose myeloablative chemotherapy and autologous transplantation. At day 12, GM-CSF was more efficient at up-regulating membrane molecules on phagocytic cells important for antibody-dependent cytotoxicity and for the uptake of immune complexes compared to treatment with G-CSF; and at day 80, a significantly higher proportion of mitogen-stimulated T cells from GM-CSF-treated patients expressed interleukin-2 receptor, and a higher proportion of these T cells were actively proliferating (CitationFattorossi et al 2001). Recently, Gazitt and colleagues showed that among 29 non-Hodgkin’s lymphoma (NHL) patients receiving cyclophosphamide plus GM-CSF, G-CSF or GM-CSF followed by G-CSF for stem cell collection, patients mobilized with the GM-CSF containing regimens mobilized higher numbers of DC, and had a higher probability of survival compared to patients receiving G-CSF alone (median of 55 months versus 15 months; p = 0.02). Of note, there was no difference in the ratio of DC1:DC2 between CSF regimens. This finding supports the hypothesis that higher numbers of DCs in the graft might be associated with prolonged survival of NHL patients after autologous transplantation (CitationGazitt et al 2006). Further studies in larger populations of patients are merited. More is known about the effects of G-CSF than the effects of GM-CSF on post-transplant immune reconstitution. Volpi and colleagues reported on the effects of G-CSF administration in the post-transplant setting among 43 patients with acute leukemia who received T-cell depleted peripheral blood progenitor cell transplants from HLA haploidentical related donors, compared to a cohort of 36 patients with acute leukemia who did not receive G-CSF after transplantation. They found significantly delayed recovery of Th1-type CD4+ T-cells (low IL-4 and IL-10, and high IL-12 receptor expression), a higher proportion of CD4+ T-cells with a Th2 phenotype (high levels of IL-4 and IL-10, and low levels of IL-12 receptor expression), and impaired production of IL-12 by dendritic cells, compared to patients who did not receive post-transplant G-CSF. T-cells from recipients of post-transplant G-CSF had significantly decreased in-vitro reactivity to fungal pathogens compared to T-cells from patients who did not receive post-transplant G-CSF. This finding suggests an increased susceptibility to opportunistic infections in the G-CSF treated cohort, given that Th1-responses are necessary for anti-fungal protection (CitationVolpi et al 2001). The effect of G-CSF on post-transplant immune reconstitution appeared to be dependent on G-CSF’s influence on DC maturation and differentiation; given that administration of G-CSF following transplantation favored the appearance of IL-12-deficient DC which polarize T-cells toward Th2 responses. Fagnoni reported a similar effect of post-transplant G-CSF in children (CitationFagnoni et al 2004). Ringden et al performed a retrospective analysis to determine the role of post-transplant treatment with G-CSF in patients with AML and acute lymphocytic leukemia (ALL) who received allogeneic BMT or peripheral blood grafts. They found that prophylactic, post-transplant treatment with G-CSF led to a higher risk of acute and chronic GVHD, higher transplant related mortality, and decreased overall survival and leukemia-free survival rates in patients who received BMT only. Post-transplant G-CSF led to faster engraftment of absolute neutrophil count but slower engraftment of platelets in transplant recipients irrespective of the type of graft (CitationRingden et al 2004). These findings suggest that post-transplant administration of G-CSF may cause an imbalance in dendritic cell content or function, resulting in impaired cellular immunity in the early post-transplant period. This may lead to an increase in the incidence of GVHD (CitationFagnoni et al 2004). Current guidelines support the use of colony stimulating factors for mobilization of autologous and allogeneic grafts and after peripheral blood progenitor cell transplantation in the autologous setting only (CitationSmith et al 2006). Further studies are needed to support the addition of GM-CSF after allogeneic transplantation.
GM-CSF may improve antibody-dependent cell-mediated cytotoxicity
The anti-CD20 antibody, rituximab, used alone or in combination with chemotherapy, is an established treatment for non-Hodgkin’s lymphoma (NHL) (CitationCvetkovic and Perry 2006). Augmenting the expression of CD20 antigen on the tumor cells may increase the cell kill and therefore increase the effectiveness of the antibody (CitationVenugopal et al 2000). Preliminary data suggest that GM-CSF can up-regulate the CD20 expression on lymphoid B cells in vitro, but these results have not been reproducible in vitro nor in vivo (CitationVenugopal et al 2000; CitationChow et al 2001; CitationYagci et al 2005).
Venugopal and colleagues performed experiments on cells from patients with chronic lymphocytic leukemia (CLL) where CLL cells were cultured with cytokines and the expression of CD 20 on the surface of the CLL cells was measured before and after cytokine exposure. They found a statistically significant up-regulation of CD20 antigen expression on CLL cells after culture with GM-CSF, IL-4, or TNF-alpha. Flow cytometry evaluation revealed an increase in fluorescence intensity as well in the percentage of cells expressing the antigen (CitationVenugopal et al 2000). This led to further studies which have revealed promising, but inconsistent results. Olivieri and his group showed the feasibility of rescuing patients with NHL relapsing after autologous transplantation with a regimen containing rituximab, CHOP chemotherapy and GM-CSF. They reported a 75% overall response rate (60% complete remission, and 15% partial response) among 20 patients with aggressive NHL who relapsed after autologous transplantation (CitationOlivieri et al 2005). Rapoport and colleagues reported promising results utilizing post-transplant rituximab and GM-CSF after autologous transplantation among a group of patients with advanced NHL and Hodgkin’s disease (CitationRapoport et al 2002). However neither up-regulation of CD20 antigen, nor a change of the proportion of CD20 positive cells was observed after culture with GM-CSF in a study by CitationYagci et al (2005) on cells from 18 patients with CLL.
GM-CSF may augment the graft versus leukemia effect of allogeneic transplantation
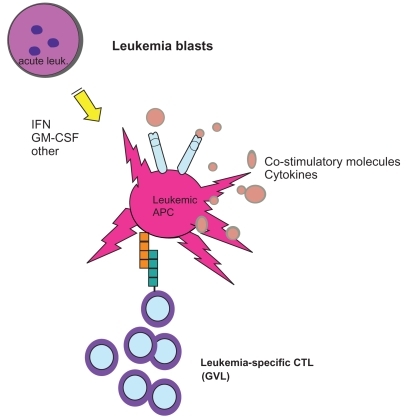
Relapse of acute leukemia after allogeneic transplantation remains a significant therapeutic challenge, affecting approximately one third of all patients with acute leukemia who receive allogeneic transplantation as a curative therapy. Salvage post-transplant maneuvers have focused on utilization of second transplants, but these are limited to a minority (10% in most series) of fit patients. Clinical and pre-clinical data has suggested a role for cytokine therapy in the induction of graft versus leukemia effects in the setting of post-transplant relapse (CitationSlavin et al 1996; CitationCortes et al 1998; CitationBoyer et al 2000; CitationMohty et al 2002; CitationKolb et al 2004; CitationLi and Waller 2004). Improving the antigen-presenting capacity of leukemic blasts may lead to clinically-significant anti-leukemic effects. The feasibility of generating DC-like leukemic antigen presenting cells upon treatment with cytokines, including GM-CSF has been demonstrated (CitationSantiago-Schwarz et al 1994; CitationMohty et al 2002). The level of co-stimulatory molecule expression on leukemic blasts has been hypothesized to play a role in the capacity of leukemic blasts to present antigen to effector cells (CitationVereecque et al 2000; CitationWhiteway et al 2003). A retrospective study at our institution reviewing the treatment of acute leukemia relapsed after allogeneic transplantation revealed promising results among a minority of patients treated with GM-CSF and interferon-alpha-2b (CitationArellano et al 2007). A prospective clinical trial at our center is currently investigating the feasibility and activity of a regimen utilizing the combination of GM-CSF and interferon-alpha-2b after cytoreduction to treat acute leukemia relapsed after allogeneic transplantation. Correlative studies will test the hypothesis that GM-CSF and interferon-alpha-2b act by up-regulating co-stimulatory molecules on leukemic blasts, and down-regulating regulatory T-cells leading to improved antigen presentation and durable graft versus leukemia effects ().
Figure 1 GM-CSF and IFN may upregulate co-stimulatory molecule expression on leukemic blasts leading to stimulation of cytotoxic T-cells and a GVL effect.
GM-CSF for the treatment of solid tumors
Role of GM-CSF in the management of renal cell carcinoma
Renal cell carcinoma (RCC) is known to be an immunogenic tumor. Interferon-alpha (IFN-alpha) has been established as the standard treatment for metastatic RCC with response rates ranging between 10 and 20%. High dose interleukin-2 has yielded similar results, but its use has been limited by significant toxicities (CitationCoppin et al 2005). Previous observations have indicated that GM-CSF can potentiate the effect of IL-2 on T-cell activation (CitationMasucci et al 1990; CitationGroenewegen and de Gast 1999). Subsequently multiple trials have tested the activity of GM-CSF combinations for RCC with modest results. summarizes activity and toxicities of GM-CSF in the treatment of renal cell carcinoma. Verra and colleagues studied the effect of simultaneous administration of low dose IL-2, IFN-alpha and GM-CSF in metastatic RCC in a phase I study and, subsequently in a multicenter phase II study, showing tolerability and promising efficacy with 19% overall responses (9% CR, 10% PR) (CitationDe Gast et al 2000; CitationVerra et al 2003). Recently, the same group conducted a phase I study of peri-operative low-dose IL-2, IFN-alpha, and GM-CSF in resectable RCC. In addition to determining the maximum tolerated dose of the cytokine combination in the peri-operative setting, the investigators studied the effects of the cytokines in the peripheral blood and at the tumor site. They found higher numbers of tumor-infiltrating T-cells and mature DC1 in tumors resected from patients who received peri-operative cytokines, compared to a control group of tumors resected from non-cytokine treated patients (CitationVerra et al 2005). The advent of targeted therapy for RCC has marked a new paradigm in the treatment of this tumor (CitationMotzer and Bukowski 2006), and further studies may find a role for combining cytokines with the newer targeted agents.
Table 1 Recent trials using GM-CSF in patients with renal cell carcinoma
Role of GM-CSF in the management of malignant melanoma
Unresectable melanoma carries a poor prognosis with limited options for treatment (CitationParmiani et al 2007). GM-CSF has been shown to induce cytotoxic T-cells and activated DC at tumor sites and draining lymph nodes (CitationParmiani et al 2007). The use of GM-CSF in combination with IL-2 or IFN-alpha has yielded promising results, but is associated with significant systemic toxicity. summarizes activity and toxicities of GM-CSF-containing regimens in the treatment of malignant melanoma. Delivery of therapy into local sites of disease may circumvent systemic toxicity and is the subject of current investigation.
Table 2 GM-CSF-containing regimens in patients with malignant melanoma
GM-CSF and cancer vaccine development
Addressing questions pertaining to the choice of vector, the specificity of the antigen, and the choice of co-stimulatory molecules is crucial to the optimal development of cancer vaccines. Tumor-associated antigens from tumor cells (both autologous and allogeneic), proteins, peptides, and nucleic acid have been used as immunogens. Genetically modified allogeneic tumor cells as well as recombinant viruses or bacterial genes have been utilized as vectors. Pre-existing immunity to the vector itself has limited the use of vaccines based on viral vectors (CitationRosenberg et al 1998). Vaccination in the absence of the co-stimulatory signals necessary to break tolerance can lead to anergy. Therefore, some vectors have been designed to express not only tumor-associated antigens, but also, co-stimulatory molecules and cytokines. CitationDranoff et al (1993) introduced GM-CSF as an important adjuvant in cancer vaccine trials, based on his observations that irradiated tumor cells expressing murine GM-CSF could stimulate potent, long-lasting, and tumor-specific immunity. In order to circumvent systemic toxicity and to increase immune responses, injection into the local tumor environment has been proposed. Hersch and colleagues used intra-tumor injection of HLA-B7/beta2-microglobulin genes as plasmid DNA in lipid into patients with malignant melanoma. In a phase I trial setting, they reported a 36% response at the locally injected tumor and a 19% systemic anti-tumor response (CitationHersh and Stopeck 1997).Vaccine trials utilizing GM-CSF or engineering tumor cells to secrete GM-CSF showed encouraging results in the treatment of solid tumors including: malignant melanoma, breast carcinoma, pancreatic cancer, renal cell carcinoma, non-small cell carcinoma of the lung and prostate cancer (CitationSchmidt et al 1997; CitationSimons et al 1997; CitationHung et al 1998; CitationSoiffer et al 1998; CitationDisis et al 1999; CitationGaudernack and Gjertsen 1999; CitationLeong et al 1999). Cassaday and colleagues performed a phase I study of immunization using particle-mediated epidermal delivery (PMED) of genes for gp100 and GM-CSF into uninvolved skin of melanoma patients. Two groups of 6 patients each were treated; group 1 received PMED with cDNA for gp100, and group 2 received PMED with cDNA for GM-CSF followed by PMED for gp100 at the same site. Biopsies were obtained and evaluated to assess transgene expression, gold-bead penetration, and dendritic cell infiltration. Exploratory studies included flow cytometric analyses of peripheral blood lymphocytes and evaluation of delayed-type hypersensitivity to gp100 peptide in HLA-A2 + patients. Local toxicity in both groups was mild and resolved within 2 weeks. No vaccine-related systemic toxicity was reported, including no induction of pathologic auto-antibodies. GM-CSF transgene expression in vaccinated skin sites was detected. GM-CSF and gp100 PMED yielded a greater infiltration of DC into vaccine sites than did gp100 PMED alone. Immunologic monitoring suggested modest activation of an anti-melanoma response (CitationCassaday et al 2007). This study demonstrated tolerability and induction of anti-melanoma immune responses with a local approach. Additional investigation utilizing this technique is warranted. Bendandi and colleagues tested the hypothesis that immune therapy is more effective in the setting of minimal residual disease (MRD). They documented clearance of the t(14,18) translocation by PCR from the peripheral circulation in 8 of 11 patients with lymphoma and MRD, after administration of a GM-CSF containing vaccine (CitationBendandi et al 1999). Currently our center participates in a multi-institutional trial of vaccine therapy for AML after remission induction. GM-CSF may enhance antigen presentation by recruiting DC to the (vaccine) site where antigen is taken up, processed, and presented to T-cells in draining lymph nodes, generating systemic tumor-specific responses (CitationBorrello and Pardoll 2002).
Cell-mediated vaccines: role of dendritic cells
DC are ideal candidates for use in vaccination, owing to their role in antigen presentation. DC can be isolated from the peripheral circulation by FACS sorting or magnetic bead isolation, or can be generated in large quantities ex-vivo from peripheral blood progenitors in media supplemented with cytokines, including GM-CSF (CitationBerthier et al 2000). These DC may then be matured with cytokine culture prior to loading with antigen. They have yielded promising results and continue to be tested in the treatment of solid and hematological malignancies. Results from phase 1 and 2 clinical trials indicate that tumor-peptide loaded DC can induce clinically significant immune responses in patients with lymphoma and melanoma (CitationHsu et al 1996; CitationHersey et al 2004). Antigen-loaded DC as cancer vaccines have been limited by uncertainty regarding the best DC subtype to use, the optimal maturation status of the DC, the best site of administration (sub-cutaneous, intravenous, or intra-nodal) and the optimal schedule of administration. More research is needed in order to answer these questions and define the optimal use of GM-CSF as an adjuvant in cell-mediated vaccines.
Table 3 Clinical trials using GM-CSF transduced tumor cells as vaccines
Future directions
Pre-clinical and clinical data support the role of GM-CSF as an immune adjuvant in the treatment of malignant solid and liquid tumors, but the challenge remains to devise combinations of cytotoxic and cytokine therapy which are synergistic in breaking immune tolerance, enhancing antigen presentation and up-regulating anti-tumor T-cell responses. Local production of GM-CSF by tumors in the setting of tumor-specific vaccination has shown promise in the induction of anti-tumor immune responses. However, the laboratory correlates of response; such as, the lymphocytic and inflammatory infiltrates that develop at the site of vaccination and cytokine injection, do not reproducibly correlate with improved clinical outcomes, and well designed translational studies are needed to better define the anti-tumor activity of GM-CSF and other cytokines. Vaccines and cytokine therapies are attractive for use in patients who cannot tolerate further cytotoxic chemotherapy, owing to their relatively low toxicity, and in patients whose tumors are in a minimal residual disease state.
The success of cytokine therapy will likely depend upon defining the most favorable combination of cytokines, the optimal site and route of administration, reaching a MRD status prior to cytokine therapy, and development of surrogate endpoints of anti-tumor activity that can be used to design subsequent clinical trials.
Disclosures
MA receives research support from Bayer Healthcare. SL has served as a consultant for, and received research support from, Berlex (now known as Bayer Healthcare).
References
- AmsenDBlanderJMLeeGR2004Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cellsCell1175152615137944
- ArellanoMLLangstonAWintonE2007Treatment of relapsed acute leukemia after allogeneic transplantation: a single center experienceBiol Blood Marrow Transplant131162317222760
- ArpinatiMGreenCLHeimfeldS2000Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cellsBlood9524849010753825
- BalkwillFMantovaniA2001Inflammation and cancer: back to Virchow?Lancet3575394511229684
- BancereauJSteinmanRM1998Dendritic cells and the control of immunityNature392245529521319
- BanchereauJBriereFCauxC2000Immunobiology of dendritic cellsAnn Rev Immunol1876781110837075
- Basak SarojKHaruiAStolinaM2002Increased dendritic cell number and function following continuous in vivo infusion of granulocyte macrophage-colony-stimulating factor and interleukin-4Blood9928697911929777
- BendandiMGockeCDKobrinCB1999Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphomaNat Med51171710502821
- BensingerWIMartinPJStorerB2001Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. [see comments]N Engl J Med3441758111172139
- BerthierRMartinon-EgoCLaharieAM2000A two-step culture method starting with early growth factors permits enhanced production of functional dendritic cells from murine splenocytesJ Immunol Methods2399510710821951
- BorrelloIPardollD2002GM-CSF-based cellular vaccines: a review of the clinical experienceCytokine Growth Factor Rev131859311900993
- BoyerMWWallerEKBrayRA2000Cytokine upregulation of the antigen presenting function of acute myeloid leukemia cellsLeukemia14412810720135
- CashenAFLinkDDevineS2004Cytokines and stem cell mobilization for autologous and allogeneic transplantationCurr Hematol Rep34061215496273
- CassadayRDSondelPMKingDM2007A phase I study of immunization using particle-mediated epidermal delivery of genes for gp100 and GM-CSF into uninvolved skin of melanoma patientsClin Cancer Res13540917255276
- ChowKUSchneiderBMitrouPS2001Influence of various cytokines on the expression of CD20 on the surface of CLL-cells in vitroLeuk Res259910011203684
- CocciaEMSeveraMGiacominiE2004Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cellsEur J Immunol3479680514991609
- CoppinCPorzsoltFAwaA2005Immunotherapy for advanced renal cell cancerCochrane Database Syst RevCD00142515674877
- CorrealePMarsiliSSabatinoM2005Immunotherapy of renal cell carcinoma with granulocyte macrophage colony stimulating factor and very low dose interleukin-2Oncol Rep13751615756453
- CortesJKantarjianHO’brienS1998GM-CSF can improve the cytogenetic response obtained with interferon-alpha therapy in patients with chronic myelogenous leukemiaLeukemia1286049639411
- CuencaAChengFWangH2003Extra-lymphatic solid tumor growth is not immunologically ignored and results in early induction of antigen-specific t-cell anergy: dominant role of cross-tolerance to tumor antigensCancer Res639007901514695219
- CvetkovicRSPerryCM2006Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemiaDrugs6679182016706552
- DavidsonJAMuskAWWoodBR1998Intralesional cytokine therapy in cancer: a pilot study of GM-CSF infusion in mesotheliomaJ Immunother21389989789201
- De GastGCBatchelorDKerstenMJ2003Temozolomide followed by combined immunotherapy with GM-CSF, low-dose IL2 and IFN alpha in patients with metastatic melanomaBritish Journal of Cancer881758012610499
- De GastGCKlumpenHJVyth-DreeseFA2000Phase I trial of combined immunotherapy with subcutaneous granulocyte macrophage colony-stimulating factor, low-dose interleukin 2, and interferon alpha in progressive metastatic melanoma and renal cell carcinomaClin Cancer Res612677210778950
- DillonSAgrawalAVan DykeT2004A toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cellsJ Immunol17247334315067049
- DisisMLGrabsteinKHSleathPR1999Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccineClin Cancer Res51289129710389911
- DranoffGCrawfordADSadelainM1994Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasisScience26471368171324
- DranoffGJaffeeELazenbyA1993Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunityProc Natl Acad Sci, USA903539438097319
- EksiogluEAMahmoodSSChangM2007GM-CSF promotes differentiation of human dendritic cells and T lymphocytes toward a predominantly type 1 proinflammatory responseExp Hematol3511637117562355
- EnzlerTGillessenSManisJP2003Deficiencies of GM-CSF and interferon gamma link inflammation and cancerJ Exp Med1971213912732663
- FagnoniFFOlivieroBGiorgianiG2004Reconstitution dynamics of plasmacytoid and myeloid dendritic cell precursors after allogeneic myeloablative hematopoietic stem cell transplantationBlood104281915010368
- FantuzziG2003Cytokine KnockoutsNew JerseyHumana Press
- FattorossiABattagliaAPierelliL2001Effects of granulocyte-colony-stimulating factor and granulocyte/macrophage-colony-stimulating factor administration on T-cell proliferation and phagocyte cell-surface molecules during hematopoietic reconstitution after autologous peripheral blood progenitor cell transplantationCancer Immunol Immunother49641811258790
- FerlazzoGKleinJPaliardX2000Dendritic cells generated from CD34+ progenitor cells with flt3 ligand, c-kit ligand, GM-CSF, IL4, and TNF-alpha are functionalantigen-presneting cells resembling monocyte-derived dendritic cellsJournal of Immunotherapy23485810687137
- FonteneauJFGillietMLarssonM2003Activation of influenza virus-specific CD4+ and CD8+ T-cells: a new role for plasmacytoid dendritic cells in adaptive immunityBlood1013520352612511409
- GajewskiTFlickingerS2000A Phase II study of outpatient chemoimmunotherapy using cisplatin and DTIC followed by GM-CSF, IL-2, and IFN-alpha-2b in patients with metastatic melanomaProc Am Soc Clin Oncol19 (abstr 2271)
- Garcia-CarboneroRMayordomoJITornamiraMV2001Granulocyte colony-stimulating factor in the treatment of high-risk febrile neutropenia: a multicenter randomized trialJ Natl Cancer Inst93313811136839
- GasparettoCGasparettoMMorseM2002Mobilization of dendritic cells from patients with breast cancer into peripheral blood stem cell leukapheresis samples using Flt-3-ligand and G-CSF or GM-CSFCytokine1881912090755
- GaudernackGGjertsenMK1999Combination of GM-CSF with antitumour vaccine strategiesEur J Cancer35S33510645220
- GazittYAkayCThomasC3rd2006No polarization of type 1 or type 2 precursor dendritic cells in peripheral blood stem cell collections of non-hodgkin’s lymphoma patients mobilized with cyclophosphamide plus G-CSF, GM-CSF, or GM-CSF followed by G-CSFStem Cells Dev152697716646673
- GibbsPO’DaySRichardsJ2000A multicenter phase ii study of modified biochemotherapy (BCT) for stage IV melanoma incorporating temozolomide, decrescendo interleukin-2 (IL-2) and GM-CSFProc Am Soc Clin Oncol (abstr 2255)19
- GillietMLiuYJ2002Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cellsJ Exp Med19569570411901196
- GroenewegenGBloemADe GastGC2002Phase I/II study of sequential chemoimmunotherapy (SCIT) for metastatic melanoma: outpatient treatment with dacarbazine, granulocyte-macrophage colony-stimulating factor, low-dose interleukin-2, and interferon-alphaCancer Immunol Immunother51630612439608
- GroenewegenGDe GastGC1999GM-CSF can cause T cell activation; results of sequential chemo-immunotherapyEur J Cancer35Suppl 3S23410645217
- GrouardGRissoanMCFilgueiraL1997The enigmatic plasma-cytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligandJ Exp Med1851101119091583
- HartDN1997Dendritic cells: unique leukocyte populations which control the primary immune responseBlood903245879345009
- HerseyPMenziesSWHallidayGM2004Phase I/II study of treatment with dendritic cell vaccines in patients with disseminated melanomaCancer Immunol Immunother531253414600790
- HershEMStopeckAT1997Advances in the biological therapy and gene therapy of malignant diseaseClin Cancer Res32623910068264
- Hoption CannSAVan NettenJPVan NettenC2002Spontaneous regression: a hidden treasure buried in timeMed Hypotheses581151911812185
- HottonKMKhorsandMHankJA2000A phase Ib/II trial of granulocyte-macrophage-colony stimulating factor and interleukin-2 for renal cell carcinoma patients with pulmonary metastases: a case of fatal central nervous system thrombosisCancer88189290110760767
- HsuFJBenikeCFagnoniF1996Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cellsNat Med25288564842
- HungKHayashiRLafond-WalkerA1998The central role of CD4(+) T cells in the antitumor immune responseJ Exp Med1882357689858522
- InabaKInabaMRomaniN1992Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factorJ Exp Med17616937021460426
- JaffeeEMHrubanRHBiedrzyckiB2001Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activationJ Clin Oncol191455611134207
- JanikJEMillerLLKornEL2001A prospective randomized phase II trial of GM-CSF priming to prevent topotecan-induced neutropenia in chemotherapy-naive patients with malignant melanoma or renal cell carcinomaBlood971942611264156
- KadowakiNHoSAntonenkoS2001Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigensJ Exp Med194863911561001
- KeilholzUWeberJFinkeJH2002Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the Society for Biological TherapyJ Immunother259713812074049
- KessingerAArmitageJOLandmarkJD1986Reconstitution of human hematopoietic function with autologous cryopreserved circulating stem cellsExp Hematol1419262868909
- KolbHJRankAChenX2004In-vivo generation of leukaemia-derived dendritic cellsBest Pract Res Clin Haematol174395115498715
- KorblingMAnderliniP2001Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter?Blood982900811698269
- KorblingMBurkePBraineH1981Successful engraftment of blood derived normal hemopoietic stem cells in chronic myelogenous leukemiaExp Hematol9684906114872
- KusumotoMUmedaSIkuboA2001Phase 1 clinical trial of irradiated autologous melanoma cells adenovirally transduced with human GM-CSF geneCancer Immunol Immunother503738111676397
- KuwanaMKaburakiJWrightTM2001Induction of antigen-specific human CD4(+) T cell anergy by peripheral blood DC2 precursorsEur J Immunol3125475711536152
- LarssonMFonteneauJFBhardwajN2003Cross-presentation of cell-associated antigens by dendritic cellsCurr Top Microbiol Immunol2762617512797452
- LeongSPEnders-ZohrPZhouYM1999Recombinant human granulocyte macrophage-colony stimulating factor (rhGM-CSF) and autologous melanoma vaccine mediate tumor regression in patients with metastatic melanomaJ Immunother221667410093041
- LevineAMReedJAKurakKE1999GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infectionJ Clin Invest103563910021465
- LiJMWallerEK2004Donor antigen-presenting cells regulate T-cell expansion and antitumor activity after allogeneic bone marrow transplantationBiol Blood Marrow Transplant105405115282532
- LieschkeGJGrailDHodgsonG1994Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilizationBlood841737467521686
- LissoniPMengoSBucovecR2003Clinical and biological effects of interleukin-2 with or without a concomitant administration of granulocyte-macrophage colony-stimulating factor in metastatic cancer patientsIn Vivo1773512655794
- LonialSHicksMRosenthalH2004A randomized trial comparing the combination of granulocyte-macrophage colony-stimulating factor plus granulocyte colony-stimulating factor versus granulocyte colony-stimulating factor for mobilization of dendritic cell subsets in hematopoietic progenitor cell productsBiol Blood Marrow Transplant108485715570253
- LonialSMcmillanSTorreC2006A randomized trial to evaluate the impact of cytokines (G-CSF or GM + G-CSF) on dendritic cell and T-cell content and function when mobilizing normal donors for allogeneic progenitor cell transplantBlood108 (abstr 3377)
- LutzkyJBlausteinAWeingradD2003Phase I/II study of GM-CSF and thalidomide in patients with high-risk malignant melanomaProc Am Soc Clin Oncol22 (abstr 2916)
- MasucciGRagnhammarPWersallP1990Granulocyte-monocyte colony-stimulating-factor augments the interleukin-2-induced cytotoxic activity of human lymphocytes in the absence and presence of mouse or chimeric monoclonal antibodies (mAb 17-1A)Cancer Immunol Immunother3123152199042
- MatzingerP1994Tolerance, Danger, and the Extended FamilyAnn Rev Immunol1299110458011301
- MetcalfD1998Cell-cell signalling in the regulation of blood cell formation and functionImmunol Cell Biol7644179797465
- MetcalfDNicolaNA1995The hematopoietic colony stimulating factorsNew York, NYCambridge University Press
- MizutaniKTakeuchiSOhashiY2003Clinical usefulness of macrophage colony-stimulating factor for ovarian cancers: Long-term prognosis after five yearsOncol Rep101273112469157
- MohtyMOliveDGauglerB2002Leukemic dendritic cells: potential for therapy and insights towards immune escape by leukemic blastsLeukemia16219720412399962
- MotzerRJBukowskiRM2006Targeted therapy for metastatic renal cell carcinomaJ Clin Oncol245601817158546
- NautsHCFowlerGABogatkoFH1953A review of the influence of bacterial infection and of bacterial products (Coley’s toxins) on malignant tumors in man; a critical analysis of 30 inoperable cases treated by Coley’s mixed toxins, in which diagnosis was confirmed by microscopic examination selected for special studyActa Med Scand Suppl44110313039964
- NelsonWGSimonsJWMikhakB2000Cancer cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer as vaccines for the treatment of genitourinary malignanciesCancer Chemother Pharmacol46SupplS677210950151
- OlivieriALucesoleMCapelliD2005A new schedule of CHOP/rituximab plus granulocyte-macrophage colony-stimulating factor is an effective rescue for patients with aggressive lymphoma failing autologous stem cell transplantationBiol Blood Marrow Transplant116273616041313
- OlweusJBitmansourAWarnkeR1997Dendritic cell ontogeny: a human dendritic cell lineage of myeloid originProc Natl Acad Sci, USA941255169356487
- PaineR3rdMorrisSBJinH2001Impaired functional activity of alveolar macrophages from GM-CSF-deficient miceAm J Physiol Lung Cell Mol Physiol281L1210811597913
- ParmianiGCastelliCSantinamiM2007Melanoma immunology: past, present and futureCurr Opin Oncol19121717272984
- PerrotIBlanchardDFreymondN2007Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stageJ Immunol1782763917312119
- RapoportAPMeisenbergBSarkodee-AdooC2002Autotrans-plantation for advanced lymphoma and Hodgkin’s disease followed by post-transplant rituxan/GM-CSF or radiotherapy and consolidation chemotherapyBone Marrow Transplant293031211896427
- RavaudADelaunayMChevreauC2001Granulocyte-macrophage colony-stimulating factor alone or with dacarbazine in metastatic melanoma: a randomized phase II trialBr J Cancer8514677111720430
- ReddyVIturraspeJATzolasAC2004Low dendritic cell count after allogeneic hematopoietic stem cell transplantation predicts relapse, death, and acute graft-versus-host diseaseBlood1034330514962904
- RepettoLBiganzoliLKoehneCH2003EORTC Cancer in the Elderly Task Force guidelines for the use of colony-stimulating factors in elderly patients with cancerEuropean Journal of Cancer3922647214556916
- RingdenOLabopinMGorinNC2004Treatment with granulocyte colony-stimulating factor after allogeneic bone marrow transplantation for acute leukemia increases the risk of graft-versus-host disease and death: a study from the acute leukemia working party of the European Group for Blood and Marrow TransplantationJ Clin Oncol224162314691124
- RissoanMCSoumelisVKadowakiN1999Reciprocal control of T helper cell and dendritic cell differentiationScience2831183610024247
- RosenbergSAZhaiYYangJC1998Immunizing patients with meta-static melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigensJ Natl Cancer Inst90189419009862627
- RoweJMAndersenJWMazzaJJ1995A randomized placebo-controlled phase III study of granulocyte-macrophage colony-stimulating factor in adult patients (>55 to 70 years of age) with acute myelogenous leukemia: a study of the Eastern Cooperative Oncology Group (E1490)Blood86457627605984
- RyanCWVogelzangNJDumasMC2000Granulocyte-macrophage-colony stimulating factor in combination immunotherapy for patients with metastatic renal cell carcinoma: results of two phase II clinical trialsCancer8813172410717612
- SalgiaRLynchTSkarinA2003Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinomaJ Clin Oncol216243012586798
- Santiago-SchwarzFBelilosEDiamondB1992TNF in combination with GM-CSF enhances the differentiation of neonatal cord blood stem cells into dendritic cells and macrophagesJ Leukoc Biol52274811387891
- Santiago-SchwarzFCoppockDLHindenburgAA1994Identification of a malignant counterpart of the monocyte-dendritic cell progenitor in an acute myeloid leukemiaBlood843054627949177
- SchachterJRakowskyESulkesA1998A sequential four-drug chemotherapy and biotherapy with interferon alpha and GM-CSF-an innovative protocol for the treatment of metastatic melanomaCancer Biother Radiopharm131556410850351
- SchmidingerMStegerGWenzelC2001Sequential administration of interferon-gamma, GM-CSF, and interleukin-2 in patients with metastatic renal cell carcinoma: results of a phase II trialJ Immunother2425762
- SchmidtWMaassGBuschleM1997Generation of effective cancer vaccines genetically engineered to secrete cytokines using adenovirus-enhanced transferrinfection (AVET)Gene190211169185869
- SchmitzNBacigalupoAHasencleverD1998Allogeneic bone marrow transplantation vs filgrastim-mobilised peripheral blood progenitor cell transplantation in patients with early leukaemia: first results of a randomised multicentre trial of the European Group for Blood and Marrow Transplantation [see comments]Bone Marrow Transplant2199510039632272
- SchmitzNBeksacMHasencleverD2002Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemiaBlood100761712130483
- SchreiberHPaulW2003Tumor ImmunologyFundamental Immunology5Lippincott, Williams, and Wilkins
- SeymourJFLieschkeGJGrailD1997Mice lacking both granulocyte colony-stimulating factor (CSF) and granulocyte-macrophage CSF have impaired reproductive capacity, perturbed neonatal granulopoiesis, lung disease, amyloidosis, and reduced long-term survivalBlood903037499376584
- SiegalFPKadowakiNShodellM1999The nature of the principal type 1 interferon-producing cells in human bloodScience2841835710364556
- SimonsJWJaffeeEMWeberCE1997Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transferCancer Res571537469108457
- SimonsJWMikhakBChangJF1999Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transferCancer Res595160810537292
- SimonsJWSacksN2006Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancerUrol Oncol244192416962494
- SlavinSNaparstekENaglerA1996Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse after allogeneic bone marrow transplantationBlood8721952048630379
- SlingluffCLJrPetroniGRYamshchikovGV2003Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cellsJ Clin Oncol2140162614581425
- Smith IiJWKurtRABaherAG2003Immune effects of escalating doses of granulocyte-macrophage colony-stimulating factor added to a fixed, low-dose, inpatient interleukin-2 regimen: a randomized phase I trial in patients with metastatic melanoma and renal cell carcinomaJ Immunother26130812616104
- SmithTJKhatcheressianJLymanGH20062006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guidelineJ Clin Oncol24318720516682719
- SoifferRHodiFSHaluskaF2003Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanomaJ Clin Oncol2133435012947071
- SoifferRLynchTMihmM1998Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanomaProc Natl Acad Sci, USA951314169789055
- StanleyELieschkeGJGrailD1994Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathologyProc Natl Acad Sci, USA91559268202532
- TateJOlenckiTFinkeJ2001Phase I trial of simultaneously administered GM-CSF and IL-6 in patients with renal-cell carcinoma: clinical and laboratory effectsAnn Oncol12655911432624
- UrbiniBArpinatiMBonifaziF2003Allogeneic graft CD34(+) cell dose correlates with dendritic cell dose and clinical outcome, but not with dendritic cell reconstitution after transplantExp Hematol319596514550812
- VaughanMMMooreJRichesPG2000GM-CSF with biochemotherapy (cisplatin, DTIC, tamoxifen, IL-2 and interferon-alpha): a phase I trial in melanomaAnn Oncol111183911061616
- VenugopalPSivaramanSHuangXK2000Effects of cytokines on CD20 antigen expression on tumor cells from patients with chronic lymphocytic leukemiaLeuk Res24411510785263
- VereecqueRBuffenoirGPreudhommeC2000Gene transfer of GM-CSF, CD80 and CD154 cDNA enhances survival in a murine model of acute leukemia with persistence of a minimal residual diseaseGene Therapy713121610918502
- VerraNDe JongDBexA2005Infiltration of activated dendritic cells and T cells in renal cell carcinoma following combined cytokine immunotherapyEur Urol485273316115526
- VerraNJansenRGroenewegenG2003Immunotherapy with concurrent subcutaneous GM-CSF, low-dose IL-2 and IFN-alpha in patients with progressive metastatic renal cell carcinomaBr J Cancer8813465112778059
- VolpiIPerruccioKTostiA2001Postgrafting administration of granulocyte colony-stimulating factor impairs functional immune recovery in recipients of human leukocyte antigen haplotype-mismatched hematopoietic transplantsBlood9725142111290617
- WallerEKRosenthalHJonesTW2001Larger numbers of CD4(bright) dendritic cells in donor bone marrow are associated with increased relapse after allogeneic bone marrow transplantationBlood9729485611342416
- WangHYWangRF2007Regulatory T cells and cancerCurrent Opinion in Immunology192172317306521
- WeberRWO’DaySRoseM2005Low-dose outpatient chemobiotherapy with temozolomide, granulocyte-macrophage colony stimulating factor, interferon-{alpha}2b, and recombinant inter-leukin-2 for the treatment of metastatic melanomaJ Clin Oncol238992900016260693
- WestermannJReichGKoppJ2001Granulocyte/macrophage-colony-stimulating-factor plus interleukin-2 plus interferon alpha in the treatment of metastatic renal cell carcinoma:a pilot studyCancer Immunol Immunother496132011225992
- WhitewayACorbettTAndersonR2003Expression of co-stimulatory molecules on acute myeloid leukaemia blasts may effect duration of first remissionBr J Haematol1204425112580958
- WosEOlenckiTTuasonL1996Phase II trial of subcutaneously administered granulocyte-macrophage colony-stimulating factor in patients with metastatic renal cell carcinomaCancer771149538635137
- YagciMAkarISucakGT2005GM-CSF does not increase CD20 antigen expression on chronic lymphocytic leukemia lymphocytesLeuk Res29735815927668
- ZhanYLieschkeGJGrailD1998Essential roles for granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF in the sustained hematopoietic response of Listeria monocytogenes-infected miceBlood9186399446646
- ZittounRSuciuSMandelliF1996Granulocyte-macrophage colony-stimulating factor associated with induction treatment of acute myelogenous leukemia:a randomized trial by the European Organization for Research and Treatment of Cancer Leukemia Cooperative GroupJ Clin Oncol14215098683249