Abstract
Taxane therapy is commonly used in the treatment of metastatic breast cancer. However, most patients will eventually become refractory to these agents. Ixabepilone is a newly approved chemotherapeutic agent for the treatment of metastatic breast cancer. Although it targets microtubules similarly to docetaxel and paclitaxel, ixabepilone has activity in patients that are refractory to taxanes. This review summarizes the pharmacology of ixapebilone and clinical trials with the drug both as a single agent and in combination. Data were obtained using searches of PubMed and abstracts of the annual meetings of the American Society of Clinical Oncology and the San Antonio Breast Cancer Symposium from 1995 to 2008. Ixapebilone is a semi-synthetic analog of epothilone B that acts to induce apoptosis of cancer cells via the stabilization of microtubules. Phase I clinical trials have employed various dosing schedules ranging from daily to weekly to 3-weekly. Dose-limiting toxicites included neuropathy and neutropenia. Responses were seen in a variety of tumor types. Phase II studies verified activity in taxane-refractory metastatic breast cancer. The FDA has approved ixabepilone for use as monotherapy and in combination with capecitabine for the treatment of metastatic breast cancer. Ixabepilone is an efficacious option for patients with refractory metastatic breast cancer. The safety profile is similar to that of taxanes, with neuropathy and neutropenia being dose-limiting. Studies are ongoing with the use of both iv and oral formulations and in combination with other chemotherapeutic and biologic agents.
Introduction
Although great strides have been made in the treatment of breast cancer, once meta-static, cure is unlikely. There are many agents available to treat metastatic breast cancer (MBC), and decisions on treatment must take into account the balance between efficacy and tolerability. Although, there are many new biological agents in clinical trials, chemotherapy is still an important backbone of the treatment. The recent approval of new agents has added to the repertoire available for the treatment of MBC (CitationGradishar et al 2005; CitationPerez et al 2007; CitationThomas et al 2007).
The taxanes are the mainstays of chemotherapy for MBC. However, because all patients will eventually become refractory to these, new strategies are needed for taxane-refractory MBC. One drug that has shown efficacy both preclinically and clinically in patients resistant to docetaxel or paclitaxel is ixabepilone (BMS-247550). This drug fills an important void in chemotherapeutic options for patients.
Pharmacology of ixapebilone
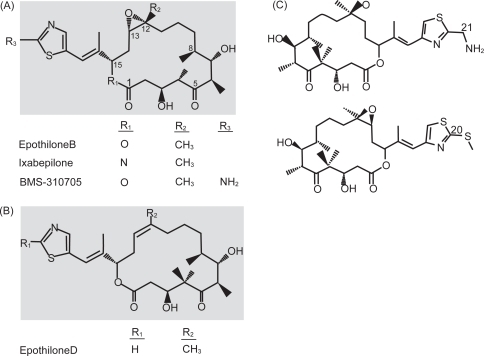
Ixapebilone is in the epothilone class of chemotherapeutic drugs. Epothilones were initially discovered when fungicidal activity was observed by the myxobacterium Sorangium cellolosum and later these agents were found to interact with and stabilize microtubules (CitationBollag et al 1995; CitationGerth et al 1996). Microtubules help form the cytoskeleton and have vital roles in various cellular processes including cell signaling and mitosis. Microtubules are made up of 2 subunits, α and β. Microtubules have been an attractive target for many chemotherapeutic drugs, which exert their cytotoxic effects via microtubule stabilization or destabilization (CitationJordan et al 2004). Ixapebilone is a semi-synthetic analog of epothilone B, created by the substitution of an azide group for oxygen in the macrolide ring (CitationFumoleau et al 2007) (). Similar to taxanes, epothilones exert their effect by binding to and subsequently polymerizing and stabilizing microtubules, thus preventing mitosis and resulting in apoptosis (CitationBollag et al 1995). Epothilones specifically bind to β-tubulin, although the exact binding site is not known (CitationGiannakakou et al 2000). Despite the fact that epothilones bind close to or at the paclitaxel binding site on tubulin (CitationNogales et al 1995; CitationOjima et al 1999; CitationGiannakakou et al 2000), there are also data from yeast studies to suggest that the binding of epothilones to tubulin and the subsequent microtubule formation may be distinct from that of paclitaxel (CitationBode et al 2002). Another important difference is that epothilones have been shown to have activity both preclinically and clinically in taxane resistance, likely due to the fact that the epothilones are not substrates for P-glycoprotein or MRP-1 and are active in β-tubulin mutations (CitationHe et al 2001a; CitationLee et al 2006). MDR-1 drug transporter expressing tumors are also sensitive to epothilones (CitationMcDaid et al 2002). In breast cancer cell lines and xenograft models, including those with multidrug resistance, ixapebilone has been shown to have cytotoxicity values ranging from 1.4 to 45 nM and has improved cytotoxicity over paclitaxel (CitationLee et al 2001, Citation2006). Both an iv and oral formulation of ixabepilone have undergone clinical studies, although it is the iv form that has been approved for the treatment of breast cancer. Further trials with the oral formulation are awaited.
Figure 1 Chemical structures of the epothilones under clinical development. (A) Structure of epothilone B, ixabepilone, and BMS-310705. (B) Structure of epothilone D. (C) Structure of epothilone D second generation. From Fumoleau P, Coudert B, Isambert N, et al. 2007. Novel tubulin-targeting agents: anticancer activity and pharmacologic profile of epothilones and related analogues. Ann Oncol, 18(Suppl 5):v9–15, by permission of Oxford University Press © 2007.
Cell lines resistant to epothilones have been reported (CitationGiannakakou et al 2000; CitationHe et al 2001b). The resistance is felt to be secondary to point mutations in the β-tubulin subunit, rather than MDR1 expression. These cells lines of various tumor types including ovarian and nonsmall-cell lung cancers have been to shown to exhibit resistance to other taxanes as well. It has been shown that these point mutations lead to less endogenous microtubule stabilization (CitationHe et al 2001a). These cell lines resistant to epothilones have also been found to have slower growth, which could be secondary to the endogenously more stable microtubules or could be due to an additional mechanism.
Pharmacokinetic studies with ixabepilone given on a daily dosing schedule have demonstrated a rapid 1-log decline after drug infusion, later followed by a prolonged elimination phase, not unlike taxanes (CitationAbraham et al 2003). The mean half-life of the drug was 16.8 hours. Clearance was rapid, dose-independent, and not associated with body weight or surface area. Dosing every 21 days showed a similar plasma concentration curve and independence of clearance, elimination, and dose (CitationMani et al 2004; CitationGadgeel et al 2005). The mean half-life was 38.5 hours at a dose of 40 mg/m2 given every 21 days (CitationMani et al 2004).
Pharmacodynamic studies with daily dosing did not show any correlation between area under the curve (AUC) and neutropenia (CitationAbraham et al 2003). Similarly, there was no correlation with AUC and neutropenia in the 21-day dosing schedule (CitationMani et al 2004). However, the percentage change in absolute neutrophil count was significantly negatively correlated with increasing doses of ixabepilone (CitationGadgeel et al 2005). Pharmacodynamic studies exploring microtubule bundle formation and the subsequent effects on plasma ixabepilone concentration and extent of neutropenia have been performed (CitationMcDaid et al 2002; CitationMani et al 2007). Microctubule bundle formation is a surrogate for ixabepilone binding to β-tubulin and drug binding increases with greater ixabepilone concentration. This binding is correlated with AUC. Interestingly, it has been shown that there were more tumor cells with microtubule bundle formation than peripheral blood mononuclear cells after ixabepilone dosing (CitationMani et al 2007). The degree of neutropenia has been found to be correlated with the extent of microtubule bundle formation in peripheral blood mononuclear cells.
Clinical trials with ixabepilone
Phase I studies ()
Numerous phase I trials have evaluated the optimum dose and toxicity with ixabepilone. There have been numerous dosing schedules in both oral and iv formulations used in these trials. The earliest phase I trial was reported by CitationAwada et al (2001) and employed a weekly iv dosing schedule. There were various tumor types in this study including breast cancer. At the time when preliminary results were presented, the current dose level was 30 mg/m2/week. Responses included stable disease in patients who were previously treated with a taxane with toxicities including fatigue, anorexia, arthralgia/myalgia, neuropathy, and myelosuppression. Another phase I study using weekly dosing has been presented (CitationBurris et al 2002). In this study the maximum tolerated dose (MTD) was 25 mg/m2/week. Although there was minimal neutropenia, grade 3 toxicities included fatigue, nausea, diarrhea, myalgia/arthralgia, and neuropathy. This study initially used a 30-minute infusion, but was later amended to allow for a 1-hour infusion time, as well as a 3 weeks on, 1 week off dosing schedule in attempts to reduce neuropathy. Responses were seen in patients who had previously received taxane therapy. CitationHao et al (2002) explored continuous weekly dosing in another phase I trial. In this study, dose-limiting toxicities were grade 3 fatigue and grade 4 neutropenia at the 20 mg/m2 and 30 mg/m2 dose levels. Neuropathy was more frequent in patients who were heavily pretreated. Decreases in tumor markers were seen in taxane-refractory patients.
Table 1 Summary of phase I studies
The use of 3-weekly dosing was also evaluated in the phase I setting. The earliest report was the preliminary data of CitationSpriggs et al (2001). In this trial, a 1-hour infusion of ixabepilone was given to patients in dose levels ranging from 7.4 mg/m2 to 65 mg/m2. MTD was determined to be 50 mg/m2. Dose-limiting toxicities included grade 3 arthralgias and myalgias, grade 3 neuropathy, grade 4 neutropenia, febrile neutropenia, pneumococcal sepsis, and 1 death. Antitumor activity was seen again in taxane pretreated patients and a complete response was seen in a patient with ovarian cancer. Another phase I study using a 1-hour infusion every 3 weeks also determined the MTD to be 50 mg/m2 (CitationAghajanian et al 2007). In this study, the most common toxicities limiting dose were neutropenia, stomatitis, myalgia, and arthralgia. A 3-hour infusion time was also investigated in this trial and no dose-limiting toxicities (DLTs) were seen at doses less than 50 mg/m2 when the drug was administered over 3 hours. Eight patients attained an objective response, many of whom had previously received taxane therapy. CitationMani et al (2004) performed a phase I study using 3-weekly dosing with 1-hour infusion times. The recommended phase II dose of ixabepilone was determined to be 40 mg/m2. Similarly to previous trials, the dose-limiting toxicities included grade 4 and febrile neutropenia; however, grade 3 abdominal pain, nausea, and vomiting were also seen. A Japanese phase I trial using a 3-hour infusion every 3 weeks also determined the tolerable dose of ixabepilone to be 40 mg/m2 (CitationShimizu et al 2008); DLT was grade 4 neutropenia. There were no objective responses, but over two-thirds of patients had stable disease. Linear pharmacokinetics were observed.
A dose of 40 mg/m2 dose was again verified as the MTD in a phase I study in patients with refractory, advanced solid tumors and the DLT was neutropenia (CitationGadgeel et al 2005). Grade 1 and 2 neuropathy was observed in 3 out of 5 patients who received greater than 28.6 mg/m2 for at least 2 cycles. Fatigue was also commonly seen. No patients met criteria for partial responses; however, there were minor responses and stable disease was seen. Pharmacokinetics were linear and there was evidence to suggest that there was a correlation between the change in neutrophil count and the time at which the ixabepilone plasma concentration was >15 ng/mL.
In attempts to augment the degree of neurotoxicity observed with 3-weekly dosing, daily administration of ixabepilone was evaluated in the phase I setting. Less neuropathy has been observed in daily compared with 3-weekly dosing. CitationAbraham et al (2003) examined a dosing schedule of treatment daily for 5 consecutive days, with 21-day dosing intervals (CitationAbraham et al 2003). Ixabepilone was given as a 1-hour infusion. Most patients had received prior taxane therapy and half had received ≥6 prior lines of treatment. MTD was 6 mg/m2. Higher doses (8 mg/m2) were complicated with neutropenia despite filgrastim support. Neurotoxicity was generally mild and no grade 3 or 4 neuropathy was observed. Five out of 27 patients enrolled achieved a partial response, and these patients had received prior taxane therapy. Preliminary data presented explored a daily dosing schedule given every 3 days where the recommended phase II dose was 8 mg/m2 for 3 days, with cycles every 21 days (CitationThambi et al 2003). A published report of a daily dosing schedule for 3 days also determined the MTD to be at a dose of 8 mg/m2/day. This was given as a 1-hour infusion and similarly to the Abraham study, the DLT was neutropenia and there were no cases of dose-limiting neurotoxicity (CitationZhuang et al 2005). Seventeen of the 26 enrolled patients had received prior taxane therapy and the median number of prior therapies received was ≥4. Although no responses were observed in this trial, patients with renal cell carcinoma, ovarian cancer, and primary peritoneal mesothelioma had prolonged stable disease up to 28 months.
Numerous phase I studies have also evaluated the toxicities associated with ixabepilone given in combination with other chemotherapeutic and biologic agents (CitationPlummer et al 2002; CitationAnderson et al 2004; CitationBunnell et al 2006; CitationHensley et al 2007). Preliminary results of a study exploring the use of ixabepilone in combination with carboplatin revealed partial responses in patients with breast cancer as well as neuroendocrine cancer (CitationPlummer et al 2002). Some patients also achieved stable disease. Patients were relatively chemotherapy-naïve as only up to 2 prior therapies were allowed. DLT was reached at 40 mg/m2 of ixabepilone and an AUC of 5 for carboplatin. A phase I/II study conducted in patients with MBC evaluated the combination of ixabepilone with capecitabine (CitationBunnell et al 2006). Eligible patients had received prior taxane and anthracycline-based treatment in the adjuvant or metastatic but could not have received greater than 3 prior regimens for metastatic disease. Two treatment schedules were evaluated; a single 3-hour infusion or daily therapy given over 1 hour for 3 days every 21 days. The recommend phase II dose for ixabepilone was determined to be 40 mg/m2 as a single infusion and for capecitabine was 2000 mg/m2 given on days 1–14 every 3 weeks. Nearly half of the patients had 2 or more therapies in the metastatic setting. Fifteen of 50 enrolled patients achieved a complete (1 patient) or partial response (14 patients) yielding an overall response rate of 30%. Responses had a median duration of nearly 7 months.
Phase II studies in breast cancer ()
Based on ample evidence of response and safety in early trials, various phase II studies were designed for patients with varying levels of previous therapies. Three published phase II trials used a daily dosing schedule. CitationLow et al (2005) used a 6 mg/m2/day dosing schedule on days 1–5 every 3 weeks in patients with taxane-refractory breast cancer. A total of 37 patients were enrolled and 43% had received between 3 and 9 prior chemotherapy regimens in the metastatic setting. The objective response rate (ORR) was 22% and patients received a median of 4 cycles (range 1–11 cycles) with a median time to progression of 80 days for all subjects. An additional 35% of patients had stable disease for at least 6 weeks. Toxicities were generally mild. Twelve patients required dose reductions secondary to toxicities including neuropathy, diarrhea, fatigue, neutropenia, and myalgia. Only 1 patient developed grade 3 neuropathy, although mild neuropathy (grades 1 or 2) was frequent (83% of total subjects).
Table 2 Summary of phase II studies in metastatic breast cancer
Another phase II study examined a daily dosing schedule for 5 consecutive days at a dose of 6 mg/m2/day given every 21 days (CitationDenduluri et al 2007a). Patients in this trial were taxane-naïve; however, any number of non-taxane prior therapies was allowed. Of the 23 patients enrolled, 70% had prior chemotherapy, which was primarily adjuvant anthracycline. ORR was 57%. Stable disease for at least 6 weeks was achieved in 26%. Median time to progression was 5.5 months, and for partial responders, the median duration of response was 5.6 months. Four patients had toxicity necessitating removal from study (grade 3 weight loss, grade 3 motor neuropathy, prolonged autonomic neuropathy, and grade 2 fatigue) and dose reductions occurred in 4 patients (prolonged neutropenia, neuropathy, and fatigue). Severe neuropathy was infrequent with 13% having grade 2 sensory neuropathy, 4% grade 2 motor neuropathy, no patients with grade 3 sensory neuropathy, and 1 patient with grade 3 motor neuropathy. The same group performed a small study using daily dosing but with 3 consecutive days of therapy at initial doses of 8 mg/m2/day which was titrated up to 10 mg/m2/day if tolerated (CitationDenduluri et al 2007b). Of the 12 patients enrolled, all had received previous taxanes. Median number of previous therapies in the metastatic setting was 3.5. The treatment had an acceptable safety profile; however, no complete or partial responses were seen and the study was stopped. Possible explanations for the lack of response may be decreased dose intensity in comparison with other studies with daily dosing.
There have also been numerous phase II studies with 3-weekly dosing in patients with MBC, in both taxane-naïve and taxane-refractory patients. CitationPerez et al (2007) examined the efficacy of ixabepilone in a heavily pretreated patient population which had received prior anthracycline, taxane, and capecitabine therapies. Dose was 40 mg/m2 given as a 3-hour infusion every 3 weeks. This was the third line of treatment in the metastatic setting for 88% of the 126 patients enrolled. An independent radiology facility assessed ORR at 11.5% and the investigator-assessed ORR was 18.3%. Stable disease was achieved in 50% of patients. The median overall survival was 8.6 months. The adverse effects encountered were generally managable. Grade 3 and 4 neutropenia was seen in about half the patients, but febrile neutropenia was uncommon. The most frequent nonhematologic toxicity was peripheral sensory neuropathy which was seen in 60%, but this generally reversed after 5 weeks.
Another study using the same dose and schedule of ixabepilone was evaluated in taxane refractory patients (CitationThomas et al 2007). Of the 66 patients, 86% had received ≥2 prior therapies and 98% of those patients had either docetaxel or paclitaxel as their most recent treatment in the metastatic setting. ORR was 12%, similar to that in the Perez study, and stable disease was achieved in 41% of patients. Fifty-five percent of patients developed grade 1 or 2 toxicity. Serious adverse effects were seen in 22% of patients. This trial was initially designed to give 50 mg/m2 of ixabepilone over 1 hour, and 38% of patients at that dose developed grade 3 sensory neuropathy. When the dose was lowered to 40 mg/m2 and extended over a 3-hour infusion time, the incidence of severe neuropathy dropped to 12%.
Ixabepilone has also been studied in the first-line metastatic setting (CitationRoche et al 2007), with a 40 mg/m2 dose given as a 3-hour infusion every 3 weeks. All 65 patients had received anthracycline-based therapy in the adjuvant setting. The primary endpoint was objective response rate, which was determined as 41.5%. There were no complete responses, but 27 patients had a partial response and 23 patients had stable disease. Median survival was 22 months. Similar to the other studies using this dosing schedule, toxicities were generally mild and managable. Twenty percent of patients developed grade 3 sensory neuropathy, and 51% had grade 1 or 2 sensory neuropathy. Although grade 3 or 4 neutropenia was seen in 58% of patients, only 6 (9%) patients had febrile neutropenia or infections related to neutropenia.
Activity in other tumor types
Based on the activity of ixabepilone in a wide variety of tumor types in phase I studies, a number of phase II studies were designed to assess disease specific activity. In patients with nonsmall-cell lung cancer refractory to platinum drugs, ORR was 14.3% with 3-weekly dosing and 11.6% with daily dosing for 5 days, which is similar to other second-line treatments in this disease. The toxicity profile was acceptable and included a 6% rate of grade 3 sensory neuropathy, and neutropenia was seen in 28% of patients receiving 3-weekly dosing and in 17% of patients receiving daily dosing (CitationVansteenkiste et al 2007). Acceptable response rates of 15%–48% with and without estramustine phosphate were seen in hormone-refractory prostate cancer. Grade 3 sensory neuropathy was seen in 13%–17% of patients and rates of grade 3/4 neutropenia were 17%–29% (CitationGalsky et al 2005; CitationHussain et al 2005). Modest activity was also seen in patients with prostate cancer in the second-line setting who had progressed on first-line docetaxel; however, half the patients developed grade 3/4 neutropenia (CitationRosenberg et al 2007). Final as well as preliminary phase II data with promising activity have also been presented in renal cell cancer (CitationFojo et al 2005), gynecologic cancers (CitationChen et al 2004), hepatobiliary cancer (CitationSingh et al 2003), gastric cancer (CitationAjani et al 2006), pancreatic cancer (CitationWhitehead et al 2006), and nonHodgkin’s lymphoma (CitationO’Connor et al 2005; CitationSmith et al 2005).
Despite some suggestions of phase I activity, there has not been any meaningful efficacy in phase II studies in melanoma, colorectal cancer, and sarcoma (CitationEng et al 2004; CitationPavlick et al 2004; CitationOkuno et al 2005).
Phase III registration trial
Based on preclinical data showing synergism between ixabepilone and capecitabine (CitationLee 2006) and the results of the phase I/II study discussed above (CitationBunnell et al 2006), a phase III study was designed to examine the response to the combination compared with single-agent capecitabine (CitationThomas et al 2007). In this trial, 752 patients were randomized to receive either capecitabine alone (2500 mg/m2 orally on days 1–14 every 21 days) or capecitabine (2000 mg/m2) in combination with ixabepilone (40 mg/m2 every 21 days). All patients had received prior anthracyclines and taxanes and almost half had received ≥2 prior therapies for metastatic disease. Dose reductions of ixabepilone or capecitabine were required in approximately half the patients in the combination arm, whereas 37% of patients in the single-agent capecitabine arm required a dose reduction. The primary endpoint was progression-free survival, which was significantly improved in the combination therapy arm (5.8 months vs 4.2 months). The hazard ratio reflected a 25% improvement in the estimated risk of disease progression favoring the combination. The objective response rate was 35% with ixabepilone and capecitabine versus 14% with capecitabine alone (p < 0.0001). In the combination arm 41% of patients had stable disease compared with 46% in the single-agent capecitabine arm. As expected, incidence of sensory neuropathy was greater in the combination arm, although rates of severe hand-foot syndrome and diarrhea were similar in each arm. Grades 3/4 neutropenia were more common in the combination arm, and any neutropenia was frequent in that arm (89%). In the single-agent capecitabine arm 43% of patients had any level of neutropenia.
Neoadjuvant trials
Ixabepilone has been used in the neoadjuvant setting in breast cancer in addition to the metastatic setting. A preliminary report using a dose of 40 mg/m2 every 21 days revealed a pathologic complete response rate (pCR) of 21% of patients. Approximately 50% of patients were ER+. Grade 3 or 4 neutropenia was seen in 21% and 13% of patients, respectively. Grade 2 neuropathy was seen in 11% of patients, whereas grade 3 neuropathy was seen in 2% (CitationBaselga et al 2005). Correlative studies revealed a 6 gene predictive model that was able to predict benefit to ixabepilone (CitationLlmobart-Cussac et al 2005). Other preliminary data suggest a higher response rate in the triple negative (ER, PR, and HER2 negative) subgroup of breast cancer patients, with a pCR rate of 26% of the 42 triple negative patients and 15% in the 119 other patients given neoadjuvant ixabepilone (CitationRoche et al 2006).
Safety
As evidenced in phase I and II trials, neuropathy and neutropenia are important potential toxicities. The neuropathy is primarily sensory and generally reversible in most patients with proper dose reductions and drug discontinuation (CitationPerez et al 2007; CitationThomas et al 2007). Patients with diabetes have been shown to be at increased risk for the development of neuropathy and it is important to note that because patients with pre-existing neuropathy were excluded from clinical trials, their potential severity of neuropathy is unknown. Numerically it appears that daily dosing (CitationAbraham et al 2003; CitationLow et al 2005; CitationDenduluri et al 2007a; CitationDenduluri et al 2007b) may be associated with less severe neuropathy (grade 3 neuropathy in 3%–4%) than in 3-weekly dosing (grade 3 in 13%–23%) (CitationPerez et al 2007; CitationRoche et al 2007; CitationThomas et al 2007). However, a study in nonsmall-lung cancer did not show any differences in neuropathy between 3-weekly and daily dosing (CitationVansteenkiste et al 2007). The use of specific neurologic function tests to characterize the neuropathy associated with ixabepilone has been described (CitationLee et al 2006). The patients enrolled in a monotherapy trial of ixabepilone in breast cancer (CitationLow et al 2005) had a number of neurologic function tests performed, including Semmes-Weinstein monofilament testing and modified Romberg stance tests, which are used to assess diabetic neuropathy, and the Jebsen Test of Hand Function (JTH) and Grooved Pegboard Test (GPT), which are used to assess hand functions particularly in patients with stroke and rheumatoid arthritis. Twenty-three percent of patients developed ≥grade 2 neuropathy (including decreased hand function, decreased sensory function, paresthesias, and motor weakness), 9 patients developing grade 2 neuropathy and 2 patients developing grade 3 neuropathy. Three patients had refractory neuropathy that did not resolve to grade 1 up to 2 years after development. The results of JTH and GPT scores were found to be identifiable with onset of grade 12 or higher peripheral neuropathy.
The most common overall adverse reactions seen in the monotherapy registration trial are summarized in (CitationPerez et al 2007). When combined with capecitabine, toxicities commonly encountered with capecitabine such as palmar-plantar erythrodysesthesia and diarrhea were not significantly increased. It is recommended that patients with known severe hypersensitivity to Cremophor® EL or derivatives, baseline ANC <1500 cells/mm3, platelets <100,000 cells/mm3, or abnormal liver function (transaminases >2.5 × ULN or bilirubin >1 × ULN) do not receive ixabepilone.
Table 3 Nonhematologic adverse events seen in monotherapy registration trial
Conclusions
Ixabepilone is an important addition to chemotherapeutic options for patients with MBC. The activity in patients who have failed previous taxanes makes this drug applicable for virtually all patients with MBC at some time in the course of their treatment. The high level of activity in the first-line setting makes it an attractive option in that setting as well. The results of ongoing trials exploring the additional of various biologic agents, such as trastuzumab and bevacizumab, are eagerly awaited (CitationCancer.gov 2008a, Citationb, Citationc). The strategy of combining chemotherapy with antiangiogenic agents is increasingly being used especially in the first-line setting for MBC and the potential for improved activity exists with the other novel microtubule agents (CitationMiller et al 2007). The taxanes in particular may potentiate the anti-angiogenic effects of these agents (CitationMiller et al 2001; CitationSweeney et al 2001). A phase III trial conducted by the Cancer and Leukemia Group B (CALGB) and the North Central Cancer Treatment Group (NCCTG) is currently underway comparing the activity of various microtubule stabilizing agents with the vascular endothelial growth factor antibody bevacizumab. This trial is comparing weekly paclitaxel (90 mg/m2), nab-paclitaxel (100 mg/m2), and ixabepilone (16 mg/m2) in combination with bevacizumab. This trial will also supply important information on the activity of these agents given in a “dose-dense” fashion, in which paclitaxel and arguably nab-paclitaxel have been found to have the greatest activity (CitationGradishar et al 2006; CitationSeidman et al 2008; CitationSparano et al 2008). There are a number of other agents that target the angiogenic as well as other important pathways with recent promising data (CitationRugo et al 2007) (CitationBurstein et al 2008), and these agents may provide other rational combinations with ixabepilone. With the advent of new and possibly improved taxane agents, the question will be in what setting are these agents best used and whether they would replace traditional taxanes as the preference for first-line therapy; or whether, perhaps because of activity in docetaxel- and paclitaxel-resistant patients, their activity may be best reserved for patient with refractory disease. The role of ixabepilone will be best ascertained when it is directly compared with the other available taxanes in a variety of dosing schedules and lines of therapy. These questions will be best answered in the setting of a phase III randomized trial such as the CALGB/NCCTG one described above.
Disclosures
Neither author has any financial disclosures related to any product discussed in this review.
References
- Cancer.gov2008aA phase II combination of trastuzumab and ixabepilone versus trastuzumab and docetaxel in patients with advanced and/or metastatic breast cancerAccessed 2 January 2008. URL: http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=558843&version=HealthProfessional&protocolsearchid=4256672
- Cancer.gov2008bPhase II study of trastuzumab (herceptin) and ixabepilone (BMS-247550) in women with HER2-positive metastatic breast cancerAccessed 2 January. 2008. URL: http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=355176&version=HealthProfessional&protocolsearchid=4352712
- Cancer.gov2008cA trial of 2 schedules of ixabepilone plus bevacizumab and paclitaxel plus bevacizumab for breast cancerAccessed 2 January 2008. URL: http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=355176&version=HealthProfessional&protocolsearchid=4256672
- AbrahamJAgrawalMBakkeS2003Phase I trial and pharmacokinetic study of BMS-247550, an epothilone B analog, administered intravenously on a daily schedule for five daysJ Clin Oncol2118667312721265
- AghajanianCBurrisHA3rdJonesS2007Phase I study of the novel epothilone analog ixabepilone (BMS-247550) in patients with advanced solid tumors and lymphomasJ Clin Oncol251082817261851
- AjaniJASafranHBokemeyerC2006A multi-center phase II study of BMS-247550 (Ixabepilone) by two schedules in patients with metastatic gastric adenocarcinoma previously treated with a taxaneInvest New Drugs24441616586011
- AndersonSDizonDSabbatiniJ2004Phase I trial of BMS-247550 and gemcitabine in patients with advanced solid tumor malignanciesProc Am Soc Clin Oncol22abstract 2098
- AwadaABleibergHdeValeriolaD2001Phase I clinical and pharmacology study of the epothilone analog BMS-247550 given weekly in patinets (pts) with advanced solid tumorsProc Am Soc Clin Oncolabstract 427
- BaselgaJGianniLLlombartA2005Predicting repsonse to ixabepilone: genomics study in patients receiving single agent ixabepilone as neoadjuvant treatment for breast cancer (BC)Breast Cancer Res Treat94s31s32
- BodeCJGuptaMLJrReiffEA2002Epothilone and paclitaxel: unexpected differences in promoting the assembly and stabilization of yeast microtubulesBiochemistry413870411900528
- BollagDMMcQueneyPAZhuJ1995Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of actionCancer Res552325337757983
- BunnellCAKlimovskyJThomasE2006Final efficacy results of a phase I/II trial of ixabepilone in combination with capecitabine in patients with metastatic breast cancer (MBC) previously treated with a taxane and an anthracyclineProc Am Soc Clin Oncol24abstract 10511
- BurrisHAAwadaAJonesS2002Phase I study of the novel epothilone BMS-247550 administered weekly in patietns (pts) with advanced malignanciesProc Am Soc Clin Oncolabstract 412
- BursteinHJEliasADRugoHS2008Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxaneJ Clin Oncol261810618347007
- ChenTMolinaAMooreS2004Epithilone B analog (BMS-247550) at the recommended Phase II dose (RPTD) in patients (pts) with gynecologic (gyn) and breast cancersJ Clin Oncol23abstract 2115
- DenduluriNLeeJJWalsheJ2007bPhase II trial of ixabepilone, an epothilone B analog, given daily for three days every three weeks, in metastatic breast cancerInvest New Drugs2563716933153
- DenduluriNLowJALeeJJ2007aPhase II trial of ixabepilone, an epothilone B analog, in patients with metastatic breast cancer previously untreated with taxanesJ Clin Oncol253421717606971
- EngCKindlerHLNattamS2004A phase II trial of the epothilone B analog, BMS-247550, in patients with previously treated advanced colorectal cancerAnn Oncol159283215151950
- FojoATMenefeeMPoruchynskyM2005A translational study of ixabepilone (BMS-247550) in renal cell cancer (RCC): assessment of its activity and demonstration of target engagement in tumor cellsJ Clin Oncol23abstract 4541
- FumoleauPCoudertBIsambertN2007Novel tubulin-targeting agents: anticancer activity and pharmacologic profile of epothilones and related analoguesAnn Oncol18Suppl 5v91517656562
- GadgeelSMWozniakABoinpallyRR2005Phase I clinical trial of BMS-247550, a derivative of epothilone B, using accelerated titration 2B designClin Cancer Res116233916144926
- GalskyMDSmallEJOhWK2005Multi-institutional randomized phase II trial of the epothilone B analog ixabepilone (BMS-247550) with or without estramustine phosphate in patients with progressive castrate metastatic prostate cancerJ Clin Oncol2314394615735119
- GerthKBedorfNHofleG1996Epothilons A and B: antifungal and cytotoxic compounds from Sorangium cellulosum (Myxobacteria). Production, physico-chemical and biological propertiesJ Antibiot (Tokyo)4956038698639
- GiannakakouPGussioRNogalesE2000A common pharmacophore for epothilone and taxanes: molecular basis for drug resistance conferred by tubulin mutations in human cancer cellsProc Natl Acad Sci USA972904910688884
- GradisharWJKrasnojonDCheporovS2006A randomized phase 2 trial of qw or q3w ABI-007 (ABX) vs. q3w solvent based docetaxel (TXT) as first-line therapy in metastatic breast cancer (MBC)Breast Cancer Res Treats21abstract 46
- GradisharWJTjulandinSDavidsonN2005Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancerJ Clin Oncol23779480316172456
- HaoDHammondLAdeBonoJS2002Continuous weekly administration of the epothilone-B derivative, BMS247,550 (NSC710428): a phase I and pharmacokinetic (PK) studyProc Am Soc Clin Oncol21abstract 411
- HeLOrrGAHorwitzSB2001aNovel molecules that interact with microtubules and have functional activity similar to taxolDrug Discov Today611536411700217
- HeLYangCPHorwitzSB2001bMutations in beta-tubulin map to domains involved in regulation of microtubule stability in epothiloneresistant cell linesMol Cancer Ther131012467233
- HensleyMLDizonDDerosaF2007A phase I trial of BMS-247550 (NSC# 710428) and gemcitabine in patients with advanced solid tumorsInvest New Drugs253354117364235
- HussainMTangenCMLaraPNJr2005Ixabepilone (epothilone B analogue BMS-247550) is active in chemotherapy-naive patients with hormone-refractory prostate cancer: a Southwest Oncology Group trial S0111J Clin Oncol238724916314632
- JordanMAWilsonL2004Microtubules as a target for anticancer drugsNat Rev Cancer42536515057285
- LeeFYBorzilleriRFairchildCR2001BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacyClin Cancer Res714293711350914
- LeeFYCamusoACastenadaS2006Preclinical studies of ixabepilone (BMS-247550) demonstrate optimal antitumor activity against both chemotherapy-sensitive and -resistant tumor types [abstract]Proceedings 97th Annual Meeting of the American Association for Cancer ResearchWashington DC. Philadelphia (PA)2006 Apr 1–5abstract 503
- LeeFYCamusoACastenadaC2006Preclinical efficacy evaluation of ixbepilone (BMS-247550) in combination with cetuximab or capecitabine in human colon and lung carcinoma xenograftsJ Clin Oncol24abstract 12017
- LeeJJLowJACroarkinE2006Changes in neurologic function tests may predict neurotoxicity caused by ixabepiloneJ Clin Oncol2420849116648510
- Llmobart-CussacABaselgaGManikhasG2005Phase II genomics study in patients receiving Ixabepilone as neoadjuvant treatment for breast cancer (BC): Preliminary efficacy and safety dataJ Clin Oncol23abstract 586
- LowJAWedamSBLeeJJ2005Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in metastatic and locally advanced breast cancerJ Clin Oncol2327263415837987
- McDaidHMManiSShenHJ2002Validation of the pharmacodynamics of BMS-247550, an analogue of epothilone B, during a phase I clinical studyClin Cancer Res820354312114401
- ManiSMcDaidHHamiltonA2004Phase I clinical and pharmacokinetic study of BMS-247550, a novel derivative of epothilone B, in solid tumorsClin Cancer Res1012899814977827
- ManiSMcDaidHMGrossmanA2007Peripheral blood mononuclear and tumor cell pharmacodynamics of the novel epothilone B analogue, ixabepiloneAnn Oncol18190517018704
- MillerKWangMGralowJ2007Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancerN Engl J Med35726667618160686
- MillerKDSweeneyCJSledgeGWJr2001Redefining the target: chemotherapeutics as antiangiogenicsJ Clin Oncol19119520611181686
- NogalesEWolfSGKhanI1995Structure of tubulin at 6.5 A and location of the Taxol-binding siteNature37542477760939
- O’ConnorOAStrausDMoskowitzC2005Targeting the microtubule apparatus in indolent and mantle cell lymphoma with the novel epothilone analog BMS 247550 induces major and durable remissions in very drug resistant diseaseJ Clin Oncol23abstract 6569
- OjimaIChakravartySInoueT1999A common pharmacophore for cytotoxic natural products that stabilize microtubulesProc Natl Acad Sci USA9642566110200249
- OkunoSMaplesWJMahoneyMR2005Evaluation of epothilone B analog in advanced soft tissue sarcoma: a phase II study of the phase II consortiumJ Clin Oncol2330697315860865
- PavlickACMillwardMFarrellK2004A phase II study of the epothilone B analog (EpoB)-BMS 247550 (NSC710428) in stage IV malignant melanomaJ Clin Oncol23abstract 7542
- PerezEALerzoGPivotX2007Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabineJ Clin Oncol2534071417606974
- PlummerRMolifeRVerrillM2002Phase I and pharmacokinetic study of BMS-247550 in combination with carboplatin in patients with advanced solid malignanciesProc Am Soc Clin Oncol21abstract 2125
- RocheHPerezEALlombart-CussacA2006Ixabepilone, an epothilone B analog, is effective in ER, PR, and HER-2 negative (triple negative) patinets (pts): Data from neoadjuvant and metastatic breast cancer (MBC) trialsAnn Oncol17abstract 256P
- RocheHYelleLCognettiF2007Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapyJ Clin Oncol2534152017606972
- RosenbergJEWeinbergVKKellyWK2007Activity of second-line chemotherapy in docetaxel-refractory hormone-refractory prostate cancer patients: randomized phase 2 study of ixabepilone or mitoxantrone and prednisoneCancer1105566317577218
- RugoHSStopeckAJoyAA2007A radomized, double-blind phase II study of the oral tyrosine kinase inhibitor (TKI) axitinib (AG-013736) in combination with docetaxel (DOC) compared to DOC plus placebo (PL) in metastatic breast cancer (MBC)Proc Am Soc Clin Oncol25abstract 1003
- SeidmanADBerryDCirrincioneC2008Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of the Cancer and Leukemia Group B protocol 9840J Clin Oncol261642918375893
- ShimizuTYamamotoNYamadaY2008Phase I clinical and pharmacokinetic study of 3-weekly, 3-h infusion of ixabepilone (BMS-247550), an epothilone B analog, in Japanese patients with refractory solid tumorsCancer Chemother Pharmacol61751817594093
- SinghDAKindlerHLEngC2003Phase II trial of the epothilone B analog BMS-247550 in patients with hepatobiliary cancerProc Am Soc Clin Oncol22abstract 1127
- SmithSMProBVan BesienK2005A phase II study of epothilone B analog BMS-247550 (NSC 710428) in patients with relapsed aggressive non-Hodgkin‘s lymphomaJ Clin Oncol23abstract 6625
- SparanoJAWangMMartinoS2008Weekly paclitaxel in the adjuvant treatment of breast cancerN Engl J Med35816637118420499
- SpriggsDSoignetSBienvenuB2001Phase I first-in-man study of the epothilone B Analog BMS-247550 in patients with advanced cancerProc Am Soc Clin Oncol20abstract 428
- SweeneyCJMillerKDSissonsSE2001The antiangiogenic property of docetaxel is synergsitic with a recombinant humanized monoclonal antobody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growht factorsCancer Res6133697211309294
- ThambiPMEdgerlyMAgarwalM2003A phase I clinical trial of BMS-247550 (NSC 71028), an epothilone B derivative, given daily for 3 days on a 21 day cycle in patients with refractory neoplasmsProc Am Soc Clin Oncol22abstract 540
- ThomasETaberneroJFornierM2007Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxaneresistant metastatic breast cancerJ Clin Oncol25339940617606975
- ThomasESGomezHLLiRK2007Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatmentJ Clin Oncol255210717968020
- VansteenkisteJLaraPNJrLe ChevalierT2007Phase II clinical trial of the epothilone B analog, ixabepilone, in patients with non small-cell lung cancer whose tumors have failed first-line platinum-based chemotherapyJ Clin Oncol2534485517606973
- WhiteheadRPMcCoySRivkinSE2006A Phase II trial of epothilone B analogue BMS-247550 (NSC #710428) ixabepilone, in patients with advanced pancreas cancer: a Southwest Oncology Group studyInvest New Drugs245152016699973
- ZhuangSHAgrawalMEdgerlyM2005A Phase I clinical trial of ixabepilone (BMS-247550), an epothilone B analog, administered intravenously on a daily schedule for 3 daysCancer1031932815800893