Abstract
Introduction
Mucopolysaccharidosis type VI (MPS VI, Maroteaux-Lamy syndrome) is an autosomal recessive lysosomal storage disorder, characterized primarily by skeletal dysplasia and joint contracture. It is caused by a deficiency of N-acetylgalactosamine-4-sulfatase (arylsulfatase B), for which a recombinant formulation (galsulfase) is available as replacement therapy.
Objective
To evaluate the effectiveness and safety of galsulfase compared to placebo or no interventions, for treating MPS VI. We also considered studies evaluating different doses of galsulfase.
Methods
A systematic review of the literature was conducted. A computerized electronic search in MEDLINE, EMBASE, CENTRAL, SciELO, and LILACS was carried on to identify any randomized trials that met our inclusion criteria.
Results
Two studies were included in the review. Because the number of studies was small, our analysis probably did not find any statistically significant difference. Long-term follow-up will be required to ascertain full clinical benefit, on both event-free survival and quality of life measures.
Conclusions
There is some evidence to support the use of galsulfase in the treatment of MPS VI; however due to the very low quantity of included studies we could not analyze it in an appropriate way. This review highlights the need for continued research into the use of enzyme replacement therapy for MPS VI.
Introduction
The mucopolysaccharidoses represent a group of lysosomal disorders characterized by the progressive accumulation of glycosaminoglycans (GAG) in multiple cell types; which occurs as a consequence of distinct deficiencies in the enzyme responsible for GAG degradation. Each subtype is assigned a number, based on its chronologic description, and an eponym, in recognition of the clinician(s) involved in its initial delineation. As a group, the incidence of MPS disorders has been estimated at 1 in 25,000. Enzyme replacement therapy is available for MPS types I, II, and VI.
Mucopolysaccharidosis VI (MPS VI), also known as Maroteaux-Lamy syndrome, is caused by the deficiency of N-acetylgalactosamine-4-sulfatase (arylsulfatase B, ASB) and the resultant tissue storage of dermatan sulfate. Clinical manifestations include distinctive facial features, skeletal dysplasia leading to short stature, joint contractures, and cardio-pulmonary involvement. Patients have reduced exercise capacity and endurance, and limitations in joint range of motion. It is a relatively rare disorder, with an estimated incidence of 1 in 248,000 to 1 in 300,000.
In clinical trials, a recombinant formulation (galsulfase, rhASB; Naglazyme®) has been shown to be safe and effective in the treatment of MPS VI, when compared to placebo or no interventions.
After the performance of a rigorous search strategy in the electronic databases it was not verified a systematic review about this topic. Therefore, we proposed to summarize and organize studies about galsulfase for MPS VI through a systematic review, because of its potential internal validity and to provide assistance to physicians and consumers about the best evidence available in the literature.
Method
Literature search
There was no language restriction. Trials were obtained from the following sources: Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, issue 1, 2009), Publisher’s MEDLINE (Pubmed; 1966–2009), Excerpta Medica database (EMBASE; 1980–2009), Scientific Electronic Library Online (SciELO; 2009) and, Literatura Latino-Americana e do Caribe em Ciências da Saúde (LILACS; 1982–2009) to identify randomized and quasi-randomized controlled clinical trials that met our inclusion criteria. The date of the last search was March 2009.
The following databases of ongoing trials were also searched: National Institutes of Health database of Ongoing Clinical trials (www.clinicaltrials.gov) and Current Controlled Trials (www.controlled-trials.com).
The databases were searched using a comprehensive search strategy for mucopolysaccharidosis VI and galsulfase including an exhaustive list of synonyms. The search strategy was adapted for each database in order to achieve more sensitivity. References in the relevant studies identified were also scrutinized for additional citations. The summary of the bibliographic search strategies for type of clinical situation and intervention of interest are shown in .
Table 1 Summary of the bibliographic search strategies for type of clinical situation and intervention of interest
Data collection
The authors independently screened the trials identified by the literature search, extracted the data, assessed trial quality and analyzed the results. A standard form was initially used to extract the following information: study characteristics (type of design and randomization methods), participants, interventions, and outcomes (Appendix 1).
Study selection
We planned to include randomized and quasi-randomized controlled trials that specifically stated that the conditions under investigation were galsulfase and which involved adults and/or children diagnosed with MPS VI based on biochemical confirmation of ASB deficiency. Also, we considered studies evaluating different doses of galsulfase.
The following efficacy outcome measurements were assessed when available, in reports of studies that meet the inclusion criteria described above:
Endurance variables, such as distance walked in a 12-minute walk test (12MWT) and number of stairs climbed in a 3-minute stair climb test (3MSC);
Joint mobility (shoulder, elbow, and knee), grip and pinch strength;
Joint function;
Level of urinary GAG excretion; and
Laboratory abnormalities.
Safety was evaluated by compliance, adverse events, drug-related serious adverse events, and adherence to the treatment protocol.
Methodological quality assessment
The methodological quality of the trials included in this review was judged using the Cochrane instrument approach recommended by the Cochrane Handbook.Citation1 We assessed the following 6 separate criteria: adequate sequence generation; allocation concealment; blinding; incomplete outcome data addressed (withdrawal and/or drop outs), and other sources of bias.
Data analysis
We considered clinical trials available in the literature to plot in a meta-analysis with relative risk (RR) for dichotomous data, and weighted mean difference for continuous data; with their 95% confidence interval (CI). Intention-to-treat analysis was carried out for dichotomous data when possible. Participants who dropped out were assumed to be non-respondents.
Inconsistency among the pooled estimates was quantified using the I2. This illustrates the percentage of the variability in effect estimates resulting from heterogeneity rather than sampling error.Citation2,Citation3
Results
Description of studies
Study selection
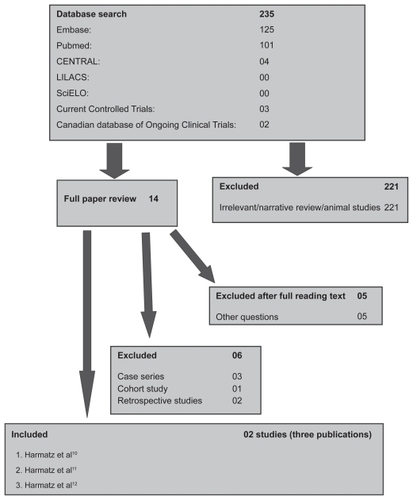
Approximately 235 titles were identified through the search strategy of the electronic databases (see ). Following assessment of 14 full text articles only nine publications were considered for inclusion in this review. Six studies were subsequently excluded,Citation4–Citation9 representing case series, cohort studies, and an open-label extension study. Two studies (3 publications) which met the minimal methodological requirements were included in the review.Citation10–Citation12
Figure 2 Representation of meta-analysis from the Harmatz 2006Citation10 study that compared 1.0 mg/kg of rhASB versus placebo. There was no statistically significant difference between both groups regarding the distance walked in a 12MW test in any of the subcategories evaluated. Note that patients initially given placebo were given rhASB during subsequent infusions after the 24 week time point.
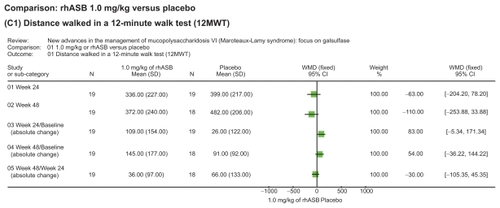
Figure 3 Representation of meta-analysis from the Harmatz 2006Citation10 study that compared 1.0 mg/kg of rhASB versus placebo. There was no statistically significant difference between both groups regarding the number of stairs climbed in a 3MSC test in any of the subcategories evaluated. Note that patients initially given placebo were given rhASB during subsequent infusions after the 24 week time point.
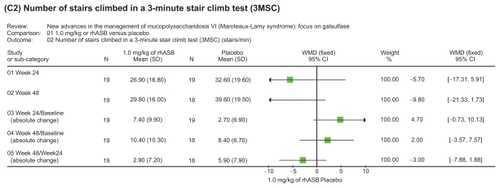
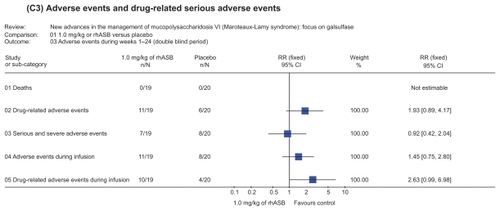
Figure 4 Representation of meta-analysis from the Harmatz 2006Citation10 study that compared 1.0 mg/kg of rhASB versus placebo. There was no statistically significant difference between both groups regarding the occurrence of adverse events during weeks 1–24 in the double blind period study. There were no reported deaths in either patient groups. Note that patients initially given placebo were given rhASB during subsequent infusions after the 24 week time point.
Included studies
Two studies (3 publications) were included in this reviewCitation10–Citation12 with a total of 46 participants.
Design of the studies
Summary details are given in .
Table 3 Summary details of included studies
The Harmatz 2006Citation10 study was a multicenter phase III randomized, double-blind, placebo-controlled trial wherein subjects were recruited from 6 clinical sites. The Harmatz 2004/Harmatz 2005Citation11,Citation12 study involved subjects enrolled in the single-site phase I/II randomized double-blind, 2-dose trial; data on the pharmacokinetics of galsulfase were presented in the latter report.
Participants and duration of trials
In the Harmatz 2006Citation10 study a total of 39 subjects participated in the study. Gender distribution: rhASB group, 12 females and 7 males; placebo group, 14 females and 6 males. Mean age: rhASB group, 13.7 years and; placebo group, 10.7 years. The follow-up period was 24 weeks; all of the patients that completed the 24 weeks study were enrolled in the open-label extension. The inclusion criteria were as follows: >7 years of age and have either a biochemical or molecular analysis result consistent with the diagnosis of MPS VI. At screening, patients should be able to walk unaided at least 5 meters (m) and no more than 270 m in the first 6 minutes, or no more than 400 m in 12 minutes, in a 12MWT. Patients who had clinically significant spinal cord compression, or a medical condition or other circumstance that could interfere with study compliance were excluded.
In Harmatz 2004/Harmatz 2005Citation11,Citation12 study a total of 7 subjects participated in the study. There were 3 females and 4 males, aged from 7 to 16 years. The follow-up period was 48 weeks (after the 24-week safety and efficacy evaluations, the study blind was removed, but all patients remained on their assigned dose until after the 48-week evaluation). The diagnosis of MPS VI was confirmed, based on decreased leukocyte ASB enzyme activity.
Types of intervention
In the Harmatz 2006Citation10 study, participants were randomized to receive weekly intravenous infusions of either rhASB 1.0 mg/kg or placebo solution during this first 24 weeks. In Harmatz 2004/Harmatz 2005Citation11,Citation12 study, patients were randomized to weekly infusions of either high (1.0 mg/kg; n = 3) or low (0.2 mg/kg; n = 4) doses of rhASB.
Types of outcome measures
The following were the primary and secondary efficacy endpoints of the Harmatz 2006Citation10 study: distance walked in a 12MWT, number of stairs climbed in a 3MSC, and the level of urinary GAG excretion. Others endpoints included: (i) assessments of joint pain, joint stiffness, and physical energy level; (ii) assessment of joint range of motion; (iii) assessment of hand dexterity as evidenced by the number of coins picked up in 1 minute; (iv) clinical parameters such as respiratory and cardiac function, and ophthalmologic tests. The safety outcomes were adverse events, serial assessment of immunologic parameters and electrocardiography, and monitoring of changes in laboratory parameters (chemistry, hematology, urinalysis, thyroid function).
The Harmatz 2004/Harmatz 2005Citation11,Citation12 study evaluated mobility and physical function, including 6MWT, joint range of motion (shoulders, elbows, and knees), spirometry, and assessment of functional status, toxicity, total GAG, pharmacokinetic parameters, and ophthalmologic and electrocardiogram evaluations. Other measurements included liver volume, lumbar vertebral trabecular bone density, polysomnography, and adverse events.
Excluded studies
Six studies are described in the ,Citation4–Citation9 which were excluded as these were non-randomized or quasi-randomized controlled trials.
Table 2 Characteristics of excluded studies
Ongoing trials
We identified one trial registered in the electronic databases of ongoing trials which is in the recruitment stage: a phase IV trial with the aim of assessing the safety and efficacy of 2 dose levels of galsulfase in infants (<1 year) who have MPS VI.
Awaiting assessment
No study is awaiting assessment.
Methodological quality of included studies
Allocation (sequence generation and allocation concealment)
The Harmatz 2006Citation10 and Harmatz 2004/Harmatz 2005Citation11,Citation12 studies did not report the generation of allocation or the allocation concealment making this study classified as ‘unclear’.
Blinding
The Harmatz 2006Citation10 study described the blinded assessment of outcomes as follows: both the investigator and staff did not participate in the efficacy assessments and were not informed of the original treatment assignments. The study ‘meets’ the criteria for blinding.
The Harmatz 2004/Harmatz 2005Citation11,Citation12 study did not describe whether investigators and/or patients were blinded to the treatment allocation as well as to the assessment of outcomes, therefore, the study was classified as ‘unclear’.
Description of drop-outs/withdrawals
The Harmatz 2006Citation10 study reported withdrawals in more than 20% (11 patients were randomized and did not fulfill inclusion criteria); therefore, the assessment of attrition bias was recorded as ‘not met’. The study used an intention-to-treat analysis of efficacy variables (eg, included all patients who were randomized); whereas safety analysis included patients who received at least one dose of the study drug.
Although the Harmatz 2004/Harmatz 2005Citation11,Citation12 study reported the occurrence of withdrawal or drop-outs (ie, 6 patients completed 24 weeks of treatment and 5 patients completed 48 weeks), the study was classified as ‘not met’. The authors did not use an intention-to-treat analysis.
Effect of interventions
It was not possible to combine the included studiesCitation10–Citation12 in a meta-analysis because the interventions compared were different: the Harmatz 2006Citation10 study compared rhASB 1.0 mg/kg versus placebo, while the Harmatz 2004/Harmatz 2005Citation11,Citation12 study evaluated different doses of rhASB (1.0 mg/kg versus 0.2 mg/kg). Therefore, we present only a representation of the meta-analysis to facilitate the interpretation of the results found in both studies.
Discussion
We have found some evidence to support the use of galsulfase in the treatment of MPS VI. Although we aimed to identify the best clinical evidence available to answer our question, and performed an extensive search and careful quality assessment, only few conclusions can be drawn from the trials we evaluated. This review has been limited by the low quantity of the trials available for inclusion. The methodological descriptions reported inadequate methods of randomization and allocation concealment, and there were limitations to blinding. Furthermore, both included trials did not address the same control groups, and for this reason the pooling of data was not possible. In addition, the small number of trials meant that our intended sensitivity analyses were not possible. Furthermore, although we did not perform any funnel plot to address the possibility of publication bias due to the very small number of clinical trials included in this review, the possibility of residual confounding due to unmeasured studies cannot be ruled out.
Enzyme therapy for MPS VI using galsulfase has been shown to result in clinical improvements in endurance (as measured by the 12MWT and 3MSC test) along with a reduction in urinary GAG levels, using the approved prescribed dose of 1.0 mg/kg of rhASB, administered weekly. In the phase III trials, patients receiving rhASB walked on average 92 m more in the 12MWT and 5.7 stairs per minute more in the 3MSC than patients receiving placebo. Continued improvement was observed during the extension study. Urinary GAG declined by −227 ± 18 μg/mg more with rhASB than placebo. Proof of therapeutic principle is based on the lowered level of urinary GAG in all treated patients, which was sustained with on-going treatment. Long term follow-up will be required to ascertain full clinical benefit, on both survival and quality of life measures.
The observation in the phase III study essentially confirmed the results noted in the phase I/II study of rhASB, using 2 different enzyme doses (0.2 and 1 mg/kg). In the latter study, reduction in total GAGs was shown to be dose dependent, and became the basis for examining safety and efficacy at the 1 mg/kg dose in subsequent trials.Citation11 Additional observations in case series studyCitation8 were an improvement in each patient on average of 155 m (98%) in the 12MWT, 64 m (62%) at the 6-minute time point of the 12-minute walk, and a gain of 48 stairs (110%) in the 3MSC versus the baseline mean values, after 24 weeks of treatment. Dose-dependent responses were not seen in the functional parameters, possibly related to the small number of patients and large range of ages and disease severity within each group.Citation8 Additional improvements after 48 weeks of treatment were noted, including mean values of 211 m (138%) in the 12MWT, 75 m (80%) at the 6-minute time point of the 12MWT, and a 61-stair (147%) gain in the 3MSC versus the baseline mean values. Joint Pain and Stiffness Questionnaire scores improved by at least 50% by week 24 and were maintained at week 48, whereas there were only small improvements in active shoulder range of motion (<10 degrees) and in the time taken to stand, walk, and turn starting from a seated position (Expanded Timed Get-Up-and-Go test). Improvement in pulmonary function based on forced vital capacity and forced expiratory volume at 1 minute in the absence of growth was observed in 3 of 6 patients, and the observed gains occurred in the 24- to 48-week treatment interval. A mean decrease of 76% in urinary excretion of GAGs was seen.
The reported increase in distance travelled, together with the increase in stairs climbed indicate that improvements in exercise capacity can be expected in MPS VI patients treated with galsulfase. The increase in performance during the extension phase of the trials indicates the results are sustained and durable. These gains are anticipated to improve affected patients’ ability to carry out activities of daily living, although this requires further longterm studies. In addition, the influence of therapy on patient survival remains to be established.
A follow up reportCitation7 in an open-label extension study has indicated that the improved endurance seen in patients after starting galsulfase was not sustained in some; which was attributed to advanced skeletal disease at study entry. The latter observation was considered to represent a high risk for disease progression in the hip and cervical spinal cord compression, which would have an adverse effect on affected patients’ performance. Intravenous administered rhASB does not penetrate the joint spaces or the central nervous system, and thus the therapy would not be expected to prevent progression of these complications.
Infusion related adverse reactions (IAR) were observed in over half the patients treated with rhASB. Anaphylactoid IARs were noted in 16% of patients; the term ‘anaphylactoid’ was used to refer to certain IARs, based on the nature of the event, its recurrence and response to treatment. These observations underscore the need to closely monitor patients given galsulfase; particularly as these patients may have pre-existing risks related to involvement of cardiopulmonary system as a consequence of ASB deficiency. The observed events were noted as consistent with immune reactions expected with infused recombinant proteins. The reactions were manageable, and responded to interruption of the infusion and adjustment of the rate of infusion as well as to the administration of supplemental antihistamines and anti-inflammatory agents such as ibuprofen and corticosteroids.
The safety profile of the treatment in clinical trials was good, with most adverse events attributed to underlying disease manifestations rather than to treatment with rhASB. However, there was no statistical significance difference between both groups for any incidence and frequency of adverse events related to study drug during weeks 1 to 24 in the representation of meta-analysis ().
Figure 5 Representation of meta-analysis from the Harmatz 2006Citation10 study that compared 1.0 mg/kg of rhASB versus placebo. There was no statistically significant difference between both groups regarding any incidence and frequency of adverse events related to study drug during weeks 1 to 24. Note that patients initially given placebo were given rhASB during subsequent infusions after the 24-week time point.
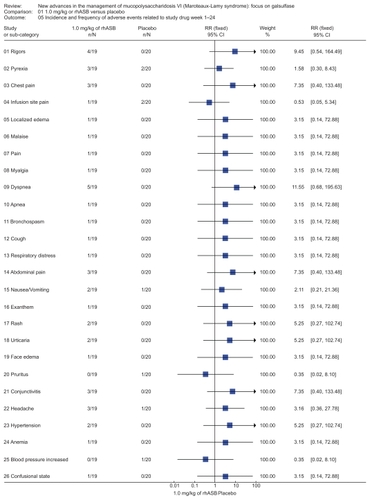
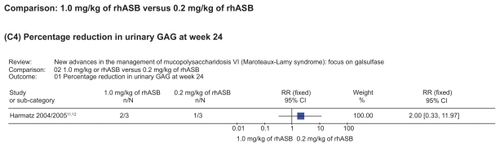
Figure 6 Representation of meta-analysis from the Harmatz 2004/2005 study that compared 1.0 mg/kg of rhASB versus 0.2 mg/kg of rhASB. There was no statistically significant difference between both groups regarding the percentage reduction in urinary GAG at week 24. Note that patients initially given placebo were given rhASB during subsequent infusions after the 24-week time point.
Investigations into disease mechanism and the extent to which these processes are modified by enzyme therapy are necessary. It is possible new insights into pathogenesis may offer novel targets for therapeutic intervention, such as the use of substrate synthesis inhibition (with genistein).Citation13
Reviewers’ conclusions
Implications for practice
There is some evidence to support the use of galsulfase in the treatment of MPS VI, however due to the very low quantity of included studies we could not analyze it in an appropriate way. Therapeutic response may be influenced by disease stage, and early intervention may lead to better outcomes, although this subject requires further investigations. It is possible that certain aspect of the disease may be modified by a longer period of treatment, and additional data is anticipated from the observational database or registry program, which has been established as a post-regulatory approval commitment made by the drug manufacturer.
Implications for research
This review highlights the need for continued research into the use of enzyme replacement therapy for MPS VI, to increase the number of studies, study participants and the period of observation so that outcome data can confirm the real benefit of the treatment. Future studies should also address the issue of compliance with treatment eg, the study must be designed to address efficacy and/or efficiency. Besides, future studies must also be adequately powered.
Disclosures
The Harmatz 2006Citation10 study was sponsored by BioMarin Pharmaceutical Inc., and was partially supported with funds provided by the National Center for Research Resources (NCRR). Drs Harmatz, Wittes, Lowe, Berger, and Yu have provided consulting support to BioMarin Pharmaceutical Inc., Novato, California. Drs Swiedler, Kakkis, and Ms Newman are employees and stockholders of BioMarin Pharmaceutical Inc.
The Harmatz 2004/Harmatz 2005Citation11,Citation12 study was sponsored by BioMarin Pharmaceutical Inc. and was also supported in part by PHS, NCRR.
References
- HigginsJPTAltmanDGHigginsJPTGreenSChapter 8: Assessing risk of bias in included studiesCochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008)The Cochrane Collaboration2008 Available from www.cochrane-handbook.org
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysisBMJ2003327741455756012958120
- HigginsJPTGreenSAssessment of study qualityCochrane Reviewers’ Handbook 425 The Cochrane Library32005Chichester, UKJohn Wiley & Sons, Ltd2005
- ScarpaMBaroneRFiumaraAAstaritaLParentiGRampazzoAMucopolysaccharidosis VI: the Italian experienceEur J Pediatr2009 [Epub ahead of print]
- BagewadiSRobertsJMercerJJonesSStephensonJWraithJEHome treatment with Elaprase and Naglazyme is safe in patients with mucopolysaccharidoses types II and VI, respectivelyJ Inherit Metab Dis200831673373718923918
- Cardoso-SantosAAzevedoACFagondesSBurinMGGiuglianiRSchwartzIVMucopolysaccharidosis type VI (Maroteaux-Lamy syndrome): assessment of joint mobility and grip and pinch strengthJ Pediatr2008842130135
- HarmatzPGiuglianiRSchwartzIVGuffonNTelesELMirandaMCLong-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: Final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfataseMol Genet Metab200894446947518502162
- HarmatzPKetteridgeDGiuglianiRGuffonNTelesELMirandaMCDirect comparison of measures of endurance, mobility, and joint function during enzyme-replacement therapy of mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): results after 48 weeks in a phase 2 open-label clinical study of recombinant human N-acetylgalactosamine 4-sulfatasePediatr20051156e681e689
- AzevedoACSchwartzIVKalakunLBrustolinSBurinMGBeheregarayAPClinical and biochemical study of 28 patients with mucopolysaccharidosis type VIClin Genet200466320821315324318
- HarmatzPGiuglianiRSchwartzIGuffonNTelesELMirandaMCEnzyme replacement therapy for mucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension studyJ Pediatr2006148453353916647419
- HarmatzPWhitleyCBWaberLPaisRSteinerRPleckoBEnzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux-Lamy syndrome)J Pediatr2004144557458015126989
- HarmatzPKramerWGHopwoodJJSimonJButenskyESwiedlerSJPharmacokinetic profile of recombinant human N-acetylgalactosamine 4-sulphatase enzyme replacement therapy in patients with mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): a phase I/II studyActa Paediatr Suppl200594447616815895715
- Jakóbkiewicz-BaneckaJPiotrowskaENarajczykMBarańskaSWegrzynGGenistein-mediated inhibition of glycosaminoglycan synthesis, which corrects storage in cells of patients suffering from mucopolysaccharidoses, acts by influencing an epidermal growth factor-dependent pathwayJ Biomed Sci2009162619272193
Appendix 1
Extraction sheet
ID – Author, year of publication:
Action
What will be asked to the author:
Methods
Design:
Multicenter or single-center:
Period:
Sample size:
Generation of allocation:
Allocation concealment:
Blinded assessment of treatment allocation:
Withdrawals and drop outs:
Intention-to-treat analysis:
Follow-up:
Participants
N:
Sex:
Age (mean):
Setting:
Inclusion criteria:
Exclusion criteria:
Intervention
Experimental group:
Control group:
Outcomes
Primary outcome:
Secondary outcome:
Continuous or dichotomous:
Notes
Conflict of interest:
Comments and notes: