Abstract
Infliximab, a monoclonal anti-TNF-α antibody, is commonly used for treatment of moderate to severe Crohn’s disease (CD). Its role in the treatment for ulcerative colitis (UC) remains controversial. We review the role of TNF-α in the pathogenesis of UC and describe the randomized, double-blind, placebo-controlled trials and systematic reviews that assess the efficacy of infliximab in the treatment of moderate to severe UC.
Introduction
Ulcerative colitis (UC) is a chronic remitting and relapsing disease characterized by mucosal inflammation of the colon. The extent of disease varies from limited involvement of the rectum to involvement of the whole colon. Rarely, there is mild involvement of the distal part of the ileum, which is believed to be an “overflow” of inflammation from the cecum and is called backwash ileitis (CitationPodolsky 2002).
UC manifests clinically as bloody diarrhea, abdominal pain, urgency, and tenesmus. In more severe cases, patients may develop systemic features, such as fever, tachycardia, nausea, vomiting, anorexia, weight loss, and an elevated CRP and erythrocyte sedimentation rate (CitationBaumgart and Sandborn 2007). Clinically mild disease is defined as disease associated with fewer than 4 bowel movements a day, with or without blood in the stool and without systemic manifestations. In these patients, laboratory results are usually within the normal range. Moderate disease describes disease associated with more than 4 bowel movements a day with minimal systemic manifestations. Severe disease describes disease associated with more than 6 bowel movements a day, with blood in the stool and with systemic involvement (CitationD’Haens et al 2007). The clinical course of UC is marked by exacerbations and remissions. Treatment of mild to moderate UC consists of oral and/or topical 5-aminosalicylates, oral and/or topical corticosteroids, and purine analogs (azathioprine and 6-mercaptopurine) (CitationCarter et al 2004; CitationLichtenstein et al 2006). In 15% of the cases, the disease manifests as an episode of severe colitis necessitating hospitalization, administration of intravenous treatment, and sometimes colectomy. A small minority of the patients develop a frequently relapsing chronic disease (CitationBinder 2004). Approximately 11% of the patients with UC develop extraintestinal manifestations over the course of their disease, which can parallel the activity of colitis or be independent of colonic disease. These manifestations include: small joint arthritis, sacroileitis, ankylosing spondylitis, erythema nodosum, pyoderma gangrenosum, apthous ulcers of the oral mucosa, episcleritis, uveitis, and sclerosing cholangitis (CitationDanese et al 2005).
Early studies reported a 30% fatality rate for patients referred for a first attack of severe UC. However, as medical and surgical treatments improved, the mortality rates declined, and currently patients with UC have an overall normal life expectancy (CitationLoftus et al 2000). Some reports even suggest decreased mortality in patients with UC compared with the general population (CitationWinther et al 2003). Despite overall good survival rates, patients have an increased risk of colectomy, development of colorectal cancer (CitationSoetikno et al 2002), and cholangiocarcinoma (CitationBroome et al 1995).
Despite major advancements in medical therapy, treatment options for patients with moderate to severe UC remain limited. Steroid treatment was introduced in the 1950s and markedly reduced mortality in severe disease (CitationTruelove and Witts 1955). The other remaining medical treatment option for patients with severe UC is the addition of cyclosporine. Although considered to be highly effective in inducing remission, double-blind, randomized, controlled trials assessing the efficacy of cyclosporine in this setting are limited (CitationLichtiger et al 1994; CitationD’Haens et al 2001). Furthermore, cyclosporine has a narrow therapeutic index with potentially serious side effects, necessitating close monitoring of cyclosporine blood levels (CitationShibolet et al 2005). If treatment with steroids and cyclosporine fails to induce remission in severe UC, patients are faced with the need for colectomy. Even if patients respond to cyclosporine, most will eventually require colectomy (CitationD’Haens et al 2001).
In the face of the paucity of treatment options it was essential to identify new therapies for moderate to severe UC that are easier to administer, have higher efficacy, are better tolerated and have a better safety profile.
The search for such drugs focused on elucidating the pathophysiology of inflammatory bowel disease (IBD) (CitationXavier and Podolsky 2007) and identifying possible drug targets (CitationKorzenik and Podolsky 2006). Data from animal models of colitis and from human studies suggested that treatment with either anti-inflammatory cytokines or with pro-inflammatory cytokine blockers may modulate the course of IBD (CitationElson et al 2005). One of the major pro-inflammatory cytokines involved in the pathogenesis of IBD was discovered to be tumor necrosis factor-α (TNF-α) (CitationPapadakis and Targan 2000). This led to a breakthrough in IBD treatment with the introduction of anti-TNF-α antibody treatment that was initially used to treat Crohn's disease (CitationSandborn and Hanauer 1999).
Pathogenesis of IBD
Inflammatory bowel disease groups together two distinctive clinical entities: UC and CD. These two diseases differ in their location (colon only for UC vs the whole length of the intestinal tract for CD), pattern of distribution (continuous vs patchy), depth of involvement (mucosal vs transmural), and histology (crypt abscesses vs granulomas). It is therefore not surprising that their pathogenesis is considerably different. The pathogenesis of IBD has been hypothesized to be caused by an inappropriate immune response against luminal antigens in a genetically susceptible host resulting in uncontrolled intestinal inflammation (CitationXavier and Podolsky 2007). Inflammatory responses are traditionally classified into Th1 or Th2 responses based on the cytokine secretion profile of differentiated T lymphocytes. Both CD4+ and CD8+ T lymphocytes can be identified as either Th1 cells that produce IFNγ, IL-2, IL-12, and IL-18 or Th2 cells that secrete IL-4, IL-5, IL-6, IL-10, and IL-13. Recently, another type of immune response dubbed Th17 and characterized by production of IL-17 by specific IL-17 producing T lymphocytes has been described and was shown to play a role in the pathogenesis of IBD (CitationPark et al 2005).
CD has been traditionally described as a Th1 mediated disease with the predominant cytokines being the pro-inflammatory cytokines IFNγ, IL-1β, and IL-12. These cytokines contribute to an increase in mucosal permeability, collagen synthesis and recruitment of inflammatory cells. Most existing animal models of experimental colitis are skewed toward a Th1 response. These include trinitrobenzene (TNBs) colitis, dextran sodium sulfate (DSS) colitis, and the IL-10 knockout spontaneous colitis models. Another Th1 mediated granulomatous colitis model has been established by the adoptive transfer of normal CD45RBhigh T lymphocytes from Balb/C mice into SCID mice (CitationBouma and Strober 2003).
UC, on the other hand, has been considered to be an atypical Th2 mediated disease characterized by CD4+ T lymphocytes bearing a natural killer (NK) T lymphocyte marker. Levels of IL-4, IL-5, IL-8, but also IL-1β, IL-12, and IFNγ, are significantly higher in patients with UC than in healthy controls with a prominence of IL-4 and IL-5. Th2 predominant animal models of experimental colitis include the TCRα and WASP deficient mice, oxazolone colitis, and spontaneous colitis in cotton-top tamarins (CitationStrober et al 2002). The recent discovery of Th17 cells and their role in IBD (CitationIwakura and Ishigame 2006) suggests that the Th1/Th2 hypothesis may represent an oversimplified model of this complex disease.
Despite the immunological and clinical differences between UC and CD, both diseases share part of their cytokine profile and exhibit elevated levels of TNF-α.
Tumor necrosis factor-α
TNF-α is a cytokine involved in inflammatory responses and is a member of the TNF-super-family. It is capable of killing tumor cells in vitro and causing hemorrhagic necrosis of transplantable tumors in mice (CitationOld 1985; CitationBazzoni and Beulter 1996). TNF-α plays a role in multiple human disease processes including septic shock (CitationCauwels et al 2003), cancer/AIDS-associated cachexia (CitationArgiles et al 1997), graft-versus-host disease (CitationJacobsohn and Vogelsang 2004), rheumatoid arthritis, and CD (CitationSiddiqui and Scott 2005).
TNF-α is first produced as a 26-kDa transmembrane protein, which can be cleaved by the metalloproteinase-desintegrin, TNF-α converting enzyme (TACE also known as ADAM17) to form a secreted, soluble 17-kDa protein (CitationBlack et al 1997). Secreted TNF-α (sTNF-α) aggregates into trimolecular complexes, which bind and activate the TNF receptors, TNF receptor type 1(TNFR1) and TNF receptor type 2 (TNFR2). TNFR1 is constitutively expressed in most tissues, and can be activated by both the membrane-bound and soluble forms of TNF-α, while TNFR2 is expressed in cells of the immune system and responds to the membrane-bound form of TNF-α. Following TNF-α binding, the receptors form trimmers and initiate intracellular signaling via 3 main pathways. Inflammatory signals are conveyed via NF-κB and MAPK pathways, while apoptotic signals are conveyed via TRADD, FADD and caspase-8 (CitationVassalli 1992).
Monocytes/macrophages are the main source of TNF-α, although T and B lymphocytes also produce significant amounts. Other cells known to produce TNF-α include NK cells, mast cells, Paneth cells, keratinocytes, astrocytes and microglial cells, smooth muscle cells, and certain tumor cell lines (CitationGuy-Grand et al 1998; CitationNilsen et al 1998; CitationBischoff et al 1999). TNF-α is also produced by intestinal epithelial cells in response to bacterial invasion (CitationJung et al 1995).
Infliximab
Infliximab is a chimeric anti-TNF-α mouse monoclonal antibody (CitationBaugh and Bucala 2001). Its Fab fragment consists of the mouse variable TNF binding region (25% of the protein) and a human Fc fragment (75% of the protein) (CitationElliott et al 1993; CitationKnight et al 1993). The antibody binds both the soluble and transmembrane forms of TNF-α with high affinity and blocks their action (association constant 1010/M), with a serum half-life of 10 days. It is administered intravenously at a dose of 5 or 10 mg/kg (Remicade®). The temporal regimens employed in IBD include administration on weeks 0, 2, and 6 and then periodically every 8 weeks (CitationRutgeerts et al 2005).
The mechanism of action of infliximab is not clearly understood. Although initially thought to be mediated via neutralization of soluble TNF-α, this is clearly not enough because treatment with other anti-TNF-α antibodies and soluble TNF-α receptors with similar or even greater neutralizing efficacy do not exert the same therapeutic effect (Remicade®; Citationvan Dullemen et al 1995; CitationHanauer et al 1998). Recent investigations suggest that the mechanism of action of TNF-α-blocking agents is mediated via apoptosis of TNF-α-expressing inflammatory cells. These studies suggest that mucosal T cells of patients with IBD are highly resistant to apoptosis (CitationIna et al 1999; CitationAtreya et al 2000). Resistance to apoptosis has been shown in both CD and UC, but the mechanism behind this phenomenon seems to differ between the two conditions. The intrinsic defect in CD occurs in the mitochondrial pathway of apoptosis (imbalance of mitochondrial bcl-2/bax), whereas in UC it results from overexpression of FLICE-inhibitory protein (FLIP) and impairment of the caspase-mediated pathway of apoptosis (CitationPeppelenbosch and van Deventer 2004). It was shown that infliximab can induce apoptosis of inflammatory cells including T cells and monocytes via a caspase-dependent mechanism. This effect was independent of Fcγ-R binding or complement activation (CitationTilg et al 2007).
Role of TNF-α in ulcerative colitis
In the intestine, the direct effects of TNF-α on intestinal epithelium include disruption of the epithelial barrier, induction of apoptosis of epithelial cells, and secretion of chemokines from intestinal epithelial cells (). TNF-α also activates the adaptive immune system of the bowel through recruitment and activation of neutrophils and macrophages (CitationGuy-Grand et al 1998; CitationNilsen et al 1998; CitationBischoff et al 1999).
Figure 1 TNF-α involvement in intestinal inflammation. After initial damage to the mucosal barrier, TNF-α is secreted by T lymphocytes, macrophages (Mac), and intestinal epithelial cells causing epithelial cell apoptosis, production of cytokines and chemokines, maturation of DCs and activation of tissue metaloproteinases from SC. This in turn causes further barrier damage, activation of neutrophils (PMN) and B lymphocytes, up-regulation of adhesion molecules, and further recruitment of inflammatory cells.
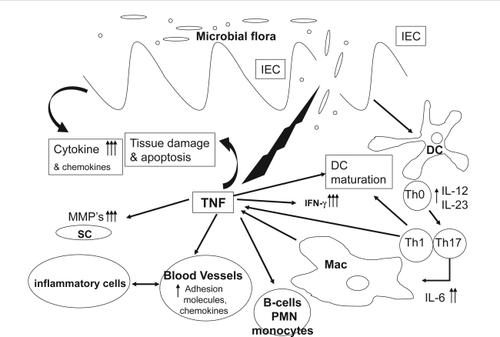
The role of TNF-α in the pathogenesis of IBD has been extensively studied and is convincingly established for CD (CitationPlevy et al 1997; Citationvan Deventer 1997). In UC, the role of TNF has been less well characterized (CitationSands and Kaplan 2007). There are several examples of a genetic association between TNF-α gene polymorphism and susceptibility to UC. The frequency of carriers for an increased TNF-α secretion polymorphism (−308A and −238G) was significantly increased in Japanese UC patients compared with healthy controls (CitationWilson et al 1993), while only weak association was observed in Chinese UC patients (CitationCao et al 2006). In contrast, in Dutch UC patients, the frequency of the same polymorphism site was markedly decreased (CitationBouma et al 1996, Citation1999). A positive transmission disequilibrium of another increased TNF-α secretion polymorphism (−857C) was shown in a UK UC Caucasian (Citationvan Heel et al 2002) patient population, while homozygosity for a TNF-α haplotype, (TNF-α, −1031T, −863C, −857C, −380G, −308G, −238G) associated with low TNF-α production was shown to be more prevalent in UC patients with limited compared with extensive colitis (CitationAhmad et al 2003).
Several studies have shown significant increase in TNF-α in colonic mucosa of UC patients (CitationMacDonald et al 1990; CitationMurch et al 1993; CitationBreese et al 1994). TNF-α production in the colon of UC patients was increased in inflamed but not in noninflamed areas (Citationvan Heel et al 2002). Similarly, TNF-α levels in stool samples from patients with active UC were significantly increased compared with healthy controls (CitationBraegger et al 1992). Serum levels of TNF-α and TNFR were also shown to be significantly higher in UC patients than those of healthy controls and their levels were shown to correlate with disease activity (CitationKomatsu et al 2001; CitationHanai et al 2004; CitationSpoettl et al 2007).
In view of the data supporting the importance of TNF-α in the pathogenesis of UC and the role infliximab plays in inducing apoptosis, it was hypothesized that infliximab may exert a beneficial effect in the treatment of moderate to severe UC. Initially, anti-TNF-α treatment did not cause disease improvement in the experimental colitis Th2 type mouse model (oxazolone colitis) (CitationShen et al 2007). However, this model does not reliably mimic human disease. In cotton-top tamarins that develop spontaneous UC-like colitis, anti-TNF-α treatment markedly improved clinical condition (CitationWatkins et al 1997). Therefore, studies were conducted to assess its efficacy in UC in humans. Although several open label studies assessed the role of infliximab in inducing remission in patients with moderate to severe UC (CitationChey et al 2001; CitationActis et al 2002; CitationKohn et al 2002; CitationSu et al 2002; CitationCastro Fernandez et al 2003; CitationGornet et al 2003; CitationArmuzzi et al 2004; CitationBermejo et al 2004; CitationLjung et al 2004; CitationOchsenkuh et al 2004; CitationRuiz et al 2004; CitationKountouras et al 2005; CitationPark et al 2005; CitationIwakura and Ishigame 2006), we chose to focus on randomized double-blind placebo-controlled studies and on systematic reviews and meta-analysis that assessed treatment efficacy.
Double blind, placebo-controlled studies (see )
We identified 5 studies and 2 systematic reviews and meta-analyses that assessed the efficacy of infliximab in moderate to severe UC as compared to placebo. These studies used different outcome measures to assess their results. Additionally, various definitions were used to describe the main outcomes. For example, clinical remission has been defined as absence of active disease symptoms using True-love and Witts criteria (the absence of blood and mucus in the stool and the absence of diarrhea), or the Lennard-Jones criteria (two or less bowel movements a day without other signs or symptoms) (CitationTruelove and Witts 1955; CitationLennard-Jones et al 1975; CitationRutgeerts et al 2005).
Table 1 Double blind, placebo-controlled studies of infliximab for UC
It is important to understand that in contrast to CD, in which the disease activity index (CDAI) is a well validated standardized tool in clinical trials, the outcome measures in UC are less well defined. Most trials use a combination of clinical, histological and endoscopic scores to describe remission. Others use combined scores which include Physician’s global assessment (PGA) scores, ulcerative colitis disease activity index (UCDAI), Mayo score, a modified Lennard-Jones criteria, Rachmilewitz criteria, or Seo index. The lack of a standardized scoring system for UC makes comparison of trial results less accurate than comparing results in CD trials and makes interpretation of individual trial results prone to bias.
We identified a systematic review (CitationGisbert et al 2007), search date 2006, and a Cochrane review (CitationLawson et al 2006), search date 2005, that assessed the efficacy of infliximab in inducing remission in moderate to severe UC. Both identified 5 randomized, double-blind, placebo-controlled studies; however, while the systematic review by Gisbert et al reported the results in terms of short- and long-term response only, the Cochrane review by Lawson et al used a more complex approach of reporting on clinical remission and response, endoscopic and mucosal responses, and colectomy rates. We will describe the studies and the various differences between the two meta-analyses.
In the first double-blind, placebo-controlled trial, CitationSands et al (2001) recruited 11 patients with active severe UC as defined by the Truelove and Witts criteria (8 men and 3 women; age range 20–63). Patients were required to have received at least 7 days of corticosteroid treatment and were permitted to be treated with immunomodulators excluding cyclosporine. Patients were randomly assigned to receive a single intravenous infusion of either placebo or infliximab at 5, 10, or 20 mg/kg. Assessment of outcome was done at week 2. The follow-up period was 4 weeks. The study was terminated prematurely because of slow enrolment.
Probert et al evaluated 43 patients with moderate UC based on ulcerative colitis symptom score (CitationSchroeder et al 1987) and a sigmoidoscopy score (F/M ratio not given; age range 29–50.5) (CitationProbert et al 2003). Patients were required to have been treated with corticosteroids for at least 1 week and were allowed to have received immunomodulators excluding cyclosporine if they were on a stable dose for at least 3 months prior to study enrollment. Patients were randomly assigned to receive an infusion of 5 mg/kg infliximab or placebo at week 0 and an identical infusion at week 2. Assessment of endpoints was done at week 6, with follow-up for the following 30 days.
Järnerot et al evaluated 45 patients with moderate to severe UC according to the Seo index (CitationSeo et al 1992) (24 men and 21 women; age range 19–61) (CitationJärnerot et al 2005). Patients were required to have received at least 4 days of intravenous corticosteroids. No specific remark concerning prior treatment was defined in the inclusion criteria and 8 patients were treated with azathioprine at the time of enrollment. Patients were randomized to receive a single infusion of 5 mg/kg 4 days after initiation of intravenous corticosteroids. Outcome measures were assessed 90 days after infliximab/placebo infusion.
Rutgeerts et al conducted the two largest studies to date that evaluated the efficacy of infliximab for induction and maintenance of remission in UC (CitationRutgeerts et al 2005). In the ACT1 study, 364 patients with moderate to severe colitis according to the Mayo Clinic score (CitationSchroeder et al 1987) with active colitis by sigmoidoscopy were recruited (222 men 142 women; age range 27–55). Patients were required to be on concurrent therapy with corticosteroids alone or with immunosuppressants. Patients were not required to be on concurrent treatment if they had previously not responded to these medications. Eligible patients were randomized to receive 5 mg/kg, 10 mg/kg of infliximab, or placebo in a 1:1:1 ratio at weeks 0, 2, and 6 and then every 8 weeks for 46 weeks overall and were followed through week 54. Outcome was measured at week 8, 30, and 54.
ACT 2 was similar in design and included 364 patients (215 men and 149 women; age range 26–53). Patients received 5 mg/kg or 10 mg/kg infliximab or placebo in a 1:1:1 ratio at weeks 0, 2, and 6 and then every 8 weeks through week 22 and were followed through week 30.
Interpretation of the results of the studies varied between the Cochrane and the systematic review. The systemic review by CitationGisbert et al (2007) evaluated short-term responses of infliximab vs placebo and found them to be 65% and 33%, respectively (95% confidence interval [CI] 61%–69% and 27%–38%, respectively). The odds ratio (OR) for the response was 3.6 (95% CI 2.67–4.95; p < 0.001), the number of treatments needed to achieve short-term response was 3 (95% CI, 3–4) without significant heterogeneity between the studies. Short-term remission was achieved in 33% (95% CI 29%–37%) and 10% (95% CI, 6.4–14%) in the infliximab and placebo groups, respectively. The OR for the remission was 4.56 (95% CI 1.98–10.5; p < 0.001) with NNT being 4 (95% CI 3–6), results being significantly heterogeneous.
Long-term response was 53% and 24% in the infliximab and placebo groups (95% CI 49%–58% and 19%–29%), respectively. The OR for the rsponse was 3.4 (95% CI 2.52–4.59; p < 0.001) with NNT to achieve long term response being 3 (95% CI 3–4) and no significant heterogeneity. Finally, long-term remission was achieved in 33% vs 14% in the infliximab and placebo groups (95% CI 29%–37% and 9%–18%), respectively. The OR for the remission was 2.72 (95% CI 1.92–3.38; p < 0.001) without significant heterogeneity and NNT to achieve long-term remission being 5 (95% CI 4–7).
The Cochrane review (CitationLawson et al 2006) adopted a slightly different approach, describing the results in terms of reaching pre-defined end points.
Clinical remission
This endpoint was described in only 4 trials. The authors combined the ACT1 and ACT2 trials for the purpose of meta-analysis and reported the other two studies separately because of different outcome measures. Infliximab was effective for the induction of clinical remission at 8 weeks (relative risk [RR] 3.22, 95% CI 2.18–4.16, NNT = 5). There was no significant difference in the results for the subgroup analysis of 5 mg/kg and 10 mg/kg of infliximab. The two small studies that reported this outcome showed a trend favoring infliximab but failed to reach statistical significance (CitationProbert et al 2003; CitationJärnerot et al 2005).
Endoscopic remission/mucosal healing
This outcome was also described in 4 studies. The two smaller studies did not reach statistical significance. The two larger studies were grouped for meta-analysis. Infliximab was effective in inducing endoscopic remission and mucosal healing at 8 weeks (RR 1.88, 95% CI 1.54–2.28, NNT = 4) without heterogeneity.
Clinical response
This endpoint was assessed in the ACT trials. infliximab effectively induced a clinical response (RR 1.99, 95% CI 1.65–2.41, NNT = 4) and there was moderate heterogeneity.
Treatment success
This outcome was assessed in only one small trial (CitationSands et al 2001). There was no statistical significance between infliximab and placebo.
Colectomy
This outcome was only described in one study (CitationJärnerot et al 2005). There was a significant reduction in rates of colectomy with infliximab (RR, 95% CI 0.22–0.78).
Quality of life
This outcome measure was assessed in one study (CitationProbert et al 2003). Using a standard questionnaire (IBDQ), the authors did not find a statistically significant difference between infliximab and placebo in improving the quality of life.
Safety
Adverse events related to infliximab were generally mild and did not differ between the treatment and placebo groups. Patients in the infliximab groups developed headache, upper respiratory tract infection, pruritus, nephrolithiasis, and catheter insertion complication. Recently, concerns regarding the issue of severe infections including tuberculosis and the development of lymphoma after infliximab treatment were raised. In the ACT1 and ACT2 studies, one patient treated with infliximab in each trial developed serious infections, one a case of tuberculosis, and the other fatal histoplasmosis. Patients in the infliximab groups were more likely to develop auto-antibodies than patients in the placebo groups.
Discussion and summary
IBD is a chronic disease with a relapsing and remitting course. Its pathogenesis involves a dysregulated immune response to luminal antigens in patients with a genetic susceptibility. IBD comprises of two distinct clinical entities, UC and CD. Despite immunologic differences between them, TNF-α, a pro-inflammatory cytokine, is hypothesized to play a key role in regulating the pathogenesis of both diseases. Therapy directed at blocking TNF-α action was shown to result in clinical improvement in patients with moderate to severe CD. In UC the role of TNF-α is less well established; however, there is mounting evidence that TNF-α gene polymorphisms are associated with disease extent and severity. Also, TNF-α levels were shown to be elevated in colonic tissue, feces, and serum of patients with UC and to correlate with disease severity. Initial studies evaluating the efficacy of anti-TNF-α treatment in moderate to severe UC showed relative success in inducing remission and in achieving clinical response. Most of these early studies were small and uncontrolled. Since then, 5 randomized placebo-controlled studies enrolling 827 patients assessed the efficacy of infliximab treatment in UC. The studies were heterogeneous. Three were small (less then 50 patients in each) and 2 were large (360 patients each). The studies utilized different outcome measures, making the results hard to compare. Two of the small studies did not show clinical benefit of infliximab (CitationProbert et al 2003; CitationSands et al 2001), raising the possibility of a type II error. The other three studies all showed clinical benefit to infliximab (CitationJärnerot et al 2005; CitationRutgeerts et al 2005); however, they did not assess the same endpoints. The smaller study by CitationJärnerot et al (2005) showed a reduction in the rates of colectomy but did not look at remission. The study was small (45 patients) and the follow-up short (13 weeks). The two larger studies by CitationRutgeerts et al (2005) showed benefit in induction of remission, clinical response, discontinuing of corticosteroids, and mucosal healing in the groups treated with infliximab, but did not assess rates of colectomy. Based on the three studies and the two systematic reviews, infliximab is effective in inducing remission and improving clinical response in patients with moderate to severe UC. One study showed reduced rates of colectomy and two small studies did not find any benefit from infliximab treatment. The proportion of patients who reported adverse events were similar between the treatment and placebo groups. Most adverse events were mild. In the ACT1 and ACT2 studies, there was a slight increase in the number of serious infections, neurological complications, and lupus-like reactions in the infliximab groups with one fatal disseminated histoplasmosis.
Until recently, the only available medical treatment for patients with moderate to severe UC that failed conventional treatment was cyclosporine. Although infliximab is sometimes considered an alternative to cyclosporine, we believe that no competition should actually exist between the two drugs: infliximab has a slower mode of action and is used to benefit moderate to severe UC in ambulatory patients. Cyclosporine continues to be the only effective alternative to emergency colectomy in patients hospitalized for severe fulminant disease. The role of combined cyclosporine and infliximab has not been assessed and would increase the risk of profound immunosuppression. There are already reports of an increase in peri-operative infections in UC patients undergoing colectomy after treatment with both infliximab and cyclosporine.
The data reviewed here emphasizes the importance of establishing a uniform scoring system to UC similar to the one used in CD. Such a scoring system will enable appropriate comparison of clinical outcomes and assist in conducting adequate drug trials in UC. Special attention should be given to elucidating the mechanism of action of infliximab. Understanding the role of TNF-α and other mediators in the pathogenesis of UC will enhance our ability to design new drugs that will target key chemokines and cytokines regulators of the intestinal inflammatory process. Although the trials provide an answer to the question concerning the place of infliximab in the treatment of moderate to severe UC, several clinical questions remain unanswered. What is the role of infliximab in inducing remission in mild to moderate UC? What is the role of infliximab in maintenance of remission? Can infliximab be used for the treatment of extra-intestinal manifestations of UC? Is there a role for infliximab in the treatment of pouchitis? These questions will have to await the results of future well designed, double-blind, placebo-controlled trials.
Abbreviations
| CRP | = | C-reactive protein |
| CI | = | confidence interval |
| IBD | = | inflammatory bowel disease |
| CD | = | Crohn’s disease |
| UC | = | ulcerative colitis |
| TNF-α | = | tumor necrosis factor-α |
Disclosures
Neither author has any conflicts of interest to disclose.
References
- ActisGCBrunoMPinna-PintorM2002Infliximab for treatment of steroid-refractory ulcerative colitisDig Liver Dis3463112405249
- AhmadTArmuzziANevilleM2003The contribution of human leucocyte antigen complex genes to disease phenotype in ulcerative colitisTissue Antigens6252714617036
- ArgilesJMLopez-SorianoJBusquetsS1997Journey from cachexia to obesity by TNFFaseb J117439271359
- ArmuzziADe PascalisBLupascuA2004Infliximab in the treatment of steroid-dependent ulcerative colitisEur Rev Med Pharmacol Sci823115638236
- AtreyaRMudterJFinottoS2000Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivoNat Med658310802717
- BaughJABucalaR2001Mechanisms for modulating TNF alpha in immune and inflammatory diseaseCurr Opin Drug Discov Devel4635
- BaumgartDCSandbornWJ2007Inflammatory bowel disease: clinical aspects and established and evolving therapiesLancet369164117499606
- BazzoniFBeutlerB1996The tumor necrosis factor ligand and receptor familiesN Engl J Med33417178637518
- BermejoFLopez-SanromanAHinojosaJ2004Infliximab induces clinical, endoscopic and histological responses in refractory ulcerative colitisRev Esp Enferm Dig969415255018
- BinderV2004Epidemiology of IBD during the twentieth century: an integrated viewBest Pract Res Clin Gastroenterol1846315157821
- BischoffSCLorentzASchwengbergS1999Mast cells are an important cellular source of tumour necrosis factor alpha in human intestinal tissueGut4464310205200
- BlackRARauchCTKozloskyCJ1997A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cellsNature3857299034190
- BoumaGCrusiusJBGarcia-GonzalezMA1999Genetic markers in clinically well defined patients with ulcerative colitis (UC)Clin Exp Immunol1152949933456
- BoumaGStroberW2003The immunological and genetic basis of inflammatory bowel diseaseNat Rev Immunol352112876555
- BoumaGXiaBCrusiusJB1996Distribution of four polymorphisms in the tumour necrosis factor (TNF) genes in patients with inflammatory bowel disease (IBD)Clin Exp Immunol1033918608636
- BraeggerCPNichollsSMurchSH1992Tumour necrosis factor alpha in stool as a marker of intestinal inflammationLancet339891345871
- BreeseEJMichieCANichollsSW1994Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel diseaseGastroenterology10614558194690
- BroomeULofbergRVeressB1995Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potentialHepatology2214047590655
- CaoQZhuQWuML2006Genetic susceptibility to ulcerative colitis in the Chinese Han ethnic population: association with TNF polymorphismsChin Med J (Engl)119119816863613
- CarterMJLoboAJTravisSP2004Guidelines for the management of inflammatory bowel disease in adultsGut53Suppl 5V115306569
- Castro FernandezMGarcia DiazERomeroM2003[Treatment of steroid-refractory ulcerative colitis with infliximab]Gastroenterol Hepatol265412525332
- CauwelsAJanssenBWaeytensA2003Caspase inhibition causes hyperacute tumor necrosis factor-induced shock via oxidative stress and phospholipase A2Nat Immunol438712652297
- CheyWYHussainARyanC2001Infliximab for refractory ulcerative colitisAm J Gastroenterol96237311513177
- DaneseSSemeraroSPapaA2005Extraintestinal manifestations in inflammatory bowel diseaseWorld J Gastroenterol11722716437620
- D’HaensGLemmensLGeboesK2001Intravenous cyclosporine versus intravenous corticosteroids as single therapy for severe attacks of ulcerative colitisGastroenterology120132311313301
- D’HaensGSandbornWJFeaganBG2007A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitisGastroenterology13276317258735
- ElliottMJMainiRNFeldmannM1993Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alphaArthritis Rheum3616818250987
- ElsonCOCongYMcCrackenVJ2005Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiotaImmunol Rev20626016048554
- GisbertJPGonzalez-LamaYMateJ2007Systematic review: Infliximab therapy in ulcerative colitisAliment Pharmacol Ther251917229218
- GornetJMCouveSHassaniZ2003Infliximab for refractory ulcerative colitis or indeterminate colitis: an open-label multicentre studyAliment Pharmacol Ther1817512869077
- Guy-GrandDDiSantoJPHenchozP1998Small bowel enter-opathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-gamma, TNF) in the induction of epithelial cell death and renewalEur J Immunol287309521083
- HanaiHWatanabeFYamadaM2004Correlation of serum soluble TNF-alpha receptors I and II levels with disease activity in patients with ulcerative colitisAm J Gastroenterol99153215307873
- HanauerSBCohenRDBeckerRV3rd1998Advances in the management of Crohn's disease: economic and clinical potential of infliximabClin Ther2010099829451
- InaKItohJFukushimaK1999Resistance of Crohn's disease T cells to multiple apoptotic signals is associated with a Bcl-2/Bax mucosal imbalanceJ Immunol163108110395708
- IwakuraYIshigameH2006The IL-23/IL-17 axis in inflammationJ Clin Invest116121816670765
- JacobsohnDAVogelsangGB2004Anti-cytokine therapy for the treatment of graft-versus-host diseaseCurr Pharm Des10119515078135
- JärnerotGHertervigEFriis-LibyI2005Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled studyGastroenterology128180515940615
- JungHCEckmannLYangSK1995A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasionJ Clin Invest95557814646
- KnightDMTrinhHLeJ1993Construction and initial characterization of a mouse-human chimeric anti-TNF antibodyMol Immunol3014438232330
- KohnAPranteraCPeraA2002Anti-tumour necrosis factor alpha (infliximab) in the treatment of severe ulcerative colitis: result of an open study on 13 patientsDig Liver Dis3462612405248
- KomatsuMKobayashiDSaitoK2001Tumor necrosis factor-alpha in serum of patients with inflammatory bowel disease as measured by a highly sensitive immuno-PCRClin Chem47129711427462
- KorzenikJRPodolskyDK2006Evolving knowledge and therapy of inflammatory bowel diseaseNat Rev Drug Discov519716518373
- KountourasJZavosCChatzopoulosD2005Anti-tumor necrosis factor therapy for ulcerative colitisGastroenterology129113816143158
- LawsonMMThomasAGAkobengAK2006Tumour necrosis factor alpha blocking agents for induction of remission in ulcerative colitisCochrane Database Syst Rev3CD00511216856078
- Lennard-JonesJERitchieJKHilderW1975Assessment of severity in colitis: a preliminary studyGut165791183857
- LichtensteinGRAbreuMTCohenR2006American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel diseaseGastroenterology13093516530531
- LichtigerSPresentDHKornbluthA1994Cyclosporine in severe ulcerative colitis refractory to steroid therapyN Engl J Med33018418196726
- LjungTKarlenPSchmidtD2004Infliximab in inflammatory bowel disease: clinical outcome in a population based cohort from Stockholm CountyGut5384915138212
- LoftusEVJrSilversteinMDSandbornWJ2000Ulcerative colitis in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survivalGut4633610673294
- MacDonaldTTHutchingsPChoyMY1990Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestineClin Exp Immunol813012117510
- MurchSHBraeggerCPWalker-SmithJA1993Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel diseaseGut3417058031350
- NilsenEMJohansenFEJahnsenFL1998Cytokine profiles of cultured microvascular endothelial cells from the human intestineGut426359659156
- OchsenkuhnTSackmannMGokeB2004Infliximab for acute, not steroid-refractory ulcerative colitis: a randomized pilot studyEur J Gastroenterol Hepatol16116715489577
- OldLJ1985Tumor necrosis factor (TNF)Science2306302413547
- PapadakisKATarganSR2000Role of cytokines in the pathogenesis of inflammatory bowel diseaseAnnu Rev Med5128910774465
- ParkHLiZYangXO2005A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17Nat Immunol6113316200068
- PeppelenboschMPvan DeventerSJ2004T cell apoptosis and inflammatory bowel diseaseGut53155615479669
- PlevySELandersCJPrehnJ1997A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn’s diseaseJ Immunol15962769550432
- PodolskyDK2002Inflammatory bowel diseaseN Engl J Med34741712167685
- ProbertCSHearingSDSchreiberS2003Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trialGut5299812801957
- Remicade package insert (Centocor–US), N., Rec 9/24/98.
- RuizPSan SalvadorPOrtiz de ZarateJ2004[Infliximab as treatment for a severe outbreak of ulcerative colitis]Gastroenterol Hepatol2743015461945
- RutgeertsPSandbornWJFeaganBG2005Infliximab for induction and maintenance therapy for ulcerative colitisN Engl J Med353246216339095
- SandbornWJHanauerSB1999Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safetyInflamm Bowel Dis511910338381
- SandsBEKaplanGG2007The role of TNFalpha in ulcerative colitisJ Clin Pharmacol4793017567930
- SandsBETremaineWJSandbornWJ2001Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot studyInflamm Bowel Dis78311383595
- SchroederKWTremaineWJIlstrupDM1987Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized studyN Engl J Med31716253317057
- SeoMOkadaMYaoT1992An index of disease activity in patients with ulcerative colitisAm J Gastroenterol879711642220
- ShenCde HertoghGBullensDM2007Remission-inducing effect of anti-TNF monoclonal antibody in TNBS colitis: mechanisms beyond neutralization?Inflamm Bowel Dis1330817206708
- ShiboletORegushevskayaEBrezisM2005Cyclosporine A for induction of remission in severe ulcerative colitisCochrane Database Syst RevCD00427715674937
- SiddiquiMAScottLJ2005Infliximab: a review of its use in Crohn’s disease and rheumatoid arthritisDrugs65217916225377
- SoetiknoRMLinOSHeidenreichPA2002Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysisGastrointest Endosc564812085034
- SpoettlTHausmannMKleblF2007Serum soluble TNF receptor I and II levels correlate with disease activity in IBD patientsInflamm Bowel Dis1372717260368
- StroberWFussIJBlumbergRS2002The immunology of mucosal models of inflammationAnnu Rev Immunol2049511861611
- SuCSalzbergBALewisJD2002Efficacy of anti-tumor necrosis factor therapy in patients with ulcerative colitisAm J Gastroenterol97257712385442
- TilgHMoschenAKaserA2007Mode of function of biological anti-TNF agents in the treatment of inflammatory bowel diseasesExpert Opin Biol Ther7105117665993
- TrueloveSCWittsLJ1955Cortisone in ulcerative colitis; final report on a therapeutic trialBr Med J2104113260656
- Van DeventerSJ1997Tumour necrosis factor and Crohn’s diseaseGut404439176068
- van DullemenHMvan DeventerSJHommesDW1995Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2)Gastroenterology1091297797011
- van HeelDAUdalovaIADe SilvaAP2002Inflammatory bowel disease is associated with a TNF polymorphism that affects an interaction between the OCT1 and NF(-kappa)B transcription factorsHum Mol Genet11128112019209
- VassalliP1992The pathophysiology of tumor necrosis factorsAnnu Rev Immunol104111590993
- WatkinsPEWarrenBFStephensS1997Treatment of ulcerative colitis in the cottontop tamarin using antibody to tumour necrosis factor alphaGut406289203942
- WilsonAGde VriesNPociotF1993An allelic polymorphism within the human tumor necrosis factor alpha promoter region is strongly associated with HLA A1, B8, and DR3 allelesJ Exp Med1775578426126
- WintherKVJessTLangholzE2003Survival and cause-specific mortality in ulcerative colitis: follow-up of a population-based cohort in Copenhagen CountyGastroenterology125157614724807
- XavierRJPodolskyDK2007Unravelling the pathogenesis of inflammatory bowel diseaseNature44842717653185