Abstract
Over one million fractures occur per year in the US and are associated with impaired healing increasing patient morbidity, stress, and economic costs. Despite improvements in surgical technique, internal fixation, and understanding of biologics, fracture healing is delayed or impaired in up to 4% of all fractures. Complications due to impaired fracture healing present therapeutic challenges to the orthopedic surgeon and often lead to chronic functional and psychological disability for the patient. As a result, it has become clinically desirable to augment mechanical fixation with biologic strategies in order to accelerate osteogenesis and promote successful arthrodesis. The discovery of bone morphogenic protein (BMP) has been pivotal in understanding the biology of fracture healing and has been a source of intense clinical research as an adjunct to fracture treatment. Multiple in vitro and in vivo studies in animals have elucidated the complex biologic interactions between BMPs and cellular receptors and have convincingly demonstrated rhBMP-2 to be a safe, effective treatment option to enhance bone healing. Multiple clinical trials in trauma surgery have provided level 1 evidence for the use of rhBMP-2 as a safe and effective treatment of fractures. Human clinical trials have provided further insight into BMP-2 dosage, time course, carriers, and efficacy in fracture healing of tibial defects. These promising results have provided hope that a new biologic field of technology has emerged as a useful adjunct in the treatment of skeletal injuries and conditions.
Introduction
The devastating effects of fractures are felt every year in the US. Over one million fractures occur per year and are associated with impaired healing increasing patient morbidity, stress, and economic cost (CitationEinhorn 1995, Citation1998). Despite improvements in surgical technique, internal fixation and understanding of biologics, fracture healing is delayed or impaired in up to 4% of all fractures (CitationWhittle et al 1992; CitationTornetta et al 1994; CitationEinhorn 1995; CitationHeckman and Sarasohn-Kahn 1997; CitationMarsh 1998; CitationKarladani et al 2000; CitationYoung and Rayan 2000). Factors affecting fracture healing include severity of injury, patient comorbidities, and surgical fixation. Complications due to impaired fracture healing present therapeutic challenges to the orthopedic surgeon and often lead to chronic functional and psychological disability for the patient. As a result, it has become clinically desirable to augment mechanical fixation with biologic strategies in order to accelerate osteogenesis and promote successful arthrodesis. The purposes of the following manuscript include: 1) to review the biology of bone morphogenic protein-2 (BMP-2, Medtronic Sofamor Danek, Memphis, TN, USA); 2) to identify important animal data that support the use of BMP-2 in fracture treatment; 3) to present recently published human clinical data on the use of BMP-2 in orthopedic fracture care.
Fracture repair
Bone is a unique organ that continuously remodels and has the capability to regenerate throughout adult life (CitationCheng et al 2003). Fracture repair includes a complex interaction between mechanical (fracture stability) and biological factors (growth factors/proteins). The physiological and biological factors responsible for the regeneration of bone are coupled to and dependent on BMPs.
Fracture of bone leads to a cascade of events including activation of the complement cascade and an inflammatory response associated with vascular injury leading to cell extravasation and signaling (CitationGautschi et al 2007). Activated macrophages release growth factors that stimulate endothelial cells to express plasminogen activator and procollagenase (CitationSchmitt et al 1999). Initiation of the clotting cascade by platelets allows the localized collection of blood to clot and form a hematoma. This hemostatic plug prevents further blood loss and provides a medium for the activity of various growth factors including platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and beta-fibroblast growth factor (β-FGF) (CitationHollinger and Wong 1996).
Approximately 3 days after a fracture, a repair blastema forms consisting of new blood vessels, macrophages, and collagen (CitationHollinger and Wong 1996). Growth factors selectively bind to collagen, forming a substrate to optimize interaction between TGF-β, β-FGF, PDGF, BMPs, and receptor cells (). Osteoprogenitor cells localized to the periosteum and endosteum of fractured bone attach to granulation tissue and differentiate into chondrocytes and osteoblasts via cell signaling. The aggregate effects of cellular transduction and enhanced cellular-growth factor interaction help to regenerate bone via osteoblastic and osteoclastic activity and ensure fracture healing by 6–8 weeks after injury (CitationHollinger and Wong 1996). Degradation products from the extracellular matrix stimulate the differentiation of macrophages into osteoclasts in order to provide additional cells for continued fracture healing (CitationSchmitt et al 1999).
Figure 1 Cell signaling in chemotaxis and cell proliferation during wound-healing: Platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and transforming growth factor-beta (TGF-β) play an integral role in the signal cascade responsible for chemotaxis and cell migration during wound-healing. The recruitment of osteoprogenitor cells and their proliferation provides a pool of cells that will respond to bone morphogenic protein (BMP). Reproduced with permission from CitationHollinger JO, et al 2008. Recombinant human platelet-derived growth factor: biology and clinical applications. J Bone Joint Surg Am, 90:48–54. Copyright © 2008. The Journal of Bone and Joint Surgery, Inc.
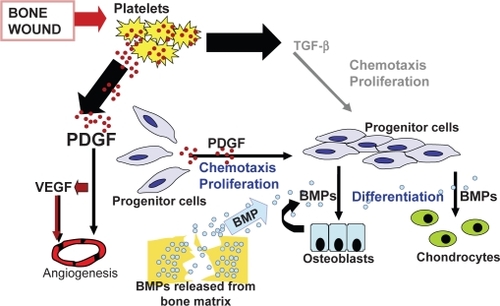
The final pathway in bone healing and regeneration depends on the interaction between BMP’s/growth factors with the various cell lines at the site of injury (CitationReddi 1994). Sufficient quantities of biologically active BMPs and competent cells interact to regenerate bone (CitationSchmitt et al 1999).
BMP biology
In 1965, Marshall R Urist discovered a substance in the extracellular bone matrix that had the ability to induce osteogenesis when implanted into extraskeletal tissue (CitationUrist 1965). Ever since this seminal discovery of bone morphogenic proteins, the role of BMP has expanded from basic biology to clinical applications. In 1998, scientists at the Genetics Institute in Cambridge, MA, USA derived the amino acid sequence of BMPs. This discovery led to the expression of complementary DNAs and recognization of BMP in the family of the transforming growth factor-β supergene family (CitationEinhorn 1998). Over the past 2 decades, 20 BMPs with varying abilities to induce cartilage or bone formation have been identified by investigators (CitationGautschi et al 2007). The structure of 16 different human BMPs have been identified and designated as BMP-1 to BMP-16. With the exception of BMP-1, the BMPs are a subgroup of the transforming growth factor-β superfamily which is a group of differentiation factors that have been shown to play an integral role in tissue repair (CitationOzkaynak et al 1992; CitationBarnes et al 1999, Citation2003; CitationTermaat et al 2005).
Enhanced fracture healing relies on two processes: mechanical and biologic intervention (CitationLinkhart et al 1996; CitationClaes et al 1998; CitationBostrom et al 1999). Biological agents recapitulate the process of both embryological bone formation and fracture healing; thus these agents have the potential to be used clinically in their natural setting (CitationMarsh 1998). Among the numerous cytokines and growth factors such as BMPs, TGF-β, FGF-1 (fibroblast growth factor), and IGF-1 (insulin like growth factor) involved in fracture healing, BMPs are regarded as the key regulators in the cascade of events required for skeletal repair (CitationOnishi et al 1998; CitationSakou 1998; CitationWozney and Rosen 1998).
In the first 5 days after a fracture, a cascade of events integral to fracture healing occurs. Progenitor cells indigenous to the fracture site are recruited through cell signaling via BMPs. The interaction of BMP-2 with these osteoprogenitor cells leads to induction of bone-forming osteoblasts. This marks a sentinel event for bone regeneration. BMPs bind and initiate a cell signal through a transmembrane receptor complex formed by type I and II serine/threonine kinase receptor proteins. Type II receptors are active continuously and function upstream of the type I receptors but cannot independently initiate cell signaling (CitationWrana et al 1994). After binding BMP-2, the type II receptor kinase phosphorylates the type I receptor, generating an intracellular response.
Studies have shown that BMP-2 through 7 and BMP-9 have the unique ability to induce differentiation of mesenchymal stem cells into osteoblasts (CitationChen et al 1991; CitationYamaguchi et al 1991; CitationHughes et al 1995; CitationMayer et al 1996). BMP-2, 6, and 9 play an important role in the early phase of differentiation of mesenchymal progenitor cells to pre-osteoblasts. In mesenchymal progenitor and osteoblastic cells, Cheng et al demonstrated the relative osteoconductivity of different BMPs at various stages in the differentiation process. Specifically, BMP-2, 6, and 9 were shown to play a pivotal role in the early phase of the differentiation of mesenchymal progenitor cells to pre-osteoblasts. These findings could implicate BMP-2, 6, and 9 as essential effectors in fracture healing where there is an abundance of pluripotent cells and pre-osteoblasts.
Although many BMP subtypes have been shown to have osteoinductive properties, only rhBMP-2 and rhBMP-7 have been developed and used for clinical applications (CitationCheng et al 2003). Multiple animal models have demonstrated that fracture healing can be accelerated by local administration of rhBMP-2 (CitationOzkaynak et al 1992; CitationBostrom et al 1999; Citationden Boer et al 2002). BMP-2 plays an important role in the early phase of differentiation of mesenchymal progenitor cells to pre-osteoblasts.
CitationTsuji et al (2006) evaluated the role of BMP-2 in fracture healing by creating transgenic mice lacking limb specific expression of BMP-2. These mice did not have defects in skeletal patterns but did develop dose-dependent defects in bone mineral density. Femoral fractures in these mice showed failure to heal by day 20 compared with heterozygotes and control groups. Mice lacking BMP-2 had delayed activation of the periosteum in response to the fracture in addition to the absence of callus formation and mesenchymal progenitor cells.
In vitro BMP-2 analysis
The functions of BMPs have been evaluated in many cell lines including osteoprogenitor cells, osteoblasts, and chondroblasts. In a study using C3H10T1/2 cells (mouse mesodermal progenitor cells), CitationWang et al (1993) demonstrated that high concentrations of BMP-2 induced differentiation into chondrocytes and bone cells. Other studies have implicated BMP-2 in the conversion of rat calvaria derived multipotent cells (ROB-C26) and clonal myoblast cells (C2C12) into cells of the osteoblast phenotype (CitationYamaguchi et al 1991; CitationKatagiri et al 1994). CitationKanatani et al (1995) showed that BMP-2 stimulated bone resorption through direct stimulation of osteoclast formation and activation of mature osteoclasts in stromal cells of mouse bone cell cultures. CitationCheng et al (2003) used osteoblastic progenitor cell lines to demonstrate that BMP-2 was able to induce both early and late osteogenic markers and matrix mineralization. These studies provide compelling evidence for the role of BMP-2 in the biology of bone regeneration, leading to further interest and study in animal models.
Lower order animal trials
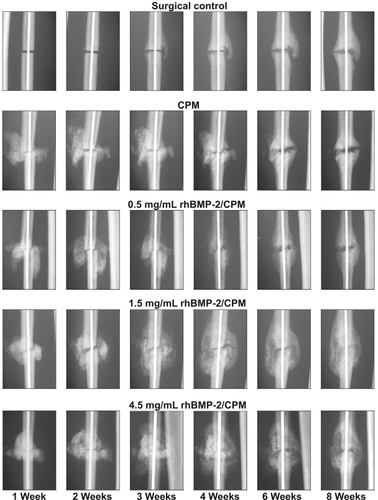
In several preclinical studies, a single percutaneous injection of recombinant human BMP-2 (rhBMP-2) in a calcium phosphate paste (alpha bone substitute material [α-BSM] accelerated osteotomy site healing in rabbit ulnar, canine tibial, and primate fibular osteotomy models (CitationFinkemeier 2002; CitationKhan et al 2005). In a study by CitationSeeherman et al (2006a), a single percutaneous administration of 1.5 mg/mL rh BMP-2/calcium phosphate matrix increased primate fibular osteotomy site callus area and accelerated radiographic evidence of healing up to 2 weeks after injury (). In this study, a 1-week delay in rhBMP-2 treatment led to accelerated healing through direct bone formation. The authors noted that treatment with rhBMP-2 led to an increase in the number of Cbfa-1 positive cells in the osteotomy site. Cbfa-1 is a transcription factor that works with Osterix (OSX) to regulate osteoblast specific genes required for osteoblast differentiation and bone formation. These findings correlate with in vitro studies that demonstrate that rhBMP-2 upregulates expression of both Cbfa-1 and OSX (CitationWozney and Rosen 1998; CitationKawaguchi et al 2001; CitationKolbeck et al 2003). On the basis of these results, CitationSeeherman et al (2006a) concluded that an injectable form of rhBMP-2/-BSM can potentially be administered at any time up to 2 weeks after fracture injury accelerating the healing of closed fractures in humans.
Figure 2 Postoperative radiographic appearance of the nonhuman primate fibular osteotomy sites that were untreated, treated with calcium phosphate matrix (CPM), and treated with 0.5, 1.5, and 4.5 mg/mL rhBMP-2/CPM administered 1 week after surgery. Reproduced with permission from CitationSeeherman H, et al 2006a. rhBMP-2/calcium phosphate matrix accelerates osteotomy-site healing in a nonhuman primate model at multiple treatment times and concentrations. J Bone Joint Surg Am, 88:144–60. Copyright © 2006. The Journal of Bone and Joint Surgery, Inc.
CitationBouxsein et al (2001) studied the effect of rhBMP-2 in fracture healing in a rabbit ulnar osteotomy model. Seventy-two rabbits had mid-ulnar osteotomies and were divided into 3 treatment groups: 1) rhBMP-2/absorbable collagen sponge (ACS), 2) absorbable collagen sponge, 3) placebo. The retention of rhBMP-2 at the osteotomy site was determined with imaging of 125I-labeled rhBMP-2. The data revealed that osteotomy sites treated with rhBMP-2 healed 33% faster than the other two groups. At each time point analyzed (3, 4, 6 weeks), enhanced healing in the rhBMP-2 treated group appeared to be more rapid with greater callus formation and advanced callus maturation. The authors concluded that the presence of larger and more mature callus in the rhBMP-2 treatment group was consistent with the increased biomechanical properties of the ulna treated with rhBMP-2.
Other animal studies have also shown enhanced fracture healing following rhBMP-2 treatment. CitationWelch et al (1998), in a tibial fracture model in goats, found that limbs treated with rhBMP-2/ACS had increased callus formation, radiographic healing parameters, and torsional stiffness at 3 and 6 weeks post-treatment. Dynamic histomorphometric analysis revealed increased callus formation consistent with recruitment of osteoprogenitor cells (CitationBoden et al 1998). CitationLuppen et al (2002) studied 49 rabbits that were injected with saline or prednisone prior to creating bilateral ulnar osteotomies. One osteotomy was treated with rhBMP-2/ACS, and the contralateral osteotomy served as a control (CitationLuppen et al 2002). Healing was assessed in these animals with computed tomography, torsional biomechanics, and histology. Although prednisolone inhibited healing in the control group, rhBMP-2/ACS enhanced fracture healing in both prednisolone- and saline-treated groups.
In a rat femur fracture model, CitationLee et al (2002) created a 2 cm defect that was filled with rhBMP-2/ACS allograft. The authors reported 75% new bone incorporation into allograft at 4 weeks and 100% at 8 weeks. CitationEinhorn et al (2003) used a femur fracture model in 144 rats to determine the effects of a single percutaneous injection of rhBMP-2 on fracture healing. The three treatment groups included a control group, a buffer vehicle group, and a rhBMP-2/buffer group. Torsional biomechanical testing indicated that the stiffness of the rhBMP-2 treated group was twice that of the two other control groups at the 2-, 3-, and 4-week time points. At 4 weeks, the strength of the rhBMP-2 treated fractures was 77% greater than that of the other groups. By 4 weeks, remodeling of the hard callus and recorticalization were observed in the rhBMP-2 treated fracture sites, whereas cartilage and/or soft tissue were still present in the control fracture sites.
Several studies have looked at the effects of rhBMP-2 on fracture healing in canine fracture models. In a study of mid-diaphyseal femoral defects filled with rhBMP2-allograft in 21 dogs, CitationZabka et al (2001) noted more balanced allograft resorption and bone formation in the rhBMP-2 group compared with the cancellous bone group and ACS groups. CitationPluhar et al (2001) studied augmentation of allograft with rhBMP-2/ACS in a canine intercalary femoral defect. The authors found that rhBMP-2/ACS allograft had results equal to or greater than those of autogenous graft. RhBMP-2 graft also resulted in greater callus formation. In a study of 18 dogs with bilateral ulnar defects, CitationCook et al (2005) compared allograft augmentation with rhBMP-2 against BMP-7. RhBMP-2 treated defects formed greater bone at early time periods, with this trend continuing throughout the study. At the 12-week interval, bridging bone formed in all rhBMP-2 treated and autograft treated groups compared with 4 of 6 BMP-7 treated defects. Mean torsional strength was measured at 78% of an intact ulna, whereas autograft showed 47% strength and BMP-7 showed 38% strength.
Although several studies have reported enhanced fracture healing in animals with rhBMP-2 delivered in a buffer, optimal bone formation requires a carrier (CitationBlokhuis et al 2001; CitationBouxein et al 2001; CitationSeeherman 2001; CitationEinhorn et al 2003). Carriers optimize BMP concentration at the pivotal stages of fracture healing, allowing osteoprogenitor cells to migrate to the site of repair, proliferate and differentiate into osteoclasts and osteoblasts. Moreover, carriers provide an osteoconductive matrix allowing for better handling properties needed for injection or implantation (CitationSeeherman 2001). Bone regeneration can be optimized in segmental defects through the use of BMP carriers providing compression resistance and appropriate elution (CitationSeeherman 2001).
A variety of BMP-carrier combinations has been used in animal models to enhance bone formation in segmental defects (Citationden Boer et al 2003). A potential problem with most BMP-carrier combinations is that a second open surgical procedure is necessary for delayed administration of BMP after initial surgery to augment the repair. Additionally, soft tissue injury may prevent adequate initial treatment and require another open procedure for BMP implantation. An injectable BMP-carrier combination may allow physicians to circumvent this potential limitation. Multiple animal studies have reported accelerated fracture healing with the use of rhBMP-2 in an injectable calcium phosphate cement carrier (CitationLi et al 2003; CitationEdwards et al 2004; CitationSeeherman et al 2006b; CitationSwiontkowski et al 2006). Currently, clinical trials are evaluating the efficacy of rhBMP-2-carrier combination in the treatment of closed fractures.
Using a novel adenoviral gene therapy technique, CitationLieberman et al (1999) used BMP-2 producing bone marrow cells to treat rats with femoral defects. The results of this group were compared with a group treated with rhBMP-2 and 3 control groups including defects treated with demineralized bone matrix, β-galactosidase transduced bone marrow cells, and untreated defects. At the 2-month time point, 22 of 24 defects in the gene therapy group and all defects in the rhBMP-2 group showed enhanced fracture healing. In contrast, 1 of 32 defects in the three control groups healed. Thus, CitationLieberman et al (1999) showed that gene therapy can potentially be used as a therapeutic delivery system for rhBMP-2 in a feasible, efficacious manner. With a similar technique, Lieberman et al noted enhanced bone healing in hindlimbs of mice treated with helper-dependent adenoviral vector producing BMP-2 (CitationAbe et al 2002).
Despite the potential use of viral vectors to express growth factors, questions remain regarding its potential for uncontrolled BMP synthesis and possible malignant cell induction. Multiple studies have questioned the impact of excess BMP-2 production in patients with osteosarcoma. With high doses of BMP, there will be more bone formation and rapid osteoinduction than desired (CitationValentin-Opran et al 2002). BMP-2 and BMP-2 receptors are expressed in a variety normal and malignant cell types, including osteosarcoma. CitationGuo et al (1999) reported expression of BMP-2 receptor mRNA was correlated with metastasis of osteosarcoma. However, no data have shown that BMPs induce malignant transformation of cells. Although some studies have suggested that BMP-2 may stimulate the proliferation of malignant cells (CitationKleeff et al 1999; CitationLangenfeld et al 2003), most studies have shown that BMP-2 inhibits or has no effect on the proliferation of malignant cells (CitationSoda et al 1998; CitationOrui et al 2000; CitationKumagai et al 2006). With these conflicting reports, rhBMP-2 should not be implanted at the site of resected tumor or in patients with active malignancy.
Clinical trials
Multiple clinical trials in trauma surgery have provided level 1 evidence for the use of rhBMP-2 as a safe and effective treatment of fractures. In a prospective randomized, controlled study, CitationJones et al (2004a, Citationb) studied the efficacy of rhBMP-2 (INFUSE bone graft; Medtronic Sofamor Danek, Memphis, TN, USA) on an absorbable collagen sponge combined with freeze-dried cancellous autograft for grafting of diaphyseal tibial defects. Thirty patients were randomly enrolled in one of two groups: 1) control group with autogenous iliac crest bone graft, 2) treatment group with cancellous allograft with rhBMP-2/ACS. Patients were followed for 12 months with treatment failures defined as the inability to heal by 12-month follow-up or the need for secondary intervention to induce fracture healing. Five patients in the control group were deemed treatment failures whereas 2 of 15 patients in the rhBMP-2 group did not obtain a solid arthrodesis. CitationJones et al (2004a, Citationb) concluded that rhBMP-2 with cancellous allograft had a similar rate of healing to that of autogenous bone graft without donor site complaints, with reduced blood loss, and with shortened surgery time.
In a landmark multicenter study by the BESTT study group (BMP-2 Evaluation in Surgery for Tibial Trauma), CitationGovender et al (2002) reported the results of a prospective, randomized controlled, single-blind study to evaluate the safety and efficacy of the use of rhBMP-2 to accelerate healing and decrease the need for secondary intervention in open tibial fractures. The 450 patients evaluated in this study were randomized to one of three groups: 1) intramedullary nail fixation and soft tissue management, 2) IM nail fixation, soft tissue management, and 0.75 mg/mL (6 mg) rhBMP-2/ACS implant, 3) IM nail fixation, soft tissue management, and 0.75 mg/mL (6 mg) rhBMP-2/ACS implant and 1.5 mg/mL (12 mg) of rhBMP-2/ACS. At the time of definitive wound closure, the rhBMP-2 was placed over the fracture. The severity of the open wound was graded according to the Gustilo-Anderson classification and was used to stratify the randomization. Stratum A comprised Gustilo Anderson types I, II, IIIA whereas Stratum B comprised Gustilo-Anderson type IIIB open fractures.
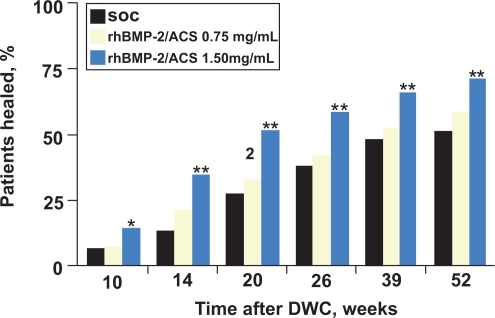
Four-hundred and twenty-one (91%) of the patients were seen at 12-month followup. At every time point (10 weeks to 12-month follow-up), the rhBMP-2 treated groups had a significantly greater percentage of patients who had successful healing without hardware failure or the need for secondary intervention to achieve union (). Compared with the control group, patients treated with 1.5 mg/mL rhBMP-2 had a 44% reduction in the risk of failure (p = 0.0005), significantly fewer invasive interventions (p = 0.0264) such as bone-grafting and nail exchange, and significantly faster fracture healing (p = 0.0022). Moreover, significantly more patients treated with 1.5 mg/mL rhBMP-2 had healing at the fracture site at postoperative visits from 10 weeks to 12 months (p = 0.008). At 6 months, the healing rate observed in the 1.5 mg/mL rhBMP-2 group was 21% higher than that in the control group. Compared with control patients, those treated with 1.5 mg/mL of rhBMP-2 also had significantly fewer hardware failures (p = 0.0174), fewer infections (in association with Gustilo-Anderson type III injuries; p = 0.0219), and faster wound-healing (83% compared with 65% had wound healing at 6 weeks; p = 0.001).
Figure 3 Rate of fracture healing. Determination of fracture-healing was based on treating surgeons’ clinical and radiographic assessment. SOC = standard of care control group with IM nail fixation and soft-tissue management, rhBMP-2/ACS = groups treated with recombinant human bone morphogenic protein-2 in an absorbable collagen sponge implant, and DWC = definitive wound closure. The Fisher exact test pairwise comparison of the control group and the 1.50 mg/mL rhBMP-2/ACS group revealed a p-value of 0.0481 at 10 weeks, < 0.0001 at 14 weeks, 0.0001 at 20 weeks, 0.0025 at 39 weeks, and 0.0009 at 52 weeks. * = p < 0.05; ** = p < 0.01. Reproduced with permission from CitationGovender S, et al 2002. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am, 84-A:2123–34. Copyright © 2002. The Journal of Bone and Joint Surgery, Inc.
The results of this study demonstrate that patients with Gustilo type IIIA or IIIB fracture who had been treated with 1.5 mg/mL rhBMP-2 implant had a significantly reduced rate of infection compared with the control. The authors speculated that this effect was likely due to earlier achievement of fracture stability. The authors also noticed accelerated soft tissue healing and reduction in pain in patients treated with rhBMP-2. This potential reduction in pain may be related to an increased vascular supply that has been observed experimentally in rhBMP-2 induced bone formation. The authors also noted that infection rates were higher in this study compared to other reports as their definition of infection was conservative and included both superficial and deep wounds. The data from this study led to the EMEA (European Agency for the Evaluation of Medicinal Products) approval of rhBMP-2/ACS in 2002 and FDA approval in 2004 for open tibial fractures treated with an IM nail (CitationMcKay et al 2007). An economic model based on the results of the BESTT study trial revealed cost savings to the payer when rhBMP-2 was reserved for patients with Gustilo type IIIA or IIIB fractures (CitationJones et al 2004b).
Between 1996 and 1999, two concurrent prospective, randomized controlled multicenter trials were performed to evaluate the efficacy of rhBMP-2 in the treatment of open tibial fractures. Along with the BESTT trial which was subsequently published, a second unpublished study was conducted with the same study design and protocol for 60 patients at 10 level-1 trauma centers in the US (CitationSwiontkowski et al 2006). Since many surgeons were unaware of this smaller study which showed that the rate of secondary intervention was reduced with the addition of rhBMP-2 to reamed or unreamed IM nailing, CitationSwiontkowski et al (2006) reported a subgroup analysis of the combined results of these two studies. With a total of 520 patients from 59 trauma centers, Swiontowski et al analyzed two subgroups: 1) 131 patients with a Gustilo-Anderson type IIIA or IIIB open tibial fractures, 2) 113 patients treated with reamed intramedullary nailing. The first subgroup demonstrated significant improvements with treatment of 1.5 mg/mL rhBMP-2 with fewer bone grafting procedures (p = 0.0005), decreased need for secondary invasive intervention (p = 0.0065), and a lower rate of infection (p = 0.0234) compared with the control group (). Analysis of subgroup II revealed that fractures treated with reamed IM nailing had no significant difference between the control and rhBMP-2 groups () In this study, CitationSwiontowksi et al (2006) noted that patients in the rhBMP-2 group were bearing weight an average of 32 days sooner than the controls. The authors of this study concluded that rhBMP-2 can be used safely and acutely in patients who present with open tibial fractures with a plan that includes IM nailing and no staged bone-grafting.
Table 1 Comparison of patient outcomes in the control group and rhBMP-2 treatment group for the Gustilo-Anderson Type-III open fracture subgroup
Table 2 Comparison of patient outcomes in control group and rhBMP-2 treatment group for the renamed nailing subgroup
Between 2000 and 2003, CitationJones et al (2006) performed a multicenter randomized controlled trial in 30 patients to investigate the benefit of rhBMP-2 with allograft compared to autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. Patients included in this study had residual fracture defect consistent with clinical recommendation for staged reconstruction with bone grafting (ie, a cortical defect measuring 1–5 cm in length and involving at least 50% of the circumference of the diaphysis) (CitationWatson et al 1995; CitationWhittle et al 1995; CitationTempleman et al 1998); and had to have had initial treatment with either intramedullary nail or external fixation. All 30 patients were enrolled 6–12 weeks after initial injury. Patients allocated to the allograft group received 1.5 mg/mL rhBMP-2/ACS as an onlay graft. Clinical evaluation of fracture healing included pain assessment with full weight bearing and fracture-site tenderness. The Short Musculoskeletal Function Assessment (SMFA) was administered before and after treatment with radiographs used to document union, the presence of bridging callus, and incorporation of bone-graft material.
Ten patients in the autograft group (66%) and 13 patients (86%) in the rhBMP-2/allograft group had healing without further intervention. The mean estimated blood loss was 67% lower in the rhBMP-2 group (mean 117 mL) than the autograft group (mean 353) (p = 0.0073). There was comparable improvement in SMFA scores between the two groups. No patient developed antibodies to BMP-2 in the rhBMP-2/allograft group. Thus, CitationJones et al (2006) demonstrated that administration of the rhBMP-2/ACS combined with cancellous allograft for the treatment of diaphyseal tibial fractures with cortical defects was comparable with the overall clinical results achieved for patients who received autogenous bone grafting. By eliminating autogenous bone graft harvesting, a rhBMP-2/allograft implant can provide significant clinical benefit with decreased intraoperative blood loss and elimination of morbidity associated with autogenous bone graft harvesting. The authors concluded that rhBMP-2/allograft is a clinically beneficial and safe alternative to autogenous bone grafting in cases of tibial fractures with extensive tibial diaphyseal bone loss.
Cost analysis
In a cost analysis study of the use of BMP-2 in open tibial fractures, Garrison et al estimated that the incremental cost per quality adjusted life-year (QALY) gained was greater than US$50,000 (CitationGarrison et al 2007). There was a 35% probability that cost per QALY gained was less than US$50,000. They concluded that the cost-effectiveness ratio is sensitive to the price of BMP and the severity of open tibial fractures. CitationJones et al (2004b) conducted an economic analysis to evaluate the cost of adding rhBMP-2 to the cost of initial fracture repair. In a comparison of treating an open tibial fracture with a nail versus a nail with rhBMP-2, they found that 10.5% of the rhBMP-2 cost would be offset by reductions in secondary interventions and infections. They estimated that the use of rhBMP-2 with intramedullary nailing would result in cost savings of US$3,570 per patient.
Conclusion
Marshall Urist’s discovery of BMP has been pivotal in understanding the biology of fracture healing and has been a source of intense clinical research as an adjunct to fracture treatment. Multiple in vitro and in vivo studies in animals have elucidated the complex biologic interactions between BMPs and cellular receptors and have convincingly demonstrated rhBMP-2 to be a safe, effective treatment option to enhance bone healing. Human clinical trials have provided further insight into BMP-2 dosage, time course, carriers, and efficacy in fracture healing. Several human clinical trials have convincingly demonstrated the positive effects of BMP-2 on fracture healing in tibial defects. These promising results have provided hope that a new biologic field of technology has emerged as a useful adjunct in the treatment of skeletal injuries and conditions. Further research and clinical studies are necessary to delineate the molecular mechanisms underlying bone formation and fracture healing.
Disclosures
No financial or material support was provided for this study, and neither author has any conflict of interest to disclose.
References
- AbeN2002Enhancement of bone repair with a helper-dependent adenoviral transfer of bone morphogenetic protein-2Biochem Biophys Res Commun297523712270126
- BarnesGL1999Growth factor regulation of fracture repairJ Bone Miner Res1418051510571679
- BarnesGL2003Osteoblast-related transcription factors Runx2 (Cbfa1/AML3) and MSX2 mediate the expression of bone sialoprotein in human metastatic breast cancer cellsCancer Res632631712750290
- BhandariMGuyattGHSwiontkowskiMF2001User’s guide to the orthopaedic literature: how to use an article about a surgical therapyJ Bone Joint Surg Am839162611407801
- BlokhuisTJ2001Biomechanical and histological aspects of fracture healing, stimulated with osteogenic protein-1Biomaterials227253011246967
- BodenSD1998Laparoscopic anterior spinal arthrodesis with rhBMP-2 in a titanium interbody threaded cageJ Spinal Disord11951019588464
- BostromMP1999Osteoinductive growth factors in preclinical fracture and long bone defects modelsOrthop Clin North Am306475810471769
- BouxseinML2001Recombinant human bone morphogenetic protein-2 accelerates healing in a rabbit ulnar osteotomy modelJ Bone Joint Surg Am83-A12193011507131
- ChenTL1991Bone morphogenetic protein-2b stimulation of growth and osteogenic phenotypes in rat osteoblast-like cells: comparison with TGF-beta 1J Bone Miner Res61387931792947
- ChengH2003Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs)J Bone Joint Surg Am85-A15445212925636
- ClaesLE1998Effects of mechanical factors on the fracture healing processClin Orthop Relat Res355SupplS132479917634
- CookSD2005Bone defect healing with an osteogenic protein-1 device combined with carboxymethylcelluloseJ Biomed Mater Res B Appl Biomater751374516035032
- den BoerFC2003Healing of segmental bone defects with granular porous hydroxyapatite augmented with recombinant human osteogenic protein-1 or autologous bone marrowJ Orthop Res21521812706026
- den BoerFC2002Effect of recombinant human osteogenic protein-1 on the healing of a freshly closed diaphyseal fractureBone311586412110429
- EdwardsRB3rd2004Percutaneous injection of recombinant human bone morphogenetic protein-2 in a calcium phosphate paste accelerates healing of a canine tibial osteotomyJ Bone Joint Surg Am86-A14253815252089
- EinhornTA2003A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repairJ Bone Joint Surg Am85-A14253512925621
- EinhornTA1995Enhancement of fracture-healingJ Bone Joint Surg Am77940567782368
- EinhornTA1998The cell and molecular biology of fracture healingClin Orthop Relat Res355SupplS7219917622
- FinkemeierCG2002Bone-grafting and bone-graft substitutesJ Bone Joint Surg Am84-A4546411886919
- GarrisonKR2007Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic reviewHealth Technol Assess111150iiiiv17669279
- GautschiOP2007Bone morphogenetic proteins in clinical applicationsANZ J Surg776263117635273
- GovenderS2002Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patientsJ Bone Joint Surg Am84-A21233412473698
- GroeneveldEHBurgerEH2000Bone morphogenetic proteins in human bone regenerationEur J Endocrinol14292110633215
- GuoW1999Expression of bone morphogenetic proteins and receptors in sarcomasClin Orthop Relat Res3651758310627702
- HeckmanJDSarasohn-KahnJ1997The economics of treating tibia fractures. The cost of delayed unionsBull Hosp Jt Dis5663729063607
- HollingerJWongME1996The integrated processes of hard tissue regeneration with special emphasis on fracture healingOral Surg Oral Med Oral Pathol Oral Radiol Endod825946068974129
- HollingerJO2008Recombinant human platelet-derived growth factor: biology and clinical applicationsJ Bone Joint Surg Am90485418292357
- HughesFJ1995The effects of bone morphogenetic protein-2, -4, and -6 on differentiation of rat osteoblast cells in vitroEndocrinology136267177750491
- JonesAL2004aProspective, randomized comparison of rhBMP-2/ACS in combination with allograft versus autogenous bone graft in healing diaphyseal tibial fractures with traumatic bone lossAnnual Meeting of the Orthopedic Trauma AssociationHollywood, FL, USA
- JonesAL2004bUse of rhBMP-2 in the treatment of open tibial shaft fractures: do improved clinical outcomes outweigh the additional expense of rhBMP-2?Annual Meeting of the Orthopedic Trauma AssociationHollywood, FL
- JonesAL2006Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trialJ Bone Joint Surg Am8814314116818967
- KanataniM1995Stimulatory effect of bone morphogenetic protein-2 on osteoclast–like cell formation and bone-resorbing activityJ Bone Miner Res101681908592944
- KarladaniA2000Displaced tibial shaft fractures: a prospective randomized study of closed intramedullary nailing versus cast treatment in 53 patientsActa Orthop Scand71160710852322
- KatagiriT1994Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineageJ Cell Biol1271755667798324
- KawaguchiH2001Acceleration of fracture healing in nonhuman primates by fibroblast growth factor-2J Clin Endocrinol Metab868758011158060
- KhanSN2005The biology of bone graftingJ Am Acad Orthop Surg13778615712985
- KleeffJ1999Bone morphogenetic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivoGastroenterology11612021610220513
- KolbeckS2003Homologous growth hormone accelerates bone healing—a biomechanical and histological studyBone336283714555268
- KumagaiT2006Bone morphogenetic protein-2 suppresses invasiveness of TSU-Pr1 cells with the inhibition of MMP-9 secretionAnticancer Res26293816475709
- LangenfeldEM2003The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cellsCarcinogenesis2414455412819188
- LeeFY2002Repair of bone allograft fracture using bone morphogenetic protein-2Clin Orthop Relat Res3971192611953604
- LiRH2003rhBMP-2 injected in a calcium phosphate paste (alpha-BSM) accelerates healing in the rabbit ulnar osteotomy modelJ Orthop Res21997100414554211
- LiebermanJR1999The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in ratsJ Bone Joint Surg Am819051710428121
- LinkhartTA1996Growth factors for bone growth and repair: IGF, TGF beta and BMPBone19Suppl 11S12S8830994
- LuppenCA2002Recombinant human bone morphogenetic protein-2 enhances osteotomy healing in glucocorticoid-treated rabbitsJ Bone Miner Res173011011811561
- MarshD1998Concepts of fracture union, delayed union, and nonunionClin Orthop Relat Res355SupplS22309917623
- MayerH1996Subtle differences in the mitogenic effects of recombinant human bone morphogenetic proteins -2 to -7 on DNA synthesis on primary bone-forming cells and identification of BMP-2/4 receptorCalcif Tissue Int58249558661956
- McKayWF2007A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE((R)) Bone Graft)Int Orthop317293417639384
- OnishiT1998Distinct and overlapping patterns of localization of bone morphogenetic protein (BMP) family members and a BMP type II receptor during fracture healing in ratsBone22605129626398
- OruiH2000Effects of bone morphogenetic protein-2 on human tumor cell growth and differentiation: a preliminary reportJ Orthop Sci5600411180925
- OzkaynakE1992Osteogenic protein-2. A new member of the transforming growth factor-beta superfamily expressed early in embryogenesisJ Biol Chem2672522071460021
- PluharGE2001The effect of recombinant human bone morphogenetic protein-2 on femoral reconstruction with an intercalary allograft in a dog modelJ Orthop Res193081711347706
- ReddiAH1994Bone and cartilage differentiationCurr Opin Genet Dev4737447849513
- SakouT1998Bone morphogenetic proteins: from basic studies to clinical approachesBone225916039626397
- SchmittJM1999Bone morphogenetic proteins: an update on basic biology and clinical relevanceJ Orthop Res172697810221845
- SeehermanH2006arhBMP-2/calcium phosphate matrix accelerates osteotomy-site healing in a nonhuman primate model at multiple treatment times and concentrationsJ Bone Joint Surg Am881446016391260
- SeehermanH2001The influence of delivery vehicles and their properties on the repair of segmental defects and fractures with osteogenic factorsJ Bone Joint Surg Am83-ASuppl 1 Pt 2S798111314799
- SeehermanHJ2006brhBMP-2 delivered in a calcium phosphate cement accelerates bridging of critical-sized defects in rabbit radiiJ Bone Joint Surg Am8815536516818982
- SodaH1998Antiproliferative effects of recombinant human bone morphogenetic protein-2 on human tumor colony-forming unitsAnticancer Drugs9327319635923
- SwiontkowskiMF2006Recombinant human bone morphogenetic protein-2 in open tibial fractures. A subgroup analysis of data combined from two prospective randomized studiesJ Bone Joint Surg Am8812586516757759
- TemplemanDC1998Update on the management of open fractures of the tibial shaftClin Orthop Relat Res35018259602796
- TermaatMF2005Bone morphogenetic proteins. Development and clinical efficacy in the treatment of fractures and bone defectsJ Bone Joint Surg Am8713677815930551
- TornettaP3rd1994Treatment of grade-IIIb open tibial fractures. A prospective randomised comparison of external fixation and non-reamed locked nailingJ Bone Joint Surg Br761398300656
- TsujiK2006BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healingNat Genet381424917099713
- UristMR1965Bone: formation by autoinductionScience15069889395319761
- Valentin-OpranA2002Clinical evaluation of recombinant human bone morphogenetic protein-2Clin Orthop Relat Res3951102011937870
- WangEA1993Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cellsGrowth Factors957718347351
- WatsonJT1995Management strategies for bone loss in tibial shaft fracturesClin Orthop Relat Res315138527634662
- WelchR1998Effect of recombinant human bone morphogenetic protein-2 on fracture healing in a goat tibial fracture modelJ Bone Miner Res131483909738522
- WhittleAP1992Treatment of open fractures of the tibial shaft with the use of interlocking nailing without reamingJ Bone Joint Surg Am741162711400544
- WhittleAPWesterWRussellTA1995Fatigue failure in small diameter tibial nailsClin Orthop Relat Res315119287634660
- WozneyJMRosenV1998Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repairClin Orthop Relat Res34626379577407
- WranaJL1994Mechanism of activation of the TGF-beta receptorNature370648834178047140
- YamaguchiA1991Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitroJ Cell Biol11368171849907
- YoungBTRayanGM2000Outcome following nonoperative treatment of displaced distal radius fractures in low-demand patients older than 60 yearsJ Hand Surg [Am]251928
- ZabkaAG2001Histomorphometric description of allograft bone remodeling and union in a canine segmental femoral defect model: a comparison of rhBMP-2, cancellous bone graft, and absorbable collagen spongeJ Orthop Res193182711347707