Abstract
Lapatinib is an oral, reversible, dual inhibitor of epidermal growth factor receptor ErbB1 (EGFR) and human epidermal growth factor receptor type 2 ErbB2 (HER2). Results of a phase III study comparing lapatinib plus capecitabine with capecitabine alone in women with ErbB2-overexpressing advanced breast cancer previously treated with an anthracycline, a taxane, and trastuzumab were reported early based on superiority of the combination in prolonging time to tumor progression (TTP). An updated analysis in 399 women supports the earlier findings. In this updated analysis, TTP (hazard ratio [HR] 0.57) favored lapatinib plus capecitabine. Survival trended in favor of the combination. The incidence of cardiac events was numerically higher in the combination arm (5 cases in the combination arm, 2 cases in the monotherapy arm).
Keywords:
Introduction
Despite substantial advances in the treatment of breast cancer, it remains the most common cause of death in women 45–55 years old (CitationAmerican Cancer Society 2007). While only 10% of cases are advanced at the time of diagnosis, after primary treatment with curative intent, 10%–20% of patients will have local recurrence within 9 years, and 10%–25% will have locally extensive or metastatic disease (CitationHeimann and Hellman 2000; CitationESMO Guidelines Working Group 2007).
Treatment of advanced disease is guided in large part by hormone-receptor status, ErbB2 (HER2) status, response to prior treatment, and co-existing conditions (eg, heart disease) that may or may not be related to prior therapy (eg, anthracyclines and trastuzumab).
Approximately 20%–25% of breast cancers overexpress human epidermal receptor type 2 (ErbB2 or HER2), which is associated with a more aggressive cancer and predicts poor clinical outcome (CitationSlamon et al 1987). Currently, women with ErbB2-positive disease are treated with trastuzumab with or without chemotherapy (CitationNational Comprehensive Cancer Network 2007), but the optimal duration of treatment remains to be determined. For example, many patients who progress during or after trastuzumab-based therapy continue to receive trastuzumab but in combination with another chemotherapy, an approach not yet demonstrated effective in randomized trials (CitationHortobogyi 2005; CitationMontemurro et al 2006). With trastuzumab now incorporated into the adjuvant setting, it is increasingly important to identify effective management strategies for patients who fail to respond to or relapse after receiving it.
Lapatinib (Tykerb®/Tyverb®, GlaxoSmithKline, Research Triangle Park, NC, USA), a small molecule inhibitor of tyrosine kinase domains of epidermal growth factor receptor (EGFR or ErbB1) and the ErbB2 (HER2) receptor, inhibits ErbB-driven cell growth (CitationRusnak et al 2001). Lapatinib binds to the ATP-binding site of tyrosine kinase, blocking phosphorylation and activation of the receptor. As a result, activation of extracellular signal-related kinase (ERK)-1/2 and phosphatidylinositol 3′ kinase (PI3K)/Akt is blocked (CitationRusnak et al 2001). In preclinical studies, lapatinib was active against trastuzumab-resistant breast cancer cells (CitationKonecny et al 2006). In a phase I study, lapatinib was active and well tolerated in women with advanced solid tumors when administered in combination with capecitabine at the optimally tolerated dose of lapatinib 1250 mg orally Days 1–21 and capecitabine 2000 mg/m2 Days 1–14 of a 21-day cycle (CitationSchwartz et al 2004). Lapatinib monotherapy has been well tolerated by women with advanced breast cancer in phase II trials (CitationBurstein et al 2004; CitationBlackwell et al 2005; CitationGomez et al 2006). The response rate was highest in the first-line setting (24%), but responses to monotherapy were also seen in heavily pretreated patients, including those previously treated with trastuzumab (4%–8%). Although lapatinib targets two distinct receptor pathways, responses in these trials were limited to patients with ErbB2-positive disease.
Lapatinib plus capecitabine for ErbB2-positive advanced breast cancer
A phase III randomized, open-label study was conducted comparing lapatinib plus capecitabine with capecitabine monotherapy in women with ErbB2-positive (3+ by immunohistochemistry [IHC] or IHC 2+ and gene amplification by fluorescence in situ hybridization [FISH]), locally advanced or metastatic breast cancer. Enrollment required that women received prior treatment that included, but was not limited to, an anthracycline, a taxane, and trastuzumab. In the combination therapy arm, lapatinib was administered continuously at a dose of 1250 mg once daily orally and capecitabine 2000 mg/m2 in 2 divided doses on Days 1 through 14. In the monotherapy arm, capecitabine was administered at a dose of 2500 mg/m2 daily in 2 divided doses on Days 1 through 14. In both arms, treatment was administered on a 21-day cycle. The primary endpoint was time to tumor progression (TTP) (CitationGeyer et al 2006).
Median age was 53 years and 14% of women were ≥65 years old. Approximately half of the women’s tumors were hormone receptor positive and nearly all (96%) had meta-static disease, with approximately 50% having metastases to three or more sites. Prior trastuzumab treatment was administered in the metastatic setting in 95% of patients (CitationGeyer et al 2006; CitationTykerb 2007).
Planned interim analysis
Study enrollment was stopped early based on a planned interim analysis of TTP that met criteria for early reporting based on superiority in the capecitabine plus lapatinib arm. At the time of this analysis a total of 324 women had been randomized and there were 49 TTP events in the combination therapy arm and 72 in the monotherapy arm, with a hazard ratio for TTP of 0.49 (95% CI, 0.34 to 0.71; p ≤ 0.001) based on an evaluation by blinded independent reviewers. The median TTP in the combination arm was 8.4 months and 4.4 months in the monotherapy arm (CitationGeyer et al 2006).
Updated analysis
By the time enrollment was suspended, an additional 75 women had been enrolled in the study. An updated analysis was conducted on this larger population of 399 women. With a total of 82 TTP events in the combination arm and 102 in the monotherapy arm by independent review, the hazard ratio for TTP remained statistically significant in favor of capecitabine plus lapatinib (HR, 0.57; 95% CI, 0.43–0.77; p = 0.00013). This corresponds to an increase in median TTP from 4.3 months in the monotherapy group to 6.2 months in the combination therapy group (). Overall response rate also favored capecitabine plus lapatinib (24% vs 14%), and fewer patients treated with the combination developed symptomatic central nervous system progression (4 vs 13 patients; p = 0.045 by Fisher’s exact test) (CitationCameron et al 2007; CitationTykerb 2007).
Figure 1 Kaplan-Meier estimates of time to progression (HR 0.57; 95% CI 0.43 to 0.77) p = 0.00013) (A) and overall survival (B) in ITT population and based on independent review. Reproduced CitationCameron D, Martin A-M, Newstat B, et al. 2007. Lapatinib (L) plus capecitabine (C) in HER2+ advanced breast cancer (ABC): updated efficacy and biomarker analysis. J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I, 25:1035.
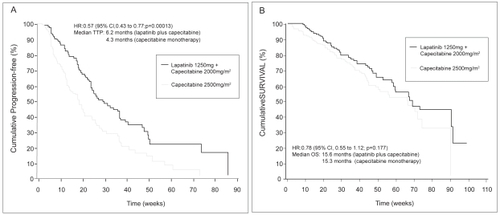
Thirty percent of patients had died by the time of the updated analysis. While overall survival trended in favor of capecitabine plus lapatinib, data were not statistically significant (HR, 0.78; 95% CI, 0.55–1.12. p = 0.177) () (CitationCameron et al 2007; CitationTykerb 2007).
Safety
The most common adverse events were diarrhea, palmarplantar erythrodysesthesia (PPE), nausea, vomiting, and rash distinct from PPE (). The incidence of these adverse events was similar in each group, with the exception of diarrhea and rash, both more common in women treated with capecitabine plus lapatinib. A similar percentage of women in each group (14% in the combination group and 14% with capecitabine alone) discontinued treatment due to adverse events (CitationTykerb 2007).
Table 1 Most frequent adverse events based on National Cancer Institute Common Terminology Criteria for Adverse Events, version 3 (CitationTykerb 2007)
Based on experience with trastuzumab, cardiac events were closely monitored. A cardiac event was defined as any decline in left ventricular ejection fraction (LVEF) that was symptomatic (ie, National Cancer Institute Common Terminology Criteria for Adverse Events [NCI CTCAE] ≥ Grade 3) or an asymptomatic decline that was ≥20% relative to baseline and below the institution’s lower limit of normal. There were no differences in the mean LVEF between the two treatment groups (CitationGeyer et al 2006). Four asymptomatic cardiac events of LVEF occurred in patients treated with the combination and two in the monotherapy group. One patient in the combination group experienced Prinzmetal’s angina, which resolved when treatment was stopped. This patient experienced a decrease in LVEF 28 days after stopping treatment with lapatinib. On follow-up, LVEF was at or above the lower limit of normal in all patients experiencing cardiac events (CitationGeyer et al 2006; CitationTykerb 2007).
Initial biomarker data
Preliminary results from a biomarker companion study to the lapatinib combination trial have recently been reported (CitationCameron et al 2007). The objectives of this study were to evaluate correlations between biomarker expression and clinical endpoints (TTP/progression-free survival [PFS]) and to characterize the biomarker predictors of benefit from lapatinib. Levels of EGFR/HER2 were analyzed using both IHC/FISH and ECD assays. Baseline EGFR/ECD levels did not affect PFS in either the combination (p = 0.88) or monotherapy arms (p = 0.32). Higher HER2 ECD levels, however, were associated with shorter PFS in the capecitabine monotherapy group (p = 0.026). This was not demonstrated in the lapatinib arm (p = 0.14). When PFS was evaluated with respect to baseline HER2 by ECD, the addition of lapatinib had a positive effect. This effect was seen regardless of ECD level, with a hazard ratio for patients with baseline ECD ≥78 of 0.271 (p = 0.0013; 95% CI 0.122, 0.621) and with baseline ECD ≤78 of 0.483 (p = 0.0018; 95% CI 0.306, 0.763). The biomarker studies are ongoing and include evaluations of the entire HER2 family, fluoropyrimidine pathway, and identification of potential markers of resistance.
Discussion
Although metastatic breast cancer (MBC) is incurable, new treatment strategies now allow it to be managed as a chronic disease. Women with MBC are typically treated with sequential regimens. When the first is no longer effective, a second regimen is tried, and then a third (CitationNational Comprehensive Cancer Network 2007). This approach might result in extended survival. To date, trastuzumab has been considered the single most effective treatment for women with ErbB2-overexpressing breast cancer. However, controversy exists regarding the optimal duration of treatment and the utility of re-treatment after progression. Results from the recently closed S0347 trial conducted by the Southwest Oncology Group, as well as other on-going phase III trials, will provide important information about the appropriateness of trastuzumab continuation with alternate chemotherapy upon disease progression. Regardless of the outcome of these studies, however, there is a need for alternative treatment options.
Lapatinib plus capecitabine offers a new option for the treatment of women with ErbB2-positive MBC whose disease has progressed following trastuzumab. The addition of lapatinib to capecitabine significantly prolongs TTP, is associated with a trend toward improved survival, and offers the convenience of oral administration. Consistent with earlier results suggesting that lapatinib crosses the blood-brain barrier, (CitationLin et al 2007), the incidence of symptomatic brain metastases as a first site of progression was significantly reduced when lapatinib was added to capecitabine therapy. Because ErbB2-overexpressing breast cancer appears to be associated with an increased risk of developing brain metastases (CitationAltaha et al 2004) and because trastuzumab does not appear to cross the blood-brain barrier (CitationClayton et al 2004; CitationStemmler et al 2006) this clinically significant finding deserves further study.
Trastuzumab is associated with an increased risk of cardiotoxicity, particularly when co-administered with anthracyclines (CitationSeidman et al 2002). Many women with advanced breast cancer will have received anthracyclines and trastuzumab. While lapatinib is not free of cardiac effects, the risk appears to be low. In an analysis of cardiac safety in 3127 patients treated with lapatinib in clinical trials, the incidence of lapatinib-associated asymptomatic LVEF decreases was 1.3%. Symptomatic decreases were rare and generally reversible (CitationPerez et al 2006).
The combination of lapatinib and capecitabine fills a void in the treatment of women with ErbB2-overexpressing advanced breast cancer who have received prior therapy with an anthracycline, a taxane, and trastuzumab. Ongoing research is investigating the use of lapatinib alone, in combination with various cytoxic and biologic agents, in combination with trastuzumab, and in adjuvant therapy for early breast cancer ().
Table 2 On-going randomized phase III trials of lapatinib in patients with breast cancer
References
- AltahaRCrowellEDucatmanB2004Risk of brain metastases in HER2/neu-positive breast cancerJ Clin Oncol, 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition)22682
- American Cancer Society2007Cancer Facts and Figures 2006 [online]Accessed 21 June 2007 URL: http://www.cancer.org/docroots/STT/content/STT_1x_Cancer_Facts_Figures_2006.asp
- BlackwellKLBursteinHPegramM2005Determining relevant biomarkers from tissue and serum that may predict response to single agent lapatinib in trastuzumab refractory metastatic breast cancerJ Clin Oncol23193s Abstract 3004. [Updated based on presentation.]
- BursteinHStornioloAMFrancoS2004A phase II, open-label, multicenter study of lapatinib in two cohorts of patients with advanced or metastatic breast cancer who have progressed while receiving trastuzumab-containing regimensAnn Oncol15suppl 327 (abstract 104O)
- CameronDMartinA-MNewstatB2007Lapatinib (L) plus capecitabine (C) in HER2+ advanced breast cancer (ABC): updated efficacy and biomarker analysisJ Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I251035
- ClaytonAJDansonSJollyS2004Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancerBr J Cancer916394315266327
- ESMO Guidelines Working Group2007Recurrent or metastatic breast cancer: ESMO Clinical Recommendations for diagnosis, treatment and follow-upAnn Oncol18Suppl 2ii9ii1117491064
- GeyerCEForsterJLindquistD2006Lapatinib plus capecitabine for HER2-positive advanced breast cancerN Engl J Med35527334317192538
- GomezHLChavezMADovalDCResults from a phase II randomized study of lapatinib as first-line treatment for patients with HER2-amplified locally advanced or metastatic breast cancerPoster presentation at the 29th Annual San Antonio Breast Cancer Symposium2006 Abstract 1090. available at:http://www.posters2view.com/sabcs06/view.php?nu=1090Accessed June 19, 2007
- HeimannRHellmanS2000Clinical progression of breast cancer malignant behavior: what to expect and when to expect itJ Clin Oncol18591910653874
- HortobagyiGN2005Continuation of trastuzumab beyond disease progression (letter to the editor)J Clin Oncol2328689
- KonecnyGEPegramMDVenkatesanN2006Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cellsCancer Res661630916452222
- LinNUDierasVPaulD2007Phase II trial of lapatinib for brain metastases in patients with HER2+ breast cancerJ Clin Oncol, ASCO Annual Meeting Proceedings Part I251012
- MontemurroFDonadioMClavarezzaM2006Outcome of patients with HER2-positive advanced breast cancer progressing during trastuzumab-based therapyThe Oncologist113182416614227
- National Comprehensive Cancer Network2007NCCN clinical practice guidelines in oncology: Breast Cancer v.2.2007 [online]Accessed 21 June 2007 URL: http://www.nccn.org
- PerezEAByrneJAHammondIW2006Cardiac safety experience in 3127 patients (pts) treated with lapatinibAnn Oncol17suppl 8ix70
- RusnakDWLackeyKAffleckK2001The effects of the novel, reversible epidermal growth factor receptor/ERbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivoMol Cancer Therap1859412467226
- SchwartzGChuQS-CHammondLA2004Phase I clinical, biology and pharmacokinetic study of the combination of GW 572016 and capecitabine in patients with advanced solid tumors (abstract 3070)J Clin Oncol2214S
- SeidmanADHudisCPierriMK2002Cardiac dysfunction in the trastuzumab clinical trials experienceJ Clin Oncol2012152111870163
- SlamonDJClarkGMWongSG1987Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogeneScience235177823798106
- StemmlerJSchmittMWillemsA2006Brain metastases in HER2-overexpressing metastatic breast cancer: comparative analysis of trastuzumab levels in serum and cerebrospinal fluid (abstract)J Clin Oncol, 2006 ASCO Annual Meeting Proceedings (Post-Meeting Edition)241525
- Tykerb (lapatinib) tablets2007Product informationResearch Triangle Park, NCGlaxoSmithKline