Abstract
Efalizumab is a monoclonal a humanized recombinant IgG1 monoclonal antibody which targets the CD11a, the alpha-subunit of LFA-1 (lymphocyte function-associated antigen-1). It acts by blocking the T-lymphocyte pathogenetic mechanisms of psoriasis. Thrombocytopenia is an adverse event that occurs during therapy. Thrombocytopenia can be mild and can occur quite early during treatment, together with leukocytosis. Both adverse events tend to normalize with ongoing therapy, or, in cases worsening, with therapy suspension. There have been multiple reports of thrombocytopenia associated with efalizumab therapy for the treatment of psoriasis. The general recommendation is to check platelet counts monthly for the first 3 months of efalizumab therapy, then every 3 months for the duration of therapy. According to our experience on a wide range of patients, it is useful to check platelets every month for the first 6 months of therapy. We report a case of efalizumab-associated thrombocytopenia that occurred after 16 weeks of therapy together with clinical worsening of skin lesions. The peculiarity of our case is the absence of signs and symptoms linked to thrombocytopenia and the quick return to normal platelet count without corticosteroid therapy.
Keywords:
Introduction
Psoriasis vulgaris, the most common form of psoriasis, is a widespread dermatological disease, affecting 2% of the world population (CitationChristophers 2001). The most typical psoriatic lesions are erythemato-squamous plaques and the general symptoms are itching and burning, which are mainly related to the appearance and extent of the lesions. As the clinical appearance of the lesions is strictly linked to epidermal changes, it was believed for a long time that psoriasis was a skin pathology linked to keratinocytes hyperproliferation and to their abnormal differentiation (CitationKrueger et al 1984). It is now known that it is an organ-specific autoimmune disease triggered by numerous cell types, among which are T-cells (CitationKrueger et al 1984, Citation2005). The crucial role of T-lymphocytes in the pathogenesis of the disease has been further emphasized through the successful results obtained with T cell-targeted agents: the biologicals.
Efalizumab is a humanized monoclonal IgG1 antibody directed against CD11a, the alpha subunit of leukocyte function-associated antigen 1 on T cells. Leukocyte function-associated antigen 1 and intercellular adhesion molecule 1 are co-stimulatory molecules expressed on T cells and antigen-presenting cells, respectively, which facilitate multiple T-cell-mediated events. Efalizumab inhibits T-cell activation, cutaneous trafficking, and adhesion to keratinocytes through the blockade of LFA-1/ICAM-1 binding, steps in the immunologic cascade that lead to the formation of the psoriatic plaques (CitationMenter et al 2005).
Many placebo-controlled trials have assessed the safety of efalizumab in patients affected by chronic psoriasis (CitationMenter et al 2005). Many studies have assessed the safety and efficacy of efalizumab in long-term trials (CitationGottlieb et al 2004; CitationLeonardi et al 2005). Moreover, efalizumab has proved to be devoid of serious adverse events, which are common in clinical practice (CitationPearce et al 2006). The most serious adverse events in the course of efalizumab therapy (apart from aseptic meningitis, which is very rare) are leukocytosis and thrombocytopenia, which tend to normalize with ongoing therapy, or, in cases worsening, with therapy suspension.
We report the clinical course of a patient with psoriasis, in comorbidity (diabetes mellitus), who was successfully treated with efalizumab for 4 months. After severe thrombocytopenia the patient had to stop the treatment and completely recovered after treatment suspension.
Case report
The patient was a 61-year-old Caucasian man with a history of psoriasis of almost 2 years. He had used many topicals (steroids, vitamin-D3 derivates, hydratation products) and systemic therapies (cyclosporine-A and methotrexate) for psoriasis.
The patient was treated with cyclosporine-A first, with a temporary improvement in his clinical conditions. After 3 months, cyclosporine-A gave no further improvement of skin lesions and blood pressure was too high. He underwent weekly treatment with methotrexate and folic acid (from the day after methotrexate injection). He had to discontinue this therapy, too, because of a sudden increase in transaminases.
The patient started efalizumab treatment in March 2008 at the first dose injection of 0.7 mg/kg and thereafter of 1 mg/kg once weekly by subcutaneous injection for 17 weeks. The baseline Psoriasis Area and Severity Index (PASI) score was 10; visual analog sale (VAS) 70; Patient evaluation of Psoriasis severity 40; Medical evaluation of Psoriasis severity 30. At the first follow up in April 2008 the patient showed a PASI score 2; VAS 0; Patient evaluation of Psoriasis severity 0; Medical evaluation of Psoriasis severity 0. In June 2008 he had a PASI score 3; VAS 0; Patient evaluation of Psoriasis severity 10; Medical evaluation of Psoriasis severity 10; with clinical worsening of his skin manifestations.
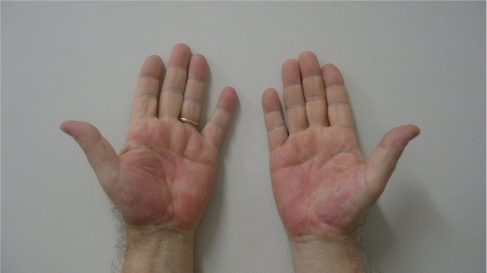
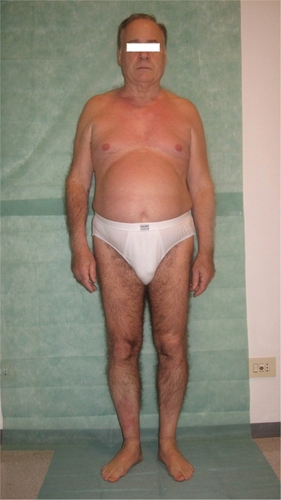
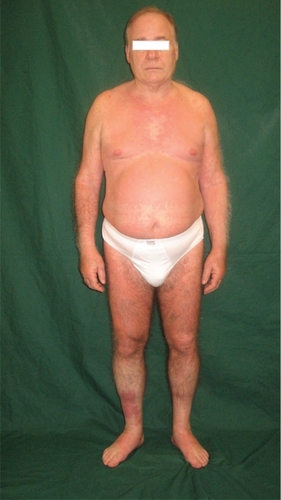
After 1 week of this follow up the patient presented erythematous papules and plaques on the trunk, particularly on the anterior part of the chest. He referred to the formation of a 10 cm circular red spot on the right leg after the 17th efalizumab injection. The previous day, before the 17th efalizumab injection, the patient began treatment with an analog of insulin (glargine 10 IU). We prescribed an urgent hematological examination, with the following results: white blood cells 14,100/mm3, platelets 48,000/mm3, ESR (erythrosedimentation rate) 48 k/μL. Meanwhile he developed a suberythrodermic manifestation () with diffuse articular pains, fever, and edema of the hands (), feet, and pretibial regions. Efalizumab treatment was stopped and he was admitted to the Dermatological Clinic. We checked hemoculture (in order to exclude septicemia from any erytrodermic skin infection), double blood microscopic observations, protidogram, Weber test, hydroelectrolitic balance, glycemia (4 per day to monitor his diabetes), Addis count and daily hematological routine. The initial therapy was teicoplanin 200 mg/day iv, paracetamol 500 mg to demand, atorvastatin 20 mg po at 21 hours, metformin 1 po after dinner, and ½ po 20 minutes before breakfast and lunch and 1 po 20 minutes before dinner. We also administered a hydrocortisone cream twice per day on the lesional skin. One week later, laboratory tests showed a reduced thrombocytopenia (platelets 126,000/mm3, with microscopic aggregates), moderate leukocytosis (12,100/mm3), neutrophilia (83.6 %), anemia (hemoglobin HGB 11.2 g/dL), ESR 90 k/μL. On the tenth day after his hospitalization there was a further improvement in hematological status: platelets (with microscopic aggregates) 131,000/mm3, with leukocytosis 14,430/mm3, neutrophilia (82%), anemia (hemoglobin 11.85 g/dL), ESR 95 k/μL. On his last day of hospitalization platelets (with microscopic aggregates) were 271,000/mm3, white blood cells 9040/mm3, neutrophils (58%), hemoglobin HGB 13.2 g/dL.
Figure 1 A suberythrodermic status involving large parts of the body. A large, red, circular edematous spot can be seen on the anterior pretibial right region.
The hemocultures were negative for any microorganisms, as were the feces for occult blood; there were no traces of blood in the urine.
The patient returned home with a medical prescription for hydrating cream twice daily and narrow band-UVB 3 times weekly.
After 3 weeks white blood cells were 6890/mm3, platelets 290,000/mm3, and all the other blood figurate elements were within the normal range. His clinical conditions were notably improved ( and ).
Discussion
We have reported a case of efalizumab-associated thrombocytopenia in a psoriatic patient with diabetes as comorbidity. Efalizumab, in this patient, was chosen for psoriasis treatment because it has proven effective and safe in the treatment of chronic plaque-type psoriasis (CitationGottlieb et al 2004; CitationPearce et al 2006). Moreover, efalizumab has proven particularly useful when psoriasis is associated with most comorbidities such as hypertension, diabetes, and dyslipidemia; many reports have demonstrated that this biologic can improve skin conditions without worsening comorbidities (CitationPrignano et al 2008). It is also the only biologic for which there is long-term study control, which provides further proof of its safety in clinical practice (CitationLeonardi et al 2008).
In some patients, mostly after many months of efalizumab therapy, papular psoriasis, which has been called “efalizumab associated popular psoriasis”, can appear. Clinically it is an aggravation of the disease and can be treated with an immunosuppressive drug such as cyclosporin-A or methotrexate in association with efalizumab for a short period according to the patient’s general condition and the experience of the dermatologist. Often, during efalizumab treatment we observed changes in some hematological parameters – moderate leukocytosis (10,000–15,000 white blood cell count per mm3), granulocytes/lymphocytes ratio, and thrombocytopenia. These adverse events usually resolved during treatment without the need to stop therapy. Dermatologists who are familiar with biologicals understand that leukocytosis and thrombocytopenia are almost always detectable during early phases of efalizumab treatment. Therefore, these adverse events are not a reason to stop treatment but to follow up patients to observe the stabilization and thereafter normalization of leukocytes and platelets. Most cases of efalizumab-associated thrombocytopenia have been documented as causing purpura, petechiae, and mucosal membrane bleeding (CitationScheinfeld 2006). The most likely mechanism able to induce thrombocytopenia is probably autoantibodies against a component of the platelet membrane, although the presence of such antibodies has never been detected in the documented cases of efalizumab-induced thrombocytopenia (Citationvan den Bemt et al 2004).
Because US clinical trials have reported some cases of efalizumab-associated thrombocytopenia between 10 and 16 weeks, US authors recommend monthly platelet counts during the first 4 months of therapy. If the total platelet count drops 50,000/mm3 or more between any two consecutive counts they suggest frequent control until the count stabilizes. We do suggest checking platelets count every month during the first 6 months of therapy. If they drop by 5000/mm3 in this time-course platelets should be counted every 10 days in order to decide whether to continue or stop therapy.
The interest in our case is linked to the fact that even when thrombocytopenia was severe (48,000/mm3), our patient presented a worsening of his psoriasis but without purpura or signs of bleeding symptoms, as reported in the literature for most patients (CitationScheinfeld 2006). Moreover, we did not administer any steroids to our patient because he suffered from diabetes and also because, being hospitalized, we could monitor continuously his platelets. Considering that the median time to normalize platelet number is 9 weeks for patients without corticosteroid intake (CitationGrim Hostetler et al 2007), our patient improved very quickly because there was a moderate increase in his platelets after 1 week and a good shift towards normality after 3 weeks of efalizumab suspension therapy (290,000/mm3).
We have considerable experience in the treatment of moderate to severe plaque type psoriasis, as in our Italian National Protocol “PsoCare” we have enrolled more than 220 patients receiving efalizumab for psoriasis. Leukocytosis and thrombocytopenia are quite common in these patients during the first 16 weeks of therapy, but this was the most severe case of thrombocytopenia.
We inform the patients before starting efalizumab treatment of the risks of thrombocytopenia and other adverse events.
According to our and other published data (CitationGrim Hostetler et al 2007; CitationLeonardi et al 2008) efalizumab can be safe and effective for treating psoriasis with proper follow-up controls. Moreover these follow-up controls are very cheap as they comprise only blood monitoring.
Disclosures
The authors have no conflicts of interest to disclose.
References
- ChristophersE2001Psoriasis-epidemiology and clinical spectrumClin Exp Dermatol263142011422182
- GordonKBPappKAHamiltonTK2003Efalizumab Study Group. Efalizumab for patients with moderate to severe plaque psoriasis: a randimized controlled trialJAMA29030738014679270
- GottliebABGordonKBLebwohlMG2004Extended efalizumab therapy sustains efficacy without increasing toxicity in patients with moderate to severe plaque psoriasisJ Drugs Dermatol3273814964744
- Grim HostetlerSZirwasMBechtelMA2007Efalizumab-associated thrombocytopeniaJ Am Acad Dermatol577071017637485
- KruegerGGBergstresserPRLoweNJ1984PsoriasisJ Am Acad Dermatol11937476389615
- KruegerJGBowcockA2005Psoriasis pathophysiology: current concepts of pathogenesisAnn Rheum Dis64306
- LeonardiCMenterAHamiltonT2008Efalizumab:results of a 3-year continuous dosing study for the long-term control of psoriasisBr J Dermatol15811071618373710
- LeonardiCLPappKAGordonKB2005Efalizumab Study Group. Extended efalizumab therapy improves chronic plaque psoriasis: Results from a randomized phase III trialJ Am Acad Dermatol524253315761420
- MenterAGordonKBCareyW2005Efficacy and safety observed during 24 weeks of efalizumab therapy in patients with moderate to severe plaque psoriasis(Reprinted) Arch Dermatol141318
- PearceDJHigginsKBStealeyKH2006Adverse events from systemic therapies for psoriasis are common in clinical practiceJ Dermatol Treat1728893
- PrignanoFBuggianiGLottiT2008Efalizumab in the treatment of psoriasis: when comorbidity is an issueDermatol Ther21S25S918837730
- ScheinfeldN2006Efalizumab: a review of events reported during clinical trials and side effectsExpert Opin Drug Saf519720916503742
- Van den BemtPMMeyboomREgbertsA2004Drug-induced immune thrombocytopeniaDrug Saf2712435215588119