Abstract
Renal cell cancer has been refractory to drug therapy in the large majority of patients. Targeted agents including sunitinib have been intensively evaluated in renal cell cancer over the past 5 years. Sunitinib is an oral small molecule inhibitor of several targets including multiple tyrosine kinase receptors of the angiogenesis pathway. This review surveys the rationale, development, validation, and clinical use of sunitinib that received conditional approval for use in North America and Europe in 2006. In patients with the clear-cell subtype of renal cell cancer and metastatic disease with good or moderate prognostic factors for survival, sunitinib 50 mg for 4 weeks of a 6-week cycle provides superior surrogate and patient-reported outcomes when compared with interferon-alfa, the previous commonly used first-line drug. Overall survival has not yet shown improvement over interferon and is problematic because of patient crossover from the control arm to sunitinib at disease progression. Toxicity is significant but manageable with experienced monitoring. Sunitinib therapy is an important step forward for this condition. High cost and limited efficacy support the ongoing search for further improved therapy.
Introduction
The systemic therapy of advanced renal cell cancer is undergoing rapid transformation as a result of the introduction of targeted agents into the clinic. Sunitinib (Sutent®, Pfizer), an oral anti-angiogenesis agent, is currently becoming the first-line standard of care for renal cancer of the clear cell type. The recent approval of sunitinib along with sorafenib for renal cell cancer represents the culmination of what is destined to become a classic example of translational research (CitationChow and Eckhardt 2007). The stepwise study of sunitinib is presented here to illustrate this logical path to progress. A systematic review of phase III trials was used as a basis for this work (CitationCoppin et al 2008), supplemented by focussed literature search using sunitinib and renal cell cancer as search terms in databases Medline, EMBase, clinicaltrials.gov, and controlled-trials. com (as of July 2007).
Relevant cell biology
Angiogenesis, the generation of new blood vessels required for normal and abnormal tissue growth, has been a slowly developing conceptual target for cancer treatment (CitationKerbel and Folkman 2002). A clue that assisted the unraveling of the angiogenesis signaling pathway came from investigation of the familial Von Hippel Lindau (VHL) syndrome, a rare dominant inherited condition that includes development of renal cell carcinomas. The gene responsible for VHL syndrome, now known as the VHL tumor-suppressor gene, was located on the short arm of chromosome 3 and subsequently cloned for functional analysis. In well-oxygenated conditions, the VHL gene product pVHL recognizes HIF1α (hypoxia inducible factor-1α) and targets it for destruction via ubiquitination (CitationRini and Small 2005). Under hypoxic conditions, HIF1α undergoes a conformational change, is not recognized by pVHL, and enters the cell nucleus. This event triggers a complex adaptive response including transcription of angiogenesis-stimulating factors VEGF (vascular endothelial growth factor) directed to receptors on microvascular endothelial cells and PDGF (platelet-derived growth factor) directed to microvascular supporting pericytes. In VHL syndrome, loss or mutation of the second VHL gene copy in renal tissue results in the angiogenesis pathway being constitutively active, and this second hit appears to underlie the pathogenesis of renal and other vascular tumors. A further critical breakthrough came with the recognition that the majority of ordinary sporadic renal cancers of the clear cell type are also associated with loss or dysfunction of both VHL gene copies (CitationNa et al 2003), and therefore that clear cell renal cancer might be especially vulnerable to anti-angiogenic therapy. As supportive evidence, VEGF expression is exceptionally high in renal cancers (CitationEscudier et al 2007b). Anti-angiogenic agents may attack blood vessel development directly at the site of normal microvascular endothelial cells and pericytes, an approach that might avoid the emergence of drug resistance resulting from genomic instability (CitationKerbel and Folkman 2002), and sunitinib is presumed to act at this location. Drugs may also act indirectly by antibody binding of elevated levels of extracellular angiogenic growth factors eg, bevacizumab (Avastin®, Genentech), acting on overexpressed receptors on tumor cells themselves eg, sunitinib, sorafenib (Nexavar®, Bayer) or by inhibiting related pathways such as mTOR upregulation of VEGF and HIF1α eg, temsirolimus (Torisel®, Wyeth).
Development and action of sunitinib
Sunitinib is an inhibitor of multiple members of the split-domain family of receptor tyrosine kinases (RTKs) especially those related to angiogenesis. Kinases activate other enzyme proteins by adding phosphate from ATP, and cascades of kinases act as the most important cellular signaling pathways central to the regulation of critical cellular functions such as growth, apoptosis, attachment, and angiogenesis. Malignant cells characteristically exhibit derangements of these controls due to mutations resulting in constitutive activation of one or more pathways, compounded by evolving progression resulting from genomic instability (CitationHanagan and Weinberg 2000). The human genome codes for over 500 kinases including over 50 RTKs (CitationManning et al 2002) that are of major interest as targets for pharmaceutical attack. The RTKs are present at the cell surface and are the initial members of signaling cascades that respond to extracellular ligands secreted by other cells or by the same cell (autocrine action). An RTK consists of an extracellular receptor domain, hydrophobic membrane anchor, and intracellular catalytic site and P-loop for the donor ATP. Ligand binding results in RTK dimerization that activates kinase function and the ensuing cascade, subject to complex regulatory controls.
In the late 1990s, the identification of all human kinases was undertaken by Sugen Inc (South San Francisco). This effort resulted in the publication of the essentially complete human “kinome” (CitationManning et al 2002). Sugen also explored the identification of inhibitors of RTKs, by synthesizing small candidate molecules that compete for the catalytic site of the RTK(s) of interest. Initial clinical results with compounds SU6668 and SU5416 were disappointing because of poor pharmacologic properties and/or too narrow RTK specificity (CitationStadler 2007a). A further series of 13 analogues were synthesized and of these, SU11248, now called sunitinib, had the most promising characteristics (CitationSun et al 2003). Specifically SU11248 had the best pharmacologic and binding potency profile for VEGFR and PDGFR at the biochemical and cellular levels. In 2003, the Sugen parent company Pharmacia was acquired by Pfizer, and Sugen was disbanded (CitationGarber 2003). Pfizer obtained patent rights to the Sugen compounds including SU11248 (sunitinib), a compound that was confirmed in preclinical models as active against tumor cell VEGFR and PDGFR (CitationAbrams et al 2003; CitationMendel et al 2003), as well as endothelial cells and angiogenesis (CitationOsusky et al 2004). Additionally, sunitinib inhibits KIT, a kinase constitutively activated in the majority of gastrointestinal stromal tumors (GIST). A randomized placebo-controlled trial as second-line therapy for GIST showed that sunitinib improved progression-free and overall survival (CitationDemetri et al 2006), resulting in regulatory approval for this indication. This trial is also the best source of information on the side-effects of sunitinib compared to placebo.
Renal cell cancer
Renal cell cancer (RCC) is not a single entity as has long been recognized from light microscopy. About 85% of renal cell cancers are of predominantly clear cell type. Recent molecular analysis (CitationLinehan et al 2005) has shown clear cell renal cancer to be the only type that has the VHL defect that creates the defined target for sunitinib. Following the introduction of survival-prolonging therapies for the commonest metastatic cancers, RCC remained essentially refractory to drug therapy. Cytotoxic chemotherapy for RCC was largely abandoned following the realization that occasional “responses” are likely spontaneous remissions seen in untreated patients (CitationOliver et al 1989; CitationGleave et al 1998), an observation that also spawned interest in immunotherapy, the main systemic therapy approach to RCC of the past two decades. In the USA, high dose interleukin-2 (hdIL2) has been the only FDA-approved systemic treatment for RCC prior to the RTK inhibitors sunitinib and sorafenib. Because of severe toxicity, the applicability of hdIL2 has been limited to the fittest patients treated in specialized centers, and furthermore did not yield better median overall survival than interferon alfa (CitationMcDermott et al 2005) though a small percentage of patients have achieved durable complete remissions with hdIL2 not seen with other therapies (CitationFyfe et al 1996). Interferon-alfa (IFNα) given by subcutaneous injection three times weekly has gradually become the de facto standard of care and clinical trial comparator (CitationMotzer et al 2002; CitationMickisch 2003) because of its safety, home-based convenience, and small survival benefit in two large studies (CitationMRC Renal Cancer Collaborators 1999; CitationPyrhonen et al 1999) and meta-analysis (CitationCoppin et al 2004). However, IFNα causes substantial fatigue in most patients, and failed to demonstrate benefit over placebo in a recent large study of advanced RCC patients with intermediate prognosis (CitationNegrier et al 2005). More effective treatment for RCC has long been needed, but few could have predicted the recent explosion of interest in RCC therapy arising from the early results of targeted therapy. At the annual meeting of the American Society of Clinical Oncology (ASCO), the number of podium and poster presentations devoted to RCC has increased from 12 in 2001 to 46 in 2007, including plenary presentations for the past 2 years.
Endpoints of targeted therapy
Ultimately the value of cancer drug therapy to the patient must be described in terms of the incremental gain in the quantity or quality of life at acceptable risk, toxicity and economic cost, when comparing standard and novel treatment options. These parameters are more problematic trial endpoints than categorical all-or-none outcomes like stroke. The clinical course of RCC is so variable that single-arm studies cannot be used to estimate efficacy though can provide encouragement for proceeding to randomized trials. The objective response rate and progression-free survival have been introduced as surrogate endpoints that have utility and convenience for comparison of trial arms but have arguable relationship to patient-centered endpoints. The situation is compounded with studies of targeted cancer agents some of which may be cytostatic rather than cytoreductive, and new methodologies are required to evaluate the clinical meaning of disease stabilization (CitationRatain and Eckhardt 2004). These methods include the following: sequential measures of tumor burden in each patient colloquially called spidergrams (CitationElaraj et al 2004); histograms of percentage changes in tumor burden less than the conventional 50% sometimes referred to as waterfall plots (CitationRatain et al 2006); and randomized discontinuation of therapy for patients with stable disease after an initial phase of treatment (CitationStadler 2007b). However, as will be seen, sunitinib is more than cytostatic so that these issues are less important for sunitinib evaluation than some other targeted agents.
Prognostic factors have been well defined in both first-line (CitationMotzer et al 2002) and second-line settings (CitationMotzer et al 2004) and have been used to create pre-defined strata in pivotal phase III studies. These factors were identified by retrospective review of patients entered on clinical trials. With first-line interferon-alfa, short overall and progression-free survival outcome was associated with impaired performance status, low hemoglobin, high corrected serum calcium, disease-free interval of less than a year, and high lactate dehydrogenase. These factors were of equivalent import, and patients could be grouped into three categories: favorable risk (zero risk factors present at start of first-line systemic therapy, median survival 30 months), intermediate risk (one or two risk factors, median survival 14 months), or poor risk (3 or more risk factors, median survival 5 months) (CitationMotzer et al 2002). In the second-line setting after cytokine failure, survival was similarly correlated with the first three of the above factors, yielding 3 prognostic strata with median overall survivals of 22, 11.9, and 5.4 months (CitationMotzer et al 2004).
Sunitinib phase I study and clinical pharmacology
One major phase I dose-escalation study of oral sunitinib in solid tumors has been reported (CitationFaivre et al 2006), and between June 2001 and September 2003 accrued twenty-seven patients evaluable for toxicity of at least one course of therapy. A schedule of 4 weeks of sunitinib followed by a 2-week rest period was used at the request of the regulatory authorities to allow recovery from possible bone marrow and adrenal toxicity seen in animal testing. Dosing was based on body surface area; however data were reported on a fixed dose basis because this method resulted in negligible increase in the wide interpatient pharmacologic variability and because only 25 mg dose increments were available at that time. Dose-limiting toxicity was observed at step 3, 75 mg po daily, with asthenia, hypertension, and thrombocytopenia probably secondary to microangiopathy (CitationFaivre et al 2006). Additional toxicities were organ perforation or hemorrhage secondary to tumor necrosis, as well as cutaneous toxicity with bullous edema and splinter nail hemorrhages. Hair depigmentation was also seen, a known marker of KIT inhibition (CitationMoss et al 2003). Remarkably for a study of this type, four partial remissions and two near-complete remissions were observed in 13 evaluable patients treated at ≥75 mg/dose, and were associated with decreased tumor vascularity after one week of therapy as assessed by Doppler ultrasound. However no remissions were seen in the 9 patients receiving 50 mg/dose suggesting a potential dose-response effect also seen in preclinical models and in a recent retrospective exposure-response analysis (CitationHouk et al 2007). Nevertheless 50 mg daily (4 weeks on, 2 off) was considered the maximum tolerated dose to go forward to phase II trial and remains standard.
Oral sunitinib malate is metabolized by cytochrome CYP3A4 into an active desethyl metabolite SU12662, with potential for clinically relevant drug interactions. The combined blood levels of these two equipotent agents had a half-life of 2–3 days. Radiolabeled sunitinib is primarily excreted in the bile with minor urinary excretion as well. Limited data are available for patients with renal or hepatic dysfunction but sunitinib pharmacokinetics were unchanged in patients with mild or moderate hepatic impairment (CitationBello et al 2006).
Phase II single arm studies of sunitinib for renal cell cancer
Two studies examined the activity of sunitinib in patients with metastatic renal cell carcinoma previously treated with cytokine therapy. The schedule was again 4 weeks on, 2 weeks off as used in phase I, at the phase I determined maximum tolerated dose of sunitinib 50 mg po daily. The first phase II trial accrued 63 patients with any renal cancer histology between January and July 2003 in a single center setting (CitationMotzer et al 2006a). The primary endpoint was the objective response rate by the RECIST (Response Evaluation Criteria in Solid Tumors) method (CitationTherasse et al 2000). Patients were treated until disease progression, unacceptable toxicity, or withdrawal of consent. Twenty-five patients (40%) achieved a partial remission, often with imaging changes consistent with tumor necrosis. Median time to disease progression for all 63 patients was 8.7 months. Fatigue was the dominant subjective toxicity (27% of patients experienced grade 2, and 11% grade 3). Serial questionnaires showed that fatigue usually reversed during each 2-week break from therapy. Additional toxicities of note included diarrhea, cytopenias, decline in cardiac ejection fraction (11 patients), and hyperlipasemia without clinical pancreatitis (15 patients). One third of the patients required dose reduction to manage subjective or laboratory toxicities, most to 37.5 mg/dose. Pharmacokinetic data demonstrated median daily trough levels within the range shown to inhibit RTKs in preclinical models. Measured plasma VEGF-A levels tended to increase and VEGFR2 levels to decrease with sunitinib therapy. These biomarkers may be useful predictors of benefit.
A second study confirmed the efficacy of second-line sunitinib in a multicenter setting (CitationMotzer et al 2006b). One hundred and six nephrectomized renal cancer patients with clear cell histology were accrued during 2004 after disease progression following cytokine therapy. Thirty-six patients (34%) had documented partial remissions as assessed by independent third-party review. Toxicities were similar to those previously described with the addition of hand-foot syndrome (15%) and mucositis (12%). A combined analysis of the two phase II studies reported an overall 42% investigator assessed remission rate, and the median progression-free survival for responders was 14.8 months (CitationMotzer 2006b).
Phase II studies in renal cell cancer have not necessarily provided a reliable guide to phase III outcomes (CitationZia et al 2005) or subsequent regulatory approval (CitationGoffin et al 2005), reasons including preferential selection of prevalent patients with indolent lung metastases for phase II. However, taken together, the two phase II studies of second-line sunitinib demonstrated a striking remission rate similar to or greater than seen in first-line phase II studies of the current standard comparator interferon-alfa, greatly exceeding the anticipated cytostatic drug action. It was therefore appropriate to proceed directly to a head-to-head comparison of sunitinib with interferon-alfa in a first-line setting.
Phase III randomized study of sunitinib for renal cell cancer
A pivotal randomized phase III study of first-line sunitinib versus interferon-alfa has been reported in detail (CitationMotzer et al 2007a) and recently updated (CitationMotzer et al 2007b). Eligibility requirements included measurable metastatic renal cell cancer of clear-cell subtype, performance status 0–1, and no prior systemic therapy. Patients with brain metastases, uncontrolled hypertension, or recent cardiovascular events were excluded. Eligible consenting patients were randomly assigned to receive either sunitinib 50 mg orally for 4 weeks of each 6-week cycle, or interferon alfa-2a (Roche) at a conventional dose of 9 MU 3 times per week by subcutaneous injection. The primary endpoint was progression-free survival as assessed by third party blinded central review of imaging studies performed every cycle for the first four cycles and alternate cycles thereafter. The study was designed to detect a 1.5 month improvement in the primary endpoint at a significance level of 0.05 and 90% power, requiring 471 events.
Seven hundred fifty patients were enrolled from 101 centers on 5 continents. Fifteen of 375 interferon-assigned patients withdrew consent without therapy but were included in the analysis. Accrual was completed October 2005. In November 2005, a second pre-planned interim analysis was performed and, with input from the independent data and safety monitoring committee, patients on the interferon alfa arm with disease progression were allowed to cross over to receive sunitinib. As of February 2007, only 6% of interferon-assigned patients had crossed to sunitinib (CitationMotzer et al 2007b). Toxicity was as expected for the two agents, with fatigue seen in patients on both arms but was more severe with interferon alfa, whereas diarrhea, hypertension, and hand-foot syndrome occurred predominantly with sunitinib. Similar proportions of patients on sunitinib or interferon alfa required treatment delays, dose reductions, or discontinued therapy for adverse events.
Independently assessed progression-free survival, the primary endpoint, was substantially better with sunitinib than interferon alfa (). As of February 2007 (CitationMotzer et al 2007b), the updated median progression-free survival was 11.0 months for sunitinib versus 5.1 months for interferon alfa, hazard ratio 0.54 (95% CI 0.44–0.66; p < 10−6). Multivariate analysis of the sunitinib arm found that diagnosis to treatment interval of less than a year, reduced patient performance status, and corrected serum calcium >10 mg/dL were independent predictors of worse progression-free survival (CitationMotzer et al 2007b).
Figure 1 Kaplan – Meier estimates of progression-free survival (independent central review). Reproduced with permission from CitationMotzer RJ, Hutson TE, Tomczak P, et al 2007a. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med, 356:115–24. Copyright © 2007 Massachusets Medical Society. All rights reserved.
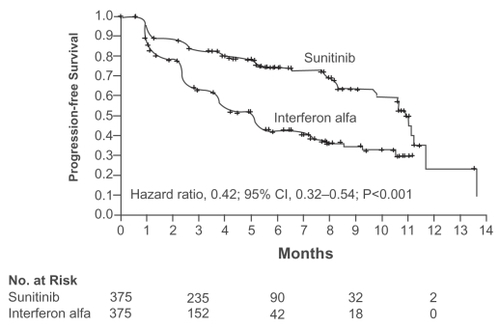
The independently assessed proportion of patients achieving a major tumor remission was 39% for sunitinib compared with 8% for interferon alfa (p < 10−6), though no confirmed complete responses were seen. The interferon alfa outcomes are consistent with previous phase III studies (CitationCoppin et al 2004). Importantly for treatments with substantial toxicity, patient-reported quality-of-life scores were statistically superior (p < 0.001) for sunitinib to a clinically meaningful degree using validated general and kidney cancer quality-of-life instruments (CitationMotzer et al 2007a, on-line appendix).
Overall survival, a secondary study outcome, is too early to fully report (CitationMotzer et al 2007b). Early overall survival at the time of the second interim analysis with 85% of patients still alive was better on the sunitinib arm, hazard ratio for death was 0.65, p = 0.02, but this did not reach the pre-specified level for statistical significance for an interim analysis (CitationMotzer et al 2007a). Thus far, 20 of 319 patients have been crossed over from interferon alfa to sunitinib following disease progression or withdrawal for an adverse event (CitationMotzer et al 2007b). This crossover will dilute any potential overall survival difference and make it more difficult to achieve statistical significance or to estimate any quantitative survival gain attributable to sunitinib.
Indirect comparison of sunitinib with other targeted therapies
Randomized trials that directly compare sunitinib with other targeted agents have not been reported. A systematic review of this rapidly changing field has been completed (CitationCoppin et al 2008). Cautious indirect comparisons may be made by inspection of randomized trials of first-line interferon alfa versus agents other than sunitinib.
Sorafenib (Nexavar®, Bayer), like sunitinib, is a small molecule multitargeted receptor tyrosine kinase inhibitor directed against angiogenesis and additional targets including raf kinase. One study randomized 189 systemically untreated patients to receive either oral sorafenib 400 mg twice daily or subcutaneous interferon alfa 9 MU three times weekly (CitationSzczylik et al 2007). Progression-free survival was not improved over interferon but the power to detect a difference was limited by the small size of this phase II trial. Additionally, sorafenib was tested well below the maximum dose of 1200–1600 mg twice daily tolerated by over 90% of patients (Amato et al 207) and further evaluation of sorafenib at the escalated dose of 600 mg twice daily or more is in progress (CitationSzczylik et al 2007).
Temsirolimus (CCI-779, Torisel®, Wyeth), an inhibitor of the mammalian target of rapamycin, has been compared with interferon-alfa in a phase III trial of 626 systemically untreated patients with advanced renal cancer (CitationHudes et al 2007). Eligibility was confined to patients with at least three of six factors predictive of short survival (including multi-organ involvement), this endpoint therefore being rapidly reached for reporting purposes. Overall survival, the primary study endpoint, was improved with temsirolimus (hazard ratio for death 0.73; p = 0.008) with median survival 10.9 months compared with 7.3 months for interferon alfa. Response rates were low in this adverse patient group. Median progression-free survival was approximately 2 months longer for temsirolimus versus interferon alfa, hazard ratio not reported. A pre-planned subset analysis suggests that the survival benefit of temsirolimus over interferon extends to patients with non-clear histologies (CitationDutcher et al 2007) that have been excluded from other pivotal trials (CitationEscudier et al 2007; CitationMotzer et al 2007). For patients with poor prognostic features including short disease-free interval, multiple organ involvement, or adverse laboratory findings, temsirolimus may be superior to sunitinib based on indirect comparison of outcomes; temsirolimus was approved by the US FDA in May 2007. High dose interleukin-2 is another option for this subset (CitationMcDermott et al 2005) but high toxicity limits its availability.
Several randomized studies have examined the addition of agents to first-line interferon alfa, and would become relevant to sunitinib if overall survival was superior in these studies to render interferon alfa alone an obsolete comparator. The addition of interferon alfa to temsirolimus (CitationHudes et al 2007) or thalidomide (CitationGordon et al 2004) resulted in additional toxicity without improved patient survival. The addition of bevacizumab or placebo to interferon alfa has been examined in a recently presented phase III study demonstrating improved progression-free survival (hazard ratio 0.63, p < 0.0001); mature survival results are awaited with interest (CitationEscudier et al 2007b).
Cost-effectiveness of sunitinib as first-line therapy
Having established that sunitinib provides better progression-free survival, quality-of-life, the convenience of oral therapy, and a favorable survival trend compared to interferon-alfa, the question of cost in relation to quantitative benefit must be addressed. In a medical environment where expensive new therapies are competing for limited financial resources, guiding principles include recognition of the issue, transparent evaluation by parties with negligible conflict of interest, and application of a standardized approach (CitationEddy 1994) such as used by the National Centre for Excellence in the UK (www.nice.org.uk). The use of a fixed cutoff such as $50–100,000 per life year, useful when only a small proportion of individuals would need chronic therapy such as hemodialysis, is no longer valid when the majority of patients with incurable cancer now have such options. The actual or imminent availability of targeted drugs for common malignancies and other conditions will only highlight the need for consistency and rigor rather than political advocacy. Published analyses almost invariably have a favorable conclusion and sunitinib is no exception (CitationRemak et al 2007); caution is advised especially in regards to assumptions and 5- to 10-year projections of immature data. A safer approach is to use available firm data. For sunitinib, in the absence of an overall survival benefit, cost per life year gained cannot be estimated and other arguments are required. The gain in progression-free expectancy, analytically equivalent to incremental gain in life expectancy, is given by the area between the progression-free curves of a randomized trial (CitationWright and Weinstein 1998): for sunitinib versus interferon alfa, this progression-free expectancy gain is approximately 3 months (derived from CitationMotzer et al 2007b). Based on such estimates of incremental benefit and cost between competing options, each jurisdiction or individual must make their own decisions for available funds. In due course, a population-based outcomes approach may also be useful for estimating sunitinib effectiveness, that is, survival impact in a real clinical setting (CitationKollmannsberger et al 2007).
Sunitinib in current clinical management of renal cancer: practical issues
Sunitinib was given accelerated approval by the US FDA in January 2006 (CitationGoodman et al 2007) on the basis of improved surrogate endpoints reasonably likely to predict clinical benefit, such as the subsequently demonstrated improvement in quality-of-life measures. Similarly, qualified approval was issued by the European Medicines Evaluation Agency in April 2006, and by Health Canada in August 2006 “for the treatment of metastatic renal cell carcinoma of clear cell histology after failure of cytokine-based therapy or in patients who are considered likely to be intolerant of such therapy” (Health Canada Notice of Compliance with Conditions, August 17, 2006). Sunitinib is available (Sutent®, Pfizer) in 12.5, 25, and 50 mg capsules.
Since these approvals, patients have been treated with sunitinib on expanded access programs providing experience from 52 countries (CitationGore et al 2007), the majority having received prior drug therapy therapy (cytokine 78%, anti-angiogenic 7%). The eligibility criteria were much broader than the pivotal phase III trial, with unrestricted performance status and renal cell subtype, and asymptomatic brain metastases permitted. The standard regimen remains 50 mg for 4 weeks of a 6-week cycle. Adverse events were as expected and in particular were no worse in patients of concern, ie, older, lower performance status or with brain metastases. Although the major remission rate of 9.3% was lower than that independently assessed in phase III for reasons possibly related to patient selection, remissions were seen in all subgroups. Many additional patients had prolonged stable disease or clinical improvement, endpoints of uncertain validity.
For poor prognosis patients with three or more adverse factors, a minority group with short median survival, temsirolimus has been shown to give overall survival superior to interferon and may therefore be the preferred treatment option for this patient subset (CitationHudes et al 2007). For the majority of clear-cell renal cancer patients who have good or intermediate prognosis disease, sunitinib is becoming the commonly preferred first-line drug (CitationAtkins 2007) and has received Category 1 designation in the US NCCN guidelines for this indication (www.nccn.org accessed 2007/8/15); in Canada, the BC Cancer Agency has implemented a similar policy on a compassionate access basis (www.bccancer.bc.ca accessed 2007/8/15). Sunitinib has some utility as second-line therapy, for example after first-line bevacizumab (CitationGeorge et al 2007) but sorafenib has better documented benefit after cytokine failure (CitationEscudier et al 2007a). With the growing consensus and availability of sunitinib for selected patients with renal cancer, practical management issues are becoming increasingly important, in particular toxicity recognition and management. As with any cancer therapy, cost and toxicity considerations obligate the provider to monitor the extent of disease on a cycle-by-cycle basis and discontinue therapy at progression.
A summary of sunitinib toxicity management issues is provided in . Dose reductions from 50 mg daily to 37.5 mg or occasionally to 25 mg were required in 28% and 9% respectively in the expanded access setting (CitationGore et al 2007). The 2-week break per cycle can assist toxicity attribution and recovery. Subjective toxicities can be patient-reported and managed symptomatically, with gastrointestinal or cutaneous symptoms being predominant. Pain at tumor sites may occur. Blood pressure must be monitored at least every 2 weeks for early cycles and appropriately managed (CitationChowdhury et al 2006). CBC must be monitored each cycle for cytopenias, and TSH about every 3 months for detection of commonly seen hypothyroidism (CitationDesai et al 2006). Left ventricular dysfunction may occur in at least 10% of patients and cardiac ejection fraction evaluation should be considered before and during sunitinib therapy especially for patients with prior cardiac history or current symptoms. Prolongation of the PR and QT interval has been described with sunitinib, so that baseline ECG should be considered and concomitant use of drugs prolonging these intervals avoided. Adrenal necrosis was seen in preclinical studies, and adrenal insufficiency monitoring has been recommended in patients with stressors such as surgery, trauma, or severe infection (CitationGoodman et al 2007). Because sunitinib is metabolized by cytochrome CYP3A4, strong inhibitors of this enzyme such as ketoconazole result in increased and potentially toxic blood levels of sunitinib and its active metabolite; conversely CYP3A4 inducers such as rifampin may reduce levels and efficacy – such interactions should be considered, avoided where possible, or managed by sunitinib dose adjustment (CitationGoodman et al 2007).
Table 1 Sunitinib toxicity monitoring checklist
Conclusions and future directions
Sunitinib has followed the idealized paradigm from laboratory to clinic. A strong rationale for suppressing the angiogenesis pathway led to screening of candidate small molecules able to inhibit multiple tyrosine kinase receptors, with sunitinib (originally SU11248) selected for further testing. Anti-tumor activity was sequentially demonstrated in preclinical models, phase I studies, and in phase II and III trials in renal cell cancer and gastrointestinal stromal tumors, eventually achieving approval in North America and elsewhere for both tumor types (CitationGoodman et al 2007). Efficacy is well established for sunitinib as initial therapy for good-to-moderate prognosis patients with metastatic renal cell cancers and a majority clear cell component. Benefits in phase III trial include improved chance of remission (39% vs 8%), longer progression-free status (hazard reduction 46%), and better quality-of-life than interferon-alfa, the previous standard in this patient population (CitationMotzer et al 2007a). In an expanded access setting, the major response rate was only 9% but applied to a broad patient population (CitationGore et al 2007) and can be achieved with adequate safety provided that attention is paid to detection and management of potentially asymptomatic toxicities. Although sunitinib was approved in 2006 for second-line use after cytokines, it is currently less well validated than sorafenib for which phase III data is now available (CitationEscudier et al 2007a).
Many additional questions must now be addressed by appropriate clinical trials. Biomarkers may help patient selection for different targeted agents. Early detection of response to sunitinib and similar agents by functional imaging techniques or biomarker measurement may permit earlier recognition of non-responsive cancers. Improved efficacy of sunitinib is needed and may come from continuous rather than interrupted scheduling (CitationSrinivas et al 2007); this is being tested in a phase III trial. The addition to sunitinib of other agents such as interferon-alfa or a variety of other targeted agents is being actively explored in current generation trials. Optimal sequencing of cytokines and targeted agents needs much clarification. Approaches that can delay or avoid the emergence of sunitinib-resistant disease are needed, most likely by targeting multiple different signaling pathways (CitationVogelzang and Sternberg 2007). In patients with the primary still in situ at the start of therapy for advanced disease, the role of nephrectomy before or after sunitinib is currently unclear (CitationRini and Campbell 2007). After sunitinib, second-line temsirolimus versus sorafenib is being examined in a multicenter North American randomized trial.
Finally, sunitinib is starting to be tested in the adjuvant setting after nephrectomy in patients at high risk of relapse from micrometastases, a situation where no established adjuvant therapy currently exists. An intergroup study led by the Eastern Co-Operative Oncology Group will compare adjuvant sunitinib with sorafenib and placebo in over 1300 high-risk patients following nephrectomy. The primary endpoint is disease-free survival, an outcome that will be reached in a reasonable timeframe and will avoid the issue of crossover by placebo-assigned patients at relapse. However improved overall survival would be needed to demonstrate that it is better to use an anti-angiogenesis agent early, rather than later when the toxicity and cost impinge only on those destined to relapse.
There is no doubt that the next several years will see much further progress in our understanding and management of renal cancer with sunitinib and other targeted agents, an exciting prospect for a condition that was, until recently, dismal to have and to treat.
Disclosures
The author has no conflicts of interest to disclose.
Notes
* virtual meeting material available at www.asco.org/virtual, accessed 2007/10/01.
References
- AbramsTJLeeLBMurrayLJ2003SU11248 inhibits KIT and platelet-derived growth factor receptor β in preclinical models of human small cell lung cancerMol Cancer Ther247147812748309
- *AmatoRJHarrisPDaltonM2007A phase II trial of intra-patient dose-escalated sorafenib in patients with metastatic renal cell cancerJ Clin Oncol2518S ASCO Proc:5026
- AtkinsMB2007Molecularly targeted therapy for advanced renal cell carcinomaUpToDate® [online]Accessed 15 Aug 2007 URL: www.uptodate.com
- BelloCLGarrettMSmergliaJ2006Pharmacokinetics of sunitinib malate (SU11248) in subjects with hepatic impairmentAnnals Oncol17S9 ESMO Proc:451P
- ChowLQMEckhardtSG2007Sunitinib: from rational design to clinical efficacyJ Clin Oncol258849617327610
- ChowdhurySSpicerJEHarperPG2006Hypertension and targeted therapy. Part 2: small molecule inhibitors of VEGFTargeted Oncol11728
- CoppinCPorzsoltFAutenriethM2004Immunotherapy for advanced renal cell cancer (review)Cochrane Database of Systematic Reviews 20042Wiley
- CoppinCLeLPorzoltF2008Targeted therapy for advanced renal cell carcinoma (review)Cochrane Database of Systematic Reviews 20082In press
- DemetriGDvan OosteromATGarrettCR2006Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trialLancet3681329133817046465
- DesaiJYassaLMarquseeEGeorgeS2006Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumorsAnn Intern Med145660417088579
- *DutcherJPSzczylikCTannirN2007Correlation of survival with tumor histology, age, and prognostic risk group for previously untreated patients with advanced renal cell carcinoma receiving temsirolimus or interferon alphaJ Clin Oncol2518S ASCO Proc:5033
- EddyDM1994Principles for making difficult decisions in difficult timesJAMA271179288196126
- ElarajDMWhiteDESteinbergSM2004A pilot study of antiangiogeneic therapy with bevacizumab and thalidomide in patients with metastatic renal cell carcinomaJ Immunother272596415235386
- EscudierBEisenTStadlerWM2007aSorafenib in advanced clear-cell renal-cell carcinomaN Engl J Med3561253417215530
- *EscudierBKoralewskiPPluzanskaA2007bA randomized, controlled, double-blind phase III study (AVOREN) of bevacizumab/interferon-a2a vs placebo/interferon-a2a as first-line therapy in metastatic renal cell carcinomaJ Clin Oncol2518S ASCO Proc:3
- FaivreSDelbaldoCVeraK2006Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancerJ Clin Oncol24253516314617
- FyfeGAFisherRIRosenbergSA1996Long term response data for 255 patients with metastatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapyJ Clin Oncol1424108708739
- GarberK2003Sugen falls as casualty of Pfizer-Pharmacia mergerNat Biotechnol21722312833079
- *GeorgeDJMichaelsonMDRosenbergJE2007Phse II trial of sunitinib in bevacizumab-refractory metastatic renal cell carcinoma: updated results and analysis of circulating biomarkersJ Clin Oncol2518S ASCO Proc:5035
- GleaveMEElhilaliMFradetY1998Interferon gamma-1b compared with placebo in metastatic renal-cell carcinomaN Engl J Med3381265719562580
- GoffinJBaralSTuD2005Objective responses in patients with malignant melanoma or renal cell cancer in early studies do not predict regulatory approvalClin Cancer Res1159283416115935
- GoodmanVLRockEPDagherR2007Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinomaClin Cancer Res1313677317332278
- *GordonMSManolaJFaircloughD2004Low dose interferon-a2b + thalidomide in patients with previously untreated renal cell cancer. Improvement in progression-free survival but not quality of life or overall survival. A phase III study of the Eastern Cooperative Oncology Group (E2898)J Clin Oncol2214S ASCO Proc:4516
- GoreMEPortaCOudardS2007Sunitinib in metastatic renal cell carcinoma: preliminary assessment of toxicity in an expanded access trial with subpopulation analysisJ Clin Oncol2518S ASCO Proc:5010
- HanaganDWeinbergRA2000The hallmarks of cancerCell100577010647931
- *HoukBEBelloCLMichaelsonMD2007Exposure-response of sunitinib in metastatic renal cell carcinoma: a population pharmacokinetic/pharmacodynamic approachJ Clin Oncol2518S ASCO Proc:15572
- HudesGCarducciMTomczakP2007Temsirolimus, interferon alfa, or both for advanced renal-cell carcinomaN Engl J Med35622718117538086
- KerbelRFolkmanJ2002Clinical translation of angiogenesis inhibitorsNat Rev Cancer27273912360276
- KollmannsbergerCKHengDYMurrayN2007A population-based study evaluating metastatic renal cell cancer patients treated with interferon (IFN) alone, first-line IFN then second-line sunitinib, or sunitinib aloneJ Clin Oncol2518S ASCO Proc:15572
- LinehanWMGrubbRLColemanJA2005The genetic basis of kidney cancer: implications for gene-specific clinical managementBJU Int95S22715720328
- ManningGWhyteDBMartinezR2002The protein kinase complement of the human genomeScience29819123412471243
- McDermottDFReganMMClarkJI2005Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinomaJ Clin Oncol231334115625368
- MendelDBLairdADXinX2003In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationshipClin Cancer Res93273712538485
- MickischGHJ2003Rational selection of a control arm for randomized trials in metastatic renal cell carcinomaEur Urol43670912767369
- MossKGTonerGCCherringtonJM2003Hair depigmentation is a biological readout for pharmacological inhibition of KIT in mice and humansJ Pharmacol Exp Ther30747648012966161
- MRC Renal Cancer Collaborators1999Interferon-alpha and survival in metastatic renal cell carcinoma: early results of a randomized controlled trialLancet353141710023944
- MotzerRJBacikJMurphyBA2002Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinomaJ Clin Oncol202899611773181
- MotzerRJBacikJSchwartzLH2004Prognostic factors for survival in previously treated patients with metastatic renal cell carcinomaJ Clin Oncol2245446314752067
- MotzerRJMichaelsonDRedmanBG2006aActivity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinomaJ Clin Oncol24162416330672
- MotzerRJRiniBIBukowskiRM2006bSunitinib in patients with metastatic renal cell carcinomaJAMA29525162416757724
- MotzerRJHutsonTETomczakP2007aSunitinib versus interferon alfa in metastatic renal-cell carcinomaN Engl J Med3561152417215529
- *MotzerRJFiglinRAHutsonTE2007bSunitinib versus interferon-alfa as first-line treatment of metastatic renal cell carcinoma: updated results and analysis of prognostic factorsJ Clin Oncol2518S ASCO Proc:5024
- NaXWuGRyanCK2003Overproduction of vascular endothelial growth factor related to von Hippel-Lindau tumor suppressor gene mutations and hypoxia-inducible factor-1α expression in renal cell carcinomasJ Urol1705889212853836
- *NegrierSPerolDRavaudA2005Do cytokines improve survival in patients with metastatic ernal cell carcinoma of intermediate prognosis? Results of the prospectively randomized PERCY Quattro trialJ Clin Oncol2316S ASCO Proc:4511
- OliverRTDNethersellABWBottomleyJM1989Unexplained spontaneous regression and alpha-inerferon as treatment for metastatic renal carcinomaBr J Urol63128312702395
- OsuskyKLHallahanDEFuA2004The receptor tyrosine kinase inhibitor SU11248 impedes endothelial cell migration, tubule formation, and blood vessel formation in vivo, but has little effect on existing tumor vesselsAngiogenesis72253315609077
- PyrhonenSSalminenERuutuM1999Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancerJ Clin Oncol1728596710561363
- RatainMJEckhardtSG2004Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECISTJ Clin Oncol224442515483011
- RatainMJEisenTStadlerWM2006Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinomaJ Clin Oncol2425051216636341
- *RemakEMullinsCDAkobunduE2007Economic evaluations of sunitinib versus interferon-alfa in first-line metastatic renal cell carcinomaJ Clin Oncol2518S ASCO Proc:6607
- RiniBISmallEJ2005Biology and clinical dvelopment of vascular endothelial growth factor-targeted therapy in renal cell carcinomaJ Clin Oncol231028104315534359
- RiniBICampbellSC2007The evolving role of surgery for advanced renal cell carcinoma in the era of molecular targeted therapyJ Urol17719788417509276
- *SrinivasSRoigasJGillessenS2007Continuous daily administration of sunitinib in patients with cytokine-refractory metastatic renal cell carcinoma: updated resultsJ Clin Oncol2518S ASCO Proc:5040
- StadlerW2007aChromosomes, hypoxia, angiogenesis, and trial design: a brief history of renal cancer drug developmentClin Cancer Res131630317363513
- StadlerWM2007bThe randomized discontinuation trial: a phase II design to assess growth-inhibitory agentsMol Cancer Ther61180517431101
- SunLLiangCShirazianS2003Discovery of 5-[5-fluoro-2-oxo-1,2-dihydroindol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinaseJ Med Chem4611161912646019
- *SzczylikCDemkowTStaehlerM2007Randomized phase II trial of first-line treatment with sorafenib versus interferon in patients with advanced renal cell carcinoma: final resultsJ Clin Oncol2518S ASCO Proc:5025
- TherassePArbuckSGEisenhauerEA2000New guidelines to evaluate the response to treatment in solid tumorsJ Natl Cancer Inst922051610655437
- VogelzangNJSternbergCN2007Signal-transduction inhibitors in renal cell carcinomaBJU Int9912899517441926
- WrightJCWeinsteinMC1998Gains in life expectancy from medical interventions - standardizing data on outcomesN Engl J Med33938069691106
- ZiaMISiuLLPondGR2005Comparison of outcomes of phase II studies and subsequent randomized control studies using identical chemotherapeutic regimensJ Clin Oncol2369829116192585