Abstract
By using a semi-quantitative immunoblotting technique, we have analyzed serum immunoglobulin G (IgG) reactivities of patients with limited cutaneous systemic sclerosis and anticentromere antibodies, patients with diffuse systemic sclerosis and antitopoisomerase 1 antibodies, patients with diffuse systemic sclerosis without antitopoisomerase 1 or anticentromere antibodies and age- and gender-matched healthy controls with normal human skin fibroblasts and HEp-2 cells antigens. Serum IgG reactivities of patients with diffuse systemic sclerosis and antitopoisomerase 1 antibodies differed significantly from those of healthy controls or systemic sclerosis patients in other groups for reactivity with fibroblast proteins. IgG from patients with antitopoisomerase 1 antibodies bound to a 90 kDa fibroblast band and to a 100 kDa protein band in a HEp-2 cell protein extract. These two bands were further identified as DNA topoisomerase 1. Our results indicate that IgG from patients with diffuse systemic sclerosis bind DNA topoisomerase 1 in normal human fibroblasts extracts.
Introduction
Systemic sclerosis (SSc) is a connective tissue disease characterized by vascular hyperreactivity, obliterative microvascular phenomena and by skin and internal organs fibrosis. Endothelial cells, fibroblasts and leukocytes, particularly monocytes and lymphocytes participate in the pathogenesis of the disease, although none of these cells has been identified as the unique initiating factor (CitationTamby et al 2003; CitationServettaz et al 2006).
Several lines of evidence suggest that unchecked activation of fibroblasts is involved in SSc. Thus, fibroblasts from SSc patients are activated to produce high amounts of extracellular matrix proteins such as types I and III collagens, proteoglycans, fibronectin, and metalloproteinase inhibitors (CitationLeask et al 2002). This might result from constitutive dysregulations of transforming growth factor beta (TGFβ) signaling pathway or from the influence of environmental factors, ie, excess of cytokines synthesis or targeting by autoantibodies.
Many autoantibodies have been detected in the serum of SSc patients, including disease specific such as anticentromere antibodies, associated with limited cutaneous SSc (CitationMoroi et al 1980; CitationSteen et al 1988); antitopoisomerase 1 antibodies associated with diffuse SSc (CitationSteen et al 1988; CitationWeiner et al 1988); and anti-RNA-polymerase III antibodies which are associated with renal involvement (CitationBunn et al 1998). In addition, non specific autoantibodies have also been identified in the serum of SSc patients, including antifibrillin 1 (CitationTan et al 1999), antiendothelial cell antibodies (AECA) (CitationIhn et al 2000; CitationSgonc et al 2000; CitationGarcia de la Pena-Lefebvre et al 2004), and antifibroblast antibodies (AFA) (CitationBrentnall et al 1982; CitationAlderuccio et al 1989; CitationChizzolini et al 2002; CitationRonda et al 2002; CitationZhou et al 2005; CitationBaroni et al 2006). Antifibroblast antibodies can activate fibroblasts and induce extracellular matrix proteins synthesis (CitationChizzolini et al 2002). DNA topoisomerase 1 was recently identified as a target of AFA (CitationHenault et al 2004). However, further experiments provided evidence that these antibodies did not target directly topoisomerase 1 expressed at the fibroblast surface but rather recognized membrane-bound topoisomerase 1 (CitationHenault et al 2004). Other target antigens of antifibroblast antibodies have been identified: namely fibrillin-1 (CitationZhou et al 2005) and platelet-derived growth factor (PDGF) receptor (CitationBaroni et al 2006).
In order to confirm these results and/or to identify specific target antigens of AFA in SSc patients, we decided to analyze reactivity patterns of immunoglobulin G (IgG) and IgM from SSc patients and age-gender-, and parity-matched healthy controls with fibroblasts.
Materials and methods
Patients
All patients gave their written informed consent. Limited cutaneous SSc was defined by skin thickening in areas solely distal to the elbows and knees, with or without facial involvement; diffuse SSc was defined by the presence of skin thickening proximal, as well as distal, to the elbows and knees, with or without facial or truncal involvement (CitationLeRoy et al 1988). Sixty patients were included in the study: 20 (16 females and 4 males, mean age 53.5 ± 15.2 years) with diffuse SSc and antitopoisomerase 1 antibodies, 20 (all females, 56.7 ± 12.8 years) with limited cutaneous SSc and anticentromere antibodies and 20 (14 females and 6 males, mean age 50.9 ± 10.9 years) with diffuse SSc and no antitopoisomerase 1 or anticentromere antibody. For these 3 groups, disease durations were measured from the onset of first non-Raynaud’s phenomenon symptom (11.3 ± 9.6 years, 9.4 ± 9.5 years, 5.6 ± 5.4 years in the 3 groups, respectively). Interstitial lung disease, assessed by chest high-resolution computed tomodensitometry and abnormal pulmonary function test values (total lung capacity or vital capacity less than 80% of predicted values and/or diffusing capacity <75% of that predicted) was present in all patients with antitopoisomerase 1 antibodies, in 13 patients without antitopoisomerase 1 or anticentromere antibody and none of the patients with anticentromere antibodies. Pulmonary arterial hypertension, defined as systolic pulmonary artery pressure was >40 mm Hg (CitationMukerjee et al 2003) as measured by Doppler echocardiography, was diagnosed in 4 patients with antitopoisomerase 1 antibodies, 2 patients with anticentromere antibodies and 3 patients without antitopoisomerase 1 or anticentromere antibody. None of the patients included had cancer or another connective tissue disease. None of the patients received corticosteroids or immunosuppressive therapy. Sixty unrelated healthy age-, gender- and parity-matched donors served as controls.
Ig sources
Serum samples were obtained from patients and controls and stored in aliquots at −80 °C until tested. Mean serum IgG and IgM concentrations determined by nephelometry were respectively: 10.6 ± 2.9 and 1.4 ± 0.8 mg/ml in patients with anticentromere antibodies, 13.9 ± 3.9, 1.7 ± 0.74 mg/ml in patients with antitopoisomerase 1 antibodies, 14.4 ± 3.3 and 1.9 ± 1.0 mg/ml in patients without specific autoantibody and 12.0 ± 2.8 and 1.6 ± 0.7 in healthy controls. Serum IgG and IgM concentrations did not differ significantly between patients in the different groups and controls. A therapeutic preparation of normal human IgG, intravenous immunoglobulin (IVIg) and normal human polyclonal IgM (LFB, Les Ulis, France) served as internal standards.
Cell culture
Fibroblasts
Normal human dermal fibroblasts were cultured from histologically normal skin obtained in 3 donors undergoing resection of basocellular carcinoma. Biopsy specimen were cut into small sections and seeded into Petri dishes and then into 162 cm2 plastic flasks. Fibroblasts were grown in Dulbecco’s modified Eagle’s medium (DMEM) with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 10 mM Hepes, 100 U/ml penicillin, 100 μg/ml streptomycin (Life Technologies Ltd, Auckland, NZ) at 37 °C in 5% CO2. When confluent, the cells were detached using 0.05% trypsin with 2 μM EDTA.
HEp-2 cells
HEp-2 cells were obtained from EuroBio (Les Ulis, France) in 162 cm2 flasks in DMEM and cultured at 37 °C in 5% CO2 supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 10 mM hepes, 20 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin (Life Technologies Ltd). When confluent, the cells were detached using 0.05% trypsin, 2 μM EDTA.
Detection of antibody reactivities
For each patient and healthy control, antinuclear antibodies were investigated by indirect immunofluorescence on HEp-2 cells and considered positive at a dilution ≥1/160. Anticentromere antibodies were characterized by a pattern of discrete dots in cells lined up on the metaphase plate in dividing interphase cells. Antitopoisomerase 1 antibodies were detected by ELISA with a commercial kit (Inova Diagnostics Inc, San Diego, CA, USA) using purified calf thymus topoisomerase 1 and sera diluted 1:100.
IgG and IgM reactivities were analyzed using a semi-quantitative immunoblotting technique with normal human dermal fibroblasts and HEp-2 cells. Cellular protein extracts were performed in a buffer containing 4% sodium dodecyl sulfate, 1.45 M 2-mercaptoethanol, 125 mM Tris/HCl pH 6.8, 1 μg/ml aprotinin, 1 μg/ml pepstatin and 1 μg/ml leupeptin and then sonicated 4 × 30 s (CitationChanseaud et al 2003; CitationGarcia de la Pena-Lefebvre et al 2004). Equal amounts of solubilized proteins were subjected to preparative sodium dodecyl sulfate–polyacrylamide gel electrophoresis through 10% polyacrylamide gels. The proteins were then transferred onto nitrocellulose membranes for 1 h at 0.8 mA/cm2 using a semi-dry electroblotter model A (Ancos, Hojby, Denmark). After blocking with phosphate buffer saline −0.2% Tween® for 90 min, the membranes were incubated with sera for 4 h at room temperature at 200 μg/mL for IgG and 20 μg/mL for IgM in a cassette miniblot system (Immunetics Inc., Cambridge, MA, USA). They were then extensively washed before being incubated with secondary rabbit antihuman Fc-γ of antihuman Fc-μ antibody coupled to alkaline phosphatase (Dakocytomation, Golstrup, Denmark) for 90 min at room temperature. Immunoreactivities were revealed using the nitroblue tetrazolium-5-bromo-4-chloro-3-indolyl-phosphate substrate (Sigma, St. Louis, MO, USA) (CitationGarcia de la Pena-Lefebvre et al 2004; CitationTamby et al 2005). Immunoreactivities were quantified by scanning the membranes with a densitometer (Epson Perfection 1200S, Seiko Epson Corporation, Nagano-Ken, Japan). The membranes were then stained with colloidal gold (Protogold;® Biocell, Cardiff, UK) and subjected to a second densitometric analysis to quantify transferred proteins. This approach allows the immunoreactivity repertoires to be compared by referring to their respective protein peaks corrected for electrophoretic migration defects by superimposing corresponding protein peaks by means of computer analysis. Standard IgM or IgG preparations included in each blot, allow the rescaling of the membranes transferred with a given protein extract and to adjust for the intensity of labelling on different membranes (CitationChanseaud et al 2003; CitationGarcia de la Pena-Lefebvre et al 2004). All experiments were performed twice.
Rabbit polyclonal antibody raised against amino acids 685–765 of human DNA topoisomerase 1 recombinant protein (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was tested in immunoblots during 90 min at room temperature at dilutions of 1:500, and 1:1000 for reactivity with fibro-blasts proteins extracts. Immunoreactivities were assayed using an alkaline phosphatase conjugated goat antirabbit IgG antibody (Santa Cruz) and revealed using nitroblue tetrazolium-5-bromo-4-chloro-3-indolyl-phosphate) (Sigma).
Protein sequencing
To identify the target antigens, we performed an additional SDS-PAGE protein electrophoresis with a fibroblast extract in 10% polyacrylamide gels and dissected bands of interest. We then performed a passive adsorption onto a polyvinyle difluoride (PVDF) membrane (CitationMesser et al 1997) and not an electrotransfer, in order to favor the protein binding onto the membrane in a nondestructive manner for subsequent analysis of the separated proteins of interest. The proteins were derivatized and sequenced by automated Edman’s degradation using a PE Applied Biosystems 492 cLC protein sequencer (Perkin Elmer, Wellesley, MA, USA) allowing the identification and quantification in the range of femtomoles of the amino acids released.
Statistical analyses
Because of the large number of reactivities identified in the blots, we decided to submit the data to multivariate statistical analysis. Therefore we used IGOR software (Igor Pro 3.16, Wavemetrics Inc, Lake Oswego, OR, USA) with specially designed software packages (CitationNobrega et al 1993), and Stat-view software (Statview 5.0; SAS institute Inc., Cary, NC, USA). Densitometry curves of IgG and IgM reactivities of each patient and control on each of the different protein extracts were divided into sections surrounding individual peaks of immunoreactivity on each substrate. In order to discriminate between groups of individuals, the peak amplitudes corresponding to these sections were submitted to principal component analysis (CitationNobrega et al 1993; CitationMouthon et al 1996). Principal component analysis data were then subjected to linear discriminant analysis, and the individual linear discriminant analysis factor 1 coordinates of each patient or healthy control in the 2 groups were compared using a paired Wilcoxon test. Repertoires of reactivities of patients in each group were compared two by two by using the same procedure, except that the individual linear discriminant analysis factor 1 coordinates of each patient in the 2 groups were compared using a Mann-Whitney test.
Results
IgG and IgM reactivities of patients and healthy controls with fibroblast antigens
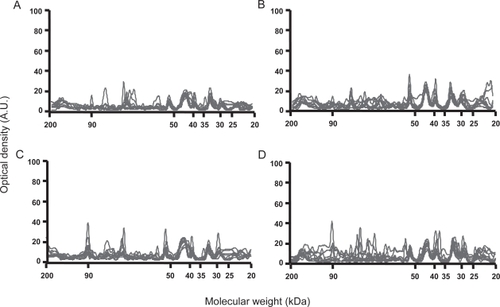
IgG and IgM reactivity patterns with fibroblast antigens were homogeneous in terms of protein bands recognized and intensity of reactivities among healthy individuals and patients in each group. As depicted in , IgG from healthy individuals bound to 5 to 8 protein bands in the fibroblast extract, with expression of three main immuno-reactivities of approximately 53, 44, and 33 kDa, respectively (). Two other minor IgG reactivities were directed against low molecular weight proteins, and some patients expressed individual specific reactivities. IgG from patients in the different groups also bound to 6 to 10 protein bands in the fibroblast extract including immunoreactivities directed toward the same 53, 44, and 33 kDa bands. In addition to these bands, IgG from patients with limited cutaneous SSc and anticentromere antibodies () bound with low intensity to a 80 kDa antigen. IgG from patients with diffuse SSc and antitopoisomerase 1 antibodies () expressed a high intensity reactivity with the 44 kDa protein band as compared with those expressed by IgG from patients in other groups and healthy controls. Interestingly, almost all patients with diffuse SSc and antitopoisomerase 1 antibodies bound to two 90 and 65 kDa protein bands, whereas IgG from patients with limited cutaneous SSc and anti anticentromere antibodies and healthy controls did not. Finally, IgG from several patients without specific autoantibody bound to the two 90 and/or 65 kDa fibroblasts protein bands, whereas other patients in this group did not (). IgG from several patients in each group expressed individual specific reactivities.
Figure 1 Densitometric profiles of immunoglobulin G (IgG) reactivity of 10 healthy controls (A), 10 patients with limited cutaneous systemic sclerosis (SSc) and anticentromere antibodies (B), 10 patients with diffuse SSc and antitopoisomerase 1 antibodies (C), 10 patients with diffuse SSc without antitopoisomerase 1 or anticentromere antibody (D) with fibroblasts antigens. Sera were diluted to an IgG concentration of 200 μg/ml. The densitometric pattern of IgG reactivities of each individual is depicted as a full line curve. Hatched areas depict the densitometric pattern observed in the presence of the secondary anti-Fcγ antibody tested alone. Molecular weights are expressed as kDa in the abscissa, and optical densities are expressed as arbitrary units (A.U.) in the ordinates.
IgM from patients and healthy individuals bound to three to five protein bands in the fibroblast extract and with a high intensity reactivity with the 53kDa protein band (data not shown).
Multiparametric analysis of IgG and IgM reactivities of patients and healthy controls with fibroblast antigens
IgG and IgM reactivities from patients in each group and healthy controls were compared by performing principal component analysis followed by linear discriminant analysis and non parametric tests, as previously described. IgG reactivities from patients with diffuse SSc and antitopoisomerase 1 antibodies with fibroblasts antigens different significantly from those of healthy controls, whereas no significant difference was observed between limited cutaneous SSc with anticentromere antibodies and their controls and diffuse SSc without antitopoisomerase 1 or anticentromere antibody and their controls ().
Table 1 Multiparametric analysis of serum immunoglobulin G (IgG) and IgM reactivities from patients with diffuse systemic sclerosis (SSc) with antitopoisomerase 1 antibodies, patients with limited cutaneous SSc with anticentromere antibodies and patients with diffuse SSc without antitopoisomerase 1 or anticentromere antibody compared to their respective healthy controls on normal human fibroblasts and HEp-2 cells antigens
IgM reactivities from patients with limited cutaneous SSc with anticentromere antibodies were significantly different from those of healthy controls, whereas no significant difference was observed between patients in other groups and their respective healthy controls ().
Multiparametric analysis of serum reactivities from patients compared two by two differed in the case of IgG from patients with diffuse SSc with antitopoisomerase 1 antibodies vs patients with diffuse SSc without antitopoisomerase 1 or anticentromere antibody and in the case of IgM from patients with diffuse SSc and antitopoisomerase 1 antibodies vs patients with limited cutaneous SSc with anticentromere antibodies ().
Table 2 Multiparametric analysis of serum immunoglobulin G (IgG) and IgM reactivities from patients with diffuse systemic sclerosis (SSc) with antitopoisomerase 1 antibodies, patients with limited cutaneous SSc with anticentromere antibodies and patients with diffuse SSc without antitopoisomerase 1 or anticentromere antibody compared two by two on normal human fibroblasts and HEp-2 cells antigens
IgG and IgM reactivities of patients and healthy controls with HEp-2 cells antigens
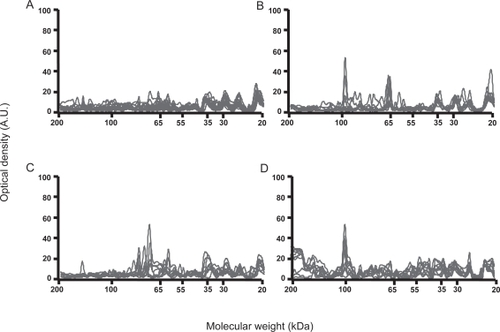
In order to better characterize the cell specificity of AFA, we further performed experiments with HEp-2 cells, the reference source of nuclear antigens. IgG reactivity patterns were relatively homogeneous among healthy individuals and among patients in each group when tested toward HEp-2 cells protein extracts. IgG from patients with antitopoisomerase 1 antibodies recognized 3–6 protein bands in HEp-2 cells extract, including one major band of 100-kDa, whereas IgG from patients with anticentromere antibodies recognized 4–8 bands, including two major bands of 65 and 80 kDa, whereas none of them bound to the 100-kDa band (). IgG from patients without antitopoisomerase 1 or anticentromere antibody recognized 6–10 HEp-2 cells bands, including the 100-kDa protein band in 5/20. IgM reactivity patterns with HEp-2 cells protein extracts were relatively homogeneous among healthy individuals and among patients in each group. No difference was observed between IgM reactivities of patients in each group and healthy individuals (data not shown).
Figure 2 Densitometric profiles of immunoglobulin G (IgG) reactivity of 10 healthy controls (A), 10 patients with limited cutaneous systemic sclerosis (SSc) and anticentromere antibodies (B), 10 patients with diffuse SSc and antitopoisomerase 1 antibodies (C), 10 patients with diffuse SSc without antitopoisomerase 1 or anticentromere antibody (D) with fibroblasts antigens. Sera were diluted to an IgG concentration of 200 μg/ml. The densitometric pattern of IgG reactivities of each individual is depicted as a full line curve. Hatched areas depict the densitometric pattern observed in the presence of the secondary anti-Fcγ antibody tested alone. Molecular weights are expressed as kDa in the abscissa, and optical densities are expressed as arbitrary units (A.U.) in the ordinates.
Multiparametric analysis of IgG and IgM reactivities of patients and healthy controls with HEp-2 cells antigens
Principal component analysis of the data discriminated between the IgG reactivity patterns of SSc patients in each group and healthy controls as well as between patients in the different groups compared two by two ( and ). IgM reactivity patterns with HEp-2 cells protein extracts were relatively homogeneous among healthy individuals and among patients in each group. No significant difference was observed between IgM reactivities of patients in each group and controls or between patients in each group tested two by two, with the exception of patients with antitopoisomerase 1 antibodies versus healthy individuals ( and ). We thus demonstrated that IgG reactivities from SSc patients in the different groups were not specific to fibroblasts antigens but also bound HEp-2 cells antigens.
Characterization of the 100-kDa target antigen in fibroblasts
We incubated the fibroblast protein extract with rabbit polyclonal antibodies raised against amino acids 685–765 of human DNA topoisomerase 1 recombinant protein as indicated in Materials and methods. This antibody bound to the same 90 kDa protein band that was recognized by serum IgG from patients with antitopoisomerase 1 antibodies (data not shown). We also tested the polyclonal goat antitopoisomerase 1 antibody on HEp-2 protein extract and observed that this Ab bound to the 100 kDa protein band (data not shown).
We further sequenced the 90-kDa protein band, as described in Materials and methods. DNA-topoisomerase I was identified by sequencing the 10 to 25 N-terminal amino acids: SQIEADFRLNDSHKH of this protein. Thus, the N-terminal sequence of the protein started at position 10, suggesting a lysis of the nine first amino acids during preparation of the protein sample (data not shown). Thus, AFA from patients with diffuse SSc with or without antitopoisomerase 1 antibodies bind to fibroblast topoisomerase 1.
Discussion
It is now well established that SSc patients express antifibroblasts antibodies (CitationBrentnall et al 1982; CitationAlderuccio et al 1989; CitationChizzolini et al 2002; CitationRonda et al 2002; CitationHenault et al 2004). CitationChizzolini and colleagues (2002) reported that the prevalence of AFA was significantly higher in diffuse SSc than in limited cutaneous SSc patients. Thus, our observation that limited cutaneous or diffuse SSc exhibit significantly different reactivity patterns with fibroblasts antigens is not unexpected. Unfortunately, in their report, Chizzolini and colleagues do not mention the prevalence of antitopoisomerase 1 antibodies in SSc patients tested.
To our knowledge, very few studies investigated the specificity of AFA in SSc patients. We here provide evidence that IgG from diffuse SSc patients with antitopoisomerase 1 antibodies express two main bands of reactivity of 65 and 90 kDa with fibroblasts antigens, whereas patients with limited cutaneous SSc and anticentromere antibodies and healthy controls do not. Our patients had IgG antitopoisomerase 1 antibodies, and we knew from the literature that antitopoisomerase 1 IgG reactivities may bind to a 70 kDa protein band corresponding to a degraded form of topoisomerase 1 in cellular extracts. Thus, we postulated that IgG immunoreactivities identified in these patients might be directed to topoisomerase. This was confirmed by N-terminal sequencing, since an amino acid sequence compatible with N-terminal sequence of DNA topoisomerase 1 was identified in the 90 kDa band. Although we did not perform any experiment to identify the same target antigen in the 65 kDa band, we postulate that this band also contains DNA topoisomerase 1. Thus, it has now been well documented for near twenty years that patients with SSc bind to a 70 kDa antigen in Hela cells extracts and that the target antigen of the anti-Scl-70 Abs is a degraded form of topoisomerase 1 (CitationKumar et al 1988), and it was postulated that reactivities directed towards Hela cells proteins of 63 to 77 kDa molecular weights bound to topoisomerase 1 (CitationBriolay et al 1989).
Importantly, our data confirm the finding of CitationHenault and colleagues (2004) who recently identified DNA topoisomerase 1 as the target of AFA, with evidence that antitopoisomerase 1 antibodies display antifibroblast activity by reacting with determinants at the fibroblast surface.
Although it has not been demonstrated yet, one may speculate that IgG antitopoisomerase 1 antibodies from patients with diffuse SSc might bind to their target antigen at the fibroblast surface and activate these cells. Indeed, in SSc patients, AFA have been shown to induce fibroblasts activation in vitro and a pro-adhesive and pro-inflammatory phenotype of these cells (CitationChizzolini et al 2002). Thus, IgG binding to the surface of fibroblasts could induce an autocrine production of cytokines such as interleukin (IL)-1α, IL-1β, and IL-6 which could up-regulate the adhesive molecule intercellular adhesion molecule-1 on fibroblast surface (CitationChizzolini et al 2002).
Interestingly, we have previously analyzed IgG antibodies repertoires in SSc patients and shown that IgG reactivity with a 100-kDa tissue and endothelial cell antigen identified as topoisomerase 1 distinguished between limited cutaneous and diffuse SSc patients (CitationGarcia de la Pena-Lefebvre et al 2004), whereas two 75- and 85-KDa protein bands identified as centromeric protein B were recognized by patients with limited cutaneous SSc (CitationServettaz et al 2006).
Prominent recognition of a 90 kDa band in fibroblasts versus a 100 kDa band in endothelial cells (CitationGarcia de la Pena-Lefebvre et al 2004) or HEp-2 cells might be the consequence of discrepancies in the amounts of proteases or free radicals in the different cell culture mediums between fibroblasts, HEp-2 cells, and endothelial cells.
In the present report, IgG from close to 50% of patients without antitopoisomerase 1 or anticentromere antibody bound to two 65 and/or 100 kDa fibroblasts protein bands, with prominent binding to the 65 kDa Hep-2 cell protein band. These data confirm those obtained in previous work performed with endothelial cell antigens that IgG from 50% of patients without antitopoisomerase 1 or anticentromere antibody, as assessed by ELISA, bound to the 100-kDa endothelial cell antigen identified as topoisomerase 1 (CitationGarcia de la Pena-Lefebvre et al 2004). Thus, IgG from SSc patients with antinuclear antibodies without documented antitopoisomerase 1 specificity as assessed ELISA may bind to 90 kDa protein bands in fibroblasts extracts and/or 100 kDa antigens in endothelial cells and HEp-2 cell extracts. Thus, in previous work, we reported that antitopoisomerase 1 antibodies were detected in 9 (8.1%) patients who had no antitopoisomerase 1 antibody as determined by ELISA. Patients with antitopoisomerase 1 antibodies had an almost similar phenotype without distinction between ELISA or immunoblot approaches (Tamby et al 2007).
Reactivities detected by immunoblotting reflect the specific binding of the variable regions of IgM and IgG antibodies to tissue antigens (CitationMouthon et al 1995). Since we used the same fibroblasts sources to test all patients, it could be argued that some of the scored reactivities could be specific to alloantigens. Moreover graft versus host disease reactions attributable to the persistence of allogeneic fetal cells in the skin of women for years after the pregnancy have been proposed to play a role in the development of SSc (CitationArtlett et al 1998; CitationNelson et al 1998). In this study, female SSc patients were matched with healthy women for parity, and in light of the absence of any difference between them, it is extremely unlikely that alloantigen recognition could explain the results obtained herein. Finally, the approach does not exclude that some of the epitopes identified by IgG in immunoblots may differ from those expressed by native proteins. The technique allows, however, for the comparative analysis of repertoires of Igs from different individuals (CitationArtlett et al 1998; CitationNelson et al 1998).
We here provide evidence that IgG from patients with limited cutaneous or diffuse SSc exhibit significantly different reactivity patterns with fibroblasts antigens, and confirm that IgG from patients with diffuse SSc bind to topoisomerase 1 in fibroblasts extracts. The function of these antibodies needs to be investigated.
Acknowledgements
This work has been presented at the 5th annual congress of European League Against Rheumatism (EULAR). 9–11 June 2004, Berlin, Germany. This work is supported by grants from the INSERM (CReS #4CR08F), the Association des Sclérodermiques de France (ASF) and the Legs POIX, Chancellerie des Universités, Académie de Paris, France. Tamby is a recipient of a grant from Actelion Pharmaceuticals France and the ASF. P. Guilpain was funded by the Fondation de la Recherche Médicale, France. A. Servettaz received a financial support from Actelion, the ASF and the direction Régionale des Affaires Sanitaires et Sociales (DRASS) de la Région Champagne Ardennes. We thank Dr. Frédéric Batteux who provided us with HEp-2 cells.
References
- AlderuccioFWitherdenDTohBH1989Autoantibody to gp50, a glycoprotein shared in common between fibroblasts and lymphocytes, in progressive systemic sclerosisClin Exp Immunol7826302805420
- ArtlettCMSmithJBJimenezSA1998Identification of fetal DNA and cells in skin lesions from women with systemic sclerosisN Engl J Med3381186919554859
- BaroniSSSantilloMBevilacquaF2006Stimulatory autoantibodies to the PDGF receptor in systemic sclerosisN Engl J Med35426677616790699
- BrentnallTJKenneallyDBarnettAJ1982Autoantibodies to fibro-blasts in sclerodermaJ Clin Lab Immunol89127047748
- BriolayJGioudMMonierJC1989Antinuclear antibodies detected by indirect immunofluorescence on HEp2 cells and by immunoblotting in patients with systemic sclerosisAutoimmunity2165762491600
- BunnCCDentonCPShi-WenX1998Anti-RNA polymerases and other autoantibody specificities in systemic sclerosisBr J Rheumatol3715209487245
- ChanseaudYPena-LefebvrePGGuilpainP2003IgM and IgG autoantibodies from microscopic polyangiitis patients but not those with other small- and medium-sized vessel vasculitides recognize multiple endothelial cell antigensClin Immunol1091657814597215
- ChizzoliniCRaschiERezzonicoR2002Autoantibodies to fibroblasts induce a proadhesive and proinflammatory fibroblast phenotype in patients with systemic sclerosisArthritis Rheum4616021312115192
- Garcia de la Pena-LefebvrePChanseaudYTambyMC2004IgG reactivity with a 100-kDa tissue and endothelial cell antigen identified as topoisomerase 1 distinguishes between limited and diffuse systemic sclerosis patientsClin Immunol1112415115183145
- HenaultJTremblayMClementI2004Direct binding of anti-DNA topoisomerase I autoantibodies to the cell surface of fibroblasts in patients with systemic sclerosisArthritis Rheum5032657415476238
- IhnHSatoSFujimotoM2000Characterization of autoantibodies to endothelial cells in systemic sclerosis (SSc): association with pulmonary fibrosisClin Exp Immunol119203910606984
- KumarVKowalewskiCKoelleM1988Scl-70 antigen stability and its effect on antibody detection in sclerodermaJ Rheumatol1514995023144599
- LeaskAHolmesAAbrahamDJ2002Connective tissue growth factor: a new and important player in the pathogenesis of fibrosisCurr Rheumatol Rep41364211890879
- LeRoyECBlackCFleischmajerR1988Scleroderma (systemic sclerosis): classification, subsets and pathogenesisJ Rheumatol1520253361530
- MesserMGriffithsMRismillerPD1997Lactose synthesis in a Monotreme, the Echidna (Tachyglossus aculeatus): isolation and amino acid sequence in Echidna a-lactalbuminComp Biochem Physiol118b40310
- MoroiYPeeblesCFritzlerMJ1980Autoantibody to centromere (kinetochore) in scleroderma seraProc Natl Acad Sci USA771627316966403
- MouthonLLacroix-DesmazesSBarreauC1996The repertoire of antibody reactivities of normal human serum IgM with self antigens is acquired in early childhood and remains stable throughout lifeScand J Immunol44243518795718
- MouthonLNobregaANicolasN1995Invariance and restriction towards a limited set of self-antigens characterize neonatal IgM antibody repertoires and prevail in autoreactive repertoires of healthy adultsProc Natl Acad Sci USA923839437731992
- MukerjeeDSt GeorgeDColeiroB2003Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approachAnn Rheum Dis6210889314583573
- NelsonJLFurstDEMaloneyS1998Microchimerism and HLA-compatible relationships of pregnancy in sclerodermaLancet351559629492775
- NobregaAHauryMGrandienA1993Global analysis of antibody repertoires. II. Evidence for specificity, self-selection and the immunological ‘homunculus’ of antibodies in normal serumEur J Immunol2328512598223861
- RondaNGattiRGiacosaR2002Antifibroblast antibodies from systemic sclerosis patients are internalized by fibroblasts via a caveolin-linked pathwayArthritis Rheum46159560112115191
- ServettazAAgardCTambyM2006Physiopathologie de la sclérodermie systémique: état des lieux sur une affection aux multiples facettesPresse Med3519031517159716
- SgoncRGruschwitzMBoeckG2000Endothelial cell apoptosis in systemic sclerosis is induced by antibody-dependent cell-mediated cytotoxicity via CD95Arthritis Rheum4325506211083280
- SteenVDPowellDLMedsgerTAJ1988Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosisArthritis Rheum311962033348823
- TambyMCChanseaudYGuillevinL2003New insights into the pathogenesis of systemic sclerosisAutoimmun Rev2152712848956
- TambyMCChanseaudYHumbertM2005Anti-endothelial cell antibodies in idiopathic and systemic sclerosis associated pulmonary arterial hypertensionThorax607657216135682
- TanFKArnettFCAntohiS1999Autoantibodies to the extra-cellular matrix microfibrillar protein, fibrillin-1, in patients with scleroderma and other connective tissue diseasesJ Immunol16310667210395706
- WeinerESEarnshawWCSenecalJL1988Clinical associations of anticentromere antibodies and antibodies to topoisomerase I. A study of 355 patientsArthritis Rheum31378852833902
- ZhouXTanFKMilewiczDM2005Autoantibodies to fibrillin-1 activate normal human fibroblasts in culture through the TGF-beta pathway to recapitulate the “scleroderma phenotype”J Immunol17545556016177099