Abstract
Drugs used for remission induction therapy for childhood precursor-B acute lymphoblastic leukemia (ALL) are nonselective for malignant cells. Several garlic compounds have been shown to induce apoptosis of cancer cells and to alter lymphocyte function. To investigate the effect of garlic on the apoptosis of ALL cells and lymphocyte immune function, cells from newly diagnosed childhood ALL patients were cultured with several commonly used chemotherapeutic agents and several garlic compounds. Apoptosis, lymphocyte proliferation and T-cell cytokine production were determined using multiparameter flow cytometry. At concentrations of garlic compounds that did not result in significant increases in Annexin V and 7-AAD staining of normal lymphocytes, there was a significant increase in apoptosis of ALL cells with no alteration of T-cell proliferation as determined by CD25/CD69 upregulation or interferonγ, interleukin-2 or tumor necrosis factor-α intracellular cytokine production. In contrast, the presence of chemotherapeutic agents resulted in nonselective increases in both lymphocyte and ALL apoptosis and a decrease in T-cell proliferation and cytokine production. In conclusion, we show selective apoptosis of malignant cells by garlic compounds that do not alter T-cell immune function and indicate the potential therapeutic benefit of garlic compounds in the treatment of childhood ALL.
Chemotherapeutic agents used in the treatment of childhood precursor-B acute lymphoblastic leukemia (ALL) have been shown to induce apoptosis of ALL cells in vitro (Rohnghe et al 2001), however, hese drugs also induce apoptosis of normal lymphocytes (CitationLeussink et al 2001). Hence there is a need for therapeutic agents that selectively reduce tumour burden while maintaining viability and immune function of normal cells.
The role of dietary compounds such as garlic in cancer prevention and treatment has been widely studied and debated (CitationLamm and Riggs 2001). There is epidemiological evidence that oral consumption of garlic is effective in reducing the incidence of esophageal and gastric cancers (CitationTakezaki et al 2001).
Ajoene, a garlic-derived compound, has recently been shown to inhibit proliferation and induce apoptosis of human leukemia cells and induce apoptosis in myeloblasts from a CML patient in blast crisis (CitationDirsch et al 1998).
Allitridium, another garlic-derived compound, has been used intravenously for over twenty years in China to successfully treat Cryptococcus neoformans and other systemic fungal infections (CitationDavis et al 2003).
The aim of this present study was to investigate the effect of garlic and two garlic compounds, ajoene and allitridium, compared with commonly used chemotherapeutic drugs on apoptosis of ALL cells and normal lymphocytes in vitro from newly diagnosed ALL patients.
As garlic compounds have been shown to stimulate immune effector cells (CitationLamm and Riggs 2001) and modulate inflammatory cytokine production (CitationHodge et al 2002), the effect of garlic compounds and commonly used chemotherapeutic drugs on T-cell function following in vitro culture was also investigated.
Patients and methods
Patient samples
Blood and bone marrow samples were collected into preservative-free heparin from fifteen children presenting with pre-B ALL at the Women’s and Children’s Hospital, Adelaide and 12 age-matched volunteers. Mononuclear cells were isolated by standard density gradient centrifugation and cells resuspended in culture medium consisting of RPMI 1640 with 2 × 103 μmol/L L-glutamine (Gibco-BRL, Sydney, Australia) supplemented with 10% fetal calf serum (FCS; CSL, Sydney, Australia), 100 IU/mL penicillin, 100 μg/mL streptomycin (Gibco BRL) (used for all experiments).
These mononuclear cells were used in all subsequent experiments.
Garlic extract
Fresh garlic extract was prepared as previously reported (CitationDavis et al 2003). Garlic extract, Allitridium (Harvest Pharmaceutical Company, Shanghai, China), Ajoene (R.A.C., Venezuela) were diluted to desired protein concentration using culture medium immediately before use. Garlic compounds were used in a range of concentrations in experiments: ajoene (0.0005, 0.005, 0.01, 0.5, 5 μg/mL); allitridium (0.0002, 0.005, 0.02, 0.2, 2.5 μg/mL); garlic extract (0.001, 0.01, 0.1, 1, 10 μg/mL protein).
Chemotherapeutic drugs
Chemotherapeutic drugs commonly used in the treatment of childhood ALL were also diluted with culture medium immediately before use in experiments to a range of concentrations likely achievable in vivo (CitationHolleman et al 2003). Prednisolone (0.08, 0.8, 8, 80, 250 μg/mL) (Pharmacia and Upjohn, Sydney, Australia); L-asparaginase (0.003, 0.03, 0.3, 3, 10 μg/mL) (Kyowa Hakko Kogyo, Tokyo, Japan); vincritine (0.005, 0.05, 0.5, 5, 50 μg/mL) (Mayne Pharma, Mulgrave, Vic, Australia); daunorubicin (0.002, 0.02, 0.2, 2.0, 20 μg/mL) (DBL, Sydney, Australia).
Cell culture assays with drugs for apoptosis measurement
Sensitivity of cells to several garlic compounds and several drugs commonly used in the treatment of childhood were tested by adding 80 μL aliquots of cell suspension (2 × 106 cells/mL) PBMC and/or BMMC to 20 μL of various concentrations of drugs in triplicate round-bottomed 96-well microculture plates. Cultures were incubated in a humidified 5% CO2/95% air atmosphere at 37 °C. At 24, 48, and 72 hrs, cells were resuspended and 100 μL aliquots removed and stained with Annexin V FITC and 7-amino-actinomycin D (7-AAD) (Sigma, Sydney, Australia) as previously reported (CitationHodge et al 2005a) and analyzed using a FACSCalibur flow cytometer using CELLQUEST software (BD).
Cell culture assays with drugs for T-cell activation
The effect of garlic compounds and drugs used in the treatment of childhood ALL on normal T-cell activation was determined by incubating these compounds with the purified cells for 1 hr in a humidified 5% CO2/95% air atmosphere at 37 °C. Phytohaemmagglutinin (10 μg) (Sigma) was then added to these cell cultures and plates reincubated in a humidified 5% CO2/95% air atmosphere at 37 °C. At 24 hrs, cell cultures were resuspended and 100 μL aliquots removed and stained with 5 μL of CD25 FITC (BD), CD69 PE (BD) and CD3 PC5 (Beckman Coulter) as previously reported (CitationHodge et al 2001).
Cell culture assays with drugs for intracellular T-cell cytokine production
The effect of garlic compounds and drugs used in the treatment of childhood ALL on T-cell cytokine production was determined by incubating these compounds with the purified cells (prepared as for the apoptosis experiments above) for 4 hrs and plates incubated at room temperature for 4 hrs. Cells were stimulated and intracellular cytokine production performed as previously reported (CitationHodge et al 2005b).
Results
Effect of drugs on ALL and lymphocyte apoptosis
Annexin V staining of normal lymphocytes from healthy controls and blasts and lymphocytes from ALL patients following 24 hrs culture is shown in .
Table 1 The percentage of Annexin V positive and 7-AAD positive CD3+ lymphocytes and acute lymphoblastic leukemia cells following culture in the presence of no drugs (Control), 2 μg/mL daunorubicin, 50 μg/mL vincristine, 10 IU/mL L-asparaginase, 250 μg/mL prednisolone, 0.1 μg/mL garlic extract, 0.01 μg/mL ajoene, 0.005 μg/mL allitridium compared with normal healthy control subjects
There was a dose-dependent increase in both Annexin V positive ALL cells and lymphocytes in the presence of all drugs (p < 0.05 for all, Mann Whitney) except lymphocytes in the presence of garlic extract (p = 0.235) () compared with control with no drug (dose dependent data not shown).
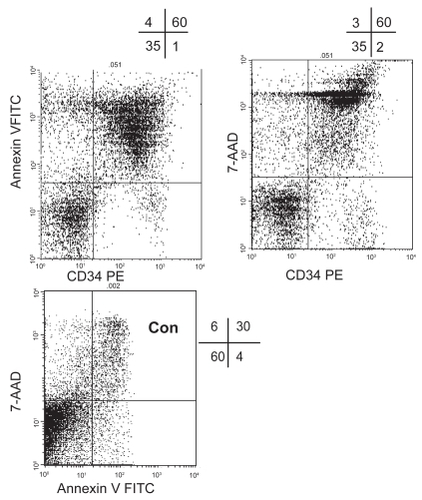
Following culture for 48 hrs, there was a significant increase in Annexin V/7AAD positive lymphocytes and ALL cells in the presence of all drugs compared with control (p < 0.05) except lymphocytes in the presence of garlic extract (p = 0.489) and ajoene (p = 0.380) (). There was a significant increase in Annexin V/7AAD positive ALL cells in the presence of garlic compared with several drugs. Representative dot plots showing Annexin V FITC and 7-AAD staining of CD34 positive ALL cells and CD34 negative lymphocytes following culture in the presence of 0.1 μg/mL garlic extract for 48 hrs are shown in . Almost all CD34 positive ALL cells stain positively for both Annexin V FITC and 7-AAD but very few CD34 negative lymphocytes show signs of apoptosis.
Figure 1 Representative dot plots showing Annexin V FITC and 7-AAD staining of CD34 positive ALL cells and CD34 negative lymphocytes following culture in the presence of 0.1 μg/mL garlic extract for 48 hrs compared with control (Con) (no drug) (gated on CD34 positive ALL cells). Almost all CD34 positive ALL cells stain positively for both Annexin V FITC and 7-AAD in the presence of 0.1 μg/mL garlic extract but very few CD34 negative lymphocytes show signs of apoptosis.
Effect of drugs on lymphocyte activation
At concentrations of garlic compounds that resulted in significant apoptosis of ALL cells but not of T-cells as determined by Annexin V and 7-AAD staining, there was no significant change in CD25 and CD69 upregulation (p > 0.05) (). In the presence of most drugs, there was a dose-dependent inhibition in CD25 and CD69 upregulation (p < 0.05) (dose-dependent data not shown). The inhibition of these cytokines was significantly greater in the presence of most drugs compared with garlic compounds. Representative dot plots showing CD25 FITC and CD69 PE staining of CD3 positive T cells in the presence of 0.1 μg/mL garlic extract and 2 μg/mL daunorubicin, compared with control without drugs following culture with 10 μg/mL PHA for 24 hrs are shown in . There was a significant decrease in both CD25 and CD69 in the presence of daunorubicin (p < 0.05) but not garlic extract compared with control with no drugs.
Figure 2 Representative dot plots showing CD25 FITC and CD69 PE staining of CD3 positive T cells in the presence of (A) 0.1 μg/mL garlic extract and (B) 2 μg/mL daunorubicin, compared with (C) Control, following culture with 10 μg/mL PHA for 24 hrs. There was a significant decrease in both CD25 and CD69 in the presence of daunorubicin (p < 0.05) but not garlic extract compared with control(C).
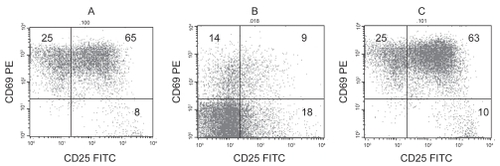
Table 2 The percentage of CD25 positive, CD69 positive, CD3 positive lymphocytes (mean ± sd) following culture in the presence of no drugs (Control), 2 μg/mL daunorubicin, 50 μg/mL vincristine, 10 IU/mL L-asparaginase, 250 μg/mL prednisolone, 0.1 μg/mL garlic extract, 0.01 μg/mL ajoene, 0.005 μg/mL allitridium compared with normal healthy control subjects
Effect of drugs on lymphocyte cytokine production
At concentrations of garlic compounds that resulted in significant apoptosis of ALL cells but not of T-cells as determined by Annexin V and 7-AAD staining, there was no significant change in intracellular interferonγ (IFNγ), interleukin-2 (IL-2), or tumor necrosis factor-α (TNFα) production (). In the presence of most drugs, there was a dose-dependent inhibition in intracellular IFNγ, IL-2, TNFα production (p < 0.05). The dose-dependent inhibition of IFNγ by drugs is shown in (dose-dependent data for IL-2 and TNFα not shown). The inhibition of these cytokines was significantly greater in the presence of most drugs compared with garlic compounds. Representative dot plots showing intracellular CD8+ and CD8− (CD4+) T-cell production of IFNγ, IL-2 and TNFα in the presence of 0.1 μg/mL garlic extract and 50 μg/mL vincristine from PBMC from a patient with ALL compared with an aged-matched control with no drugs is shown in . The percentage and mean fluorescence intensity of CD8+ and CD8− (CD4+) T-cells producing IFNγ, IL-2, and TNFα was significantly inhibited (p < 0.05) in the presence of vincristine but not garlic extract (p < 0.05) compared with control without drugs (data not shown). Intracellular CD8+ and CD8− (CD4+) T-cell production of IFNγ, IL-2, and TNFα by PBMC from the ALL patient was significantly decreased (p < 0.05) compared with the healthy age-matched control.
Figure 3 Graph showing dose-dependent inhibition of T-cell IFNγ production in the presence of increasing concentrations of D: daunorubicin (0.002, 0.02, 0.2, 2.0, 20 μg/mL), V: vincritine (0.005, 0.05, 0.5, 5, 50 μg/mL), Al: allitridium (0.0002, 0.005, 0.02, 0.2, 2.5 μg/mL), L: L-asparaginase (0.003, 0.03, 0.3, 3, 10 μg/mL), Aj: ajoene (0.0005, 0.005, 0.01, 0.5, 5 μg/mL), G: garlic extract (0.001, 0.01, 0.1, 1, 10 μg/mL) and P: Prednisolone (0.08, 0.8, 8, 80, 250 μg/mL).
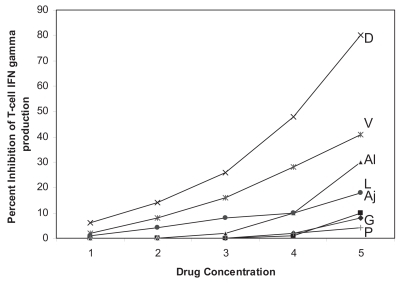
Figure 4 Representative dot plots showing intracellular CD8+ and CD8− (CD4+) T-cell production of IFNγ, IL-2 and TNFα in the presence of (A) 0.1 μg/mL garlic extract and (B) 50 μg/mL vincristine from PBMC from a patient with ALL compared with healthy aged-matched control subject (C). The percentage and mean fluorescence intenstiy of CD8+ and CD8− (CD4+) T-cell producing IFNγ, IL-2, and TNFα was significantly inhibited (p < 0.05) in the presence of vincristine but not garlic extract (p > 0.05) compared with control without drugs (data not shown). Intracellular CD8+ and CD8− (CD4+) T-cell production of IFNγ, IL-2, and TNFα by PBMC from the ALL patient was significantly decreased (p < 0.05) compared with control.
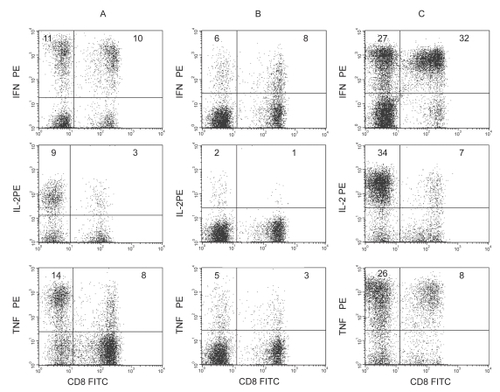
Table 3 The percentage of cytokine positive CD3 positive lymphocytes (mean ± SD) following culture in the presence of no drugs (Control), 2 μg/mL daunorubicin, 50 μg/mL vincristine, 10 IU/mL L-asparaginase, 250 μg/mL prednisolone, 0.1 μg/mL garlic extract, 0.01 μg/mL ajoene, 0.005 μg/mL allitridium compared with control subjects (n = 12)
Discussion
We report selective apoptosis of malignant cells by garlic compounds that do not alter lymphocyte viability or T-cell immune function in vitro. Drugs used for remission induction therapy for childhood pre-B ALL have been shown to be nonselective in targeting only malignant cells and result in loss of hemopoietic cells (CitationLeussink et al 2001) and our current studies confirm these findings.
Our findings regarding the apoptotic effect of currently used chemotherapeutic drugs on childhood pre-B ALL cells are consistent with a recent report (CitationHolleman et al 2003). We now show that the presence of these drugs result in a rapid transition of CD34 positive ALL cells from early (Annexin V+/7AAD−) to late apoptosis (Annexin V+/7AAD+).
However, there have been reports of CD34 negative leukemic stem cells being resistant to current therapeutic strategies and that subsequent relapses may arise from this population (Cox and Blair 2005). Further studies into the effect of garlic compounds and current chemotherapeutic drugs on this small leukemic stem cell population are required once more precise definition of this subset is available.
T-helper-type 1 (Th1) responses are important in immune response against malignant cells, viruses and microbial infections. T-cell immune function has been shown to be impaired in children with ALL before and during treatment (CitationNash et al 1993). A previous study showed that intracellular T-cell IFNγ and IL-2 production was impaired in children with ALL (CitationNash et al 1993) and our current findings of reduced intracellular T-cell IFNγ and IL-2 by the majority of these patients confirms these previous findings. Our additional findings of impaired T-cell TNFα production are important as TNFα has been shown to inhibit tumor cell growth in a variety of hematological malignancies (CitationReed and Pellechia 2005) and stimulate immune response to tumors (CitationVassalli 1992). Our current findings show that children with ALL have an impaired Th1 response at presentation and are subsequently treated with drugs that further impair the antileukemic immune response. Not surprisingly, we have previously shown that apoptotic cells have an impaired capacity for Th1 cytokine production (CitationHodge et al 2000). In contrast, the presence of garlic compounds did not impair T-cell proliferation or Th1 responses by T cells from children with ALL or normal healthy controls.
Patients with childhood ALL are susceptible to a variety of bacterial, fungal, and viral infections. Garlic compounds have been shown to have antimicrobial properties in the prevention and treatment of common infective organisms in these patients such as methicillin-resistant Staphylococcus aureus (CitationCutler and Wilson 2004), Escherichia coli (CitationSasaki et al 1999), Aspergillus spp. (CitationShadkchan et al 2004), and invasive fungal infections (CitationDavis 2005) and the mechanism of action of garlic has recently been described (CitationDavis 2005). Garlic compounds have also been shown to be effective in the clinic against CMV, a common pathogen isolated from these patients (CitationLu 1994). The intravenous use of garlic compounds in the treatment of invasive fungal infections in humans has been shown to be safe and effective (CitationDavis 2003) and anti-fungal activity has been shown in human serum and urine after ingestion of garlic (CitationCaporaso et al 1983) suggesting either form of therapy for the treatment of childhood ALL may be effective. Further investigations are warranted into the dose of garlic extracts, both oral and intravenous, required to achieve efficacy in the treatment of childhood ALL. After oral consumption or intravenous dose of garlic extracts to volunteers, serum from these volunteers could be substituted in similar experiments to that described in this manuscript to determine optimal dosage to achieve maximal killing of ALL cells while maintaining Th1 response.
Previous findings taken together with our current investigations argue for the use of garlic compounds in the therapeutic treatment of childhood pre-B ALL to selectively kill ALL cells and prevent morbidity associated with pathogenic microbial organisms while maintaining cellular immune function.
Acknowledgments
This study was supported by a Channel 7 Foundation Research Grant. The authors report no conflicts of interest.
References
- CutlerRRWilsonP2004Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin-resistant Staphylococcus aureusBr J Biomed Sci6171415250668
- CaporasoNSmithSEngR1983Antifungal activity in human serum and urine after ingestion of garlic (Allium sativum)Antimicrob Agents Chemother2370026870217
- DavisSR2005An overview of the antifungal properties of allicin and its breakdown products- the possibility of a safe and effective antifungal prophylacticMycoses489510015743425
- DavisSPerrieRApitz-CastroR2003In vitro susceptibility of S. Prolificans to ajoene, allitridium and raw extract of garlicJ Antimicrob Chemother51593712615859
- DirschVMGerbesALVollmarAM1998Ajoene, a compound of garlic, induces apoptosis in human promyelocytic cells, accompanied by generation of reactive oxygen species and activation of nuclear factor kappaBMol Pharmacol5340279495804
- HodgeGHanP2001Effect of Factor VIII concentrate on lymphocyte proliferation and apoptosis: TGFβ is a significant immunomodulatory component of FVIIIBr J Haematol1153768111703339
- HodgeGHodgeSHanP2002Allium sativum (Garlic) suppresses leucocyte inflammatory cytokine production in vitro: potential therapeutic use in the treatment of inflammatory bowel diseaseCytometry482091512210145
- HodgeSHodgeGHolmesM2005aIncreased apoptosis in the airways in COPD persists after smoking cessationEur Resp J2544754
- HodgeGHodgeSReynoldsP2005bIntracellular cytokines in blood T cells in lung transplant patients- a more relevant indicator of immunosuppression than drug levelsClin Exper Immunol1391596415606627
- HodgeGHodgeSHanP2000Increased levels of apoptosis of leucocyte subsets in PBMCs compared to whole blood as shown by Annexin V binding- relevance to cytokine productionCytokine121763811097745
- HollemanAden BoerMLKazemierKM2003Resistance to different classes of drugs is associated with impaired apoptosis in childhood acute lymphoblastic leukemiaBlood1024541612920041
- LammDLRiggsDR2001Enhanced immunocompetence by garlic: role in bladder cancer and other malignanciesJ Nutr131106770
- LeussinkVIJungSMerschdorfU2001High dose methylprednisolone therapy in multiple sclerosis induces apoptosis in peripheral blood leucocytesArch Neurol5891711176941
- LuDP1994Bone marrow transplantation in the Peoples Republic of ChinaChinese Bone Transplant Registry137034
- NashKAMohammedGNandapalanN1993T cell function in children with acute lymphoblastc leukemiaBr J Haematol83419278485047
- ReedJCPellecchiaM2005Apoptosis-based therapies for haematological malignanciesBlood1064081815797997
- RongheMBurkeGALowisSP2001Remission induction therapy for childhood acute lymphoblastc leukemia: clinical and cellular pharmacology of vincristine, corticosteroids, L-asparaginase and anthracyclinesCancer Treat Rev273273711908926
- TakezakiTGaoCMWuJZ2001Dietary protective and risk factors for oesophageal and stomach cancers in a low epidemic area for stomach cancer in Jiangsu Province, China: comparison with those in a high epidemic areaJapan J Cancer Res9211576511714439
- SasakiJKitaTIshitaK1999Antibacterial activity of garlic powder against Escherichia coli 0-157J Nutr Vitaminol (Tokyo)4578590
- ShadkchanYShemeshEMirelmanD2004Efficacy of allicin, the reactive molecule of garlic, in inhibiting Aspergillus spp. in vitro, and in a murine model of aspergillosisJ Antimicrob Chemother53832615044429
- VassalliP1992The pathophysiology of tumour necrosis factorsAnn Rev Immunol10411521590993