Abstract
There is an increasing demand for minimally-invasive cosmetic procedures to arrest the aging process. Botulinum toxin type A injections are the most commonly used nonsurgical cosmetic procedures in the United States. There has been research spanning over two decades dedicated to safety, efficacy, dosing, and complications of botulinum toxin type A. There are now two Food and Drug Administration (FDA) approved botulinum toxin type A options in the United States: Botox® and Dysport™, with new advances being made in the field.
Introduction
There is an increasing demand for a reversal of the aging process and recently more people are turning to minimally-invasive methods to meet this goal, in lieu of surgery. Botulinum toxin type A injections are the most commonly used nonsurgical cosmetic procedures in the United States, with 4.6 million procedures in 2007.Citation1 Glabellar lines occur naturally with facial animation, a continuous practice that facilitates communication. Contraction of the procerus and corrugator supercilli muscles produces creasing of the glabellar skin and ultimately permanent rhytids develop. Administration of low doses of botulinum toxin type A into bilateral corrugator supercilii and procerus muscles paralyze muscular activity, thus diminishing the appearance of dynamic rhytids of the glabella. Botulinum toxin injection is a minimally invasive procedure with relatively quick onset of action seen within three days to two weeks of administration. The effects of botulinum toxin type A commonly last for three to six months, although there is a report of duration as long as twelve months.Citation2 Botulinum toxin type A is a successful treatment clinically, but more importantly patient satisfaction is consistently high with its use.Citation3 The purpose of this review is to discuss the important topics surrounding and pertaining to the role of botulinum toxin type A in the management of glabellar rhytids, as well as, to compare and contrast the FDA approved commercial botulinum toxin type A products.
Botulinum toxin is a neurotoxic protein produced by Clostridium botulinum, a Gram-negative anaerobic bacterium. In the 1980’s, Dr Alan Scott was the first to utilize botulinum toxin clinically with his research on strabismus and blepharospasm, after using it successfully in experiments using monkeys in the 1970’s. Now botulinum toxin is used to treat many medical conditions including cervical dystonia, hyperhidrosis, strabismus, and blepharospasm. The first studyCitation4 indicating the utility of botulinum toxin type A for the treatment of hyperfunctional facial lines occurred in the early 1990’s.
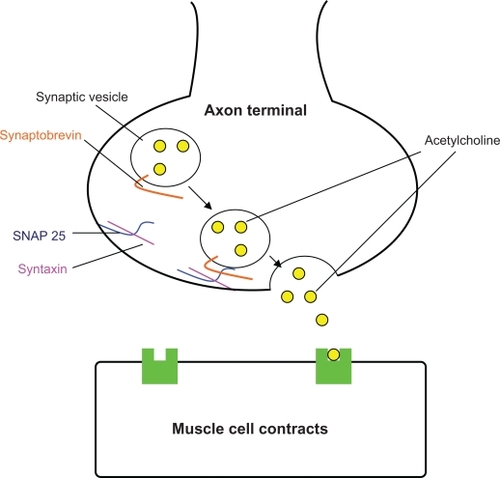
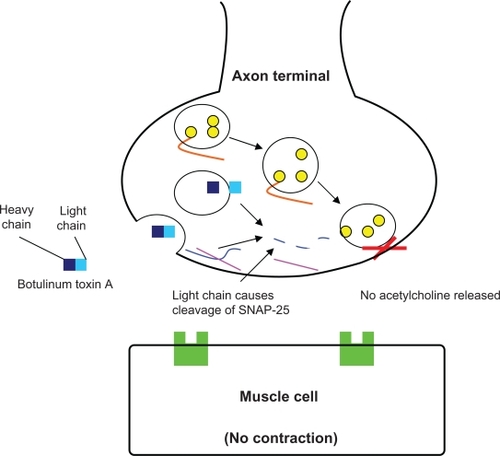
Normal facial muscle cell contraction occurs when acetylcholine released from the nerve terminal, diffuses across the synaptic cleft, and attaches to its receptor on the muscle cell (). After injection of botulinum toxin type A into the muscle, the neurotoxin is taken up by the adjacent nerve terminal. Within the terminal it prevents proper binding of the synaptic vesicle containing acetylcholine. The neurotoxin accomplishes this by cleaving SNAP-25, a protein that is crucial for docking of the vesicle to the nerve ending. Thus, neurotransmitter release into the synaptic cleft is inhibited and muscle contraction cannot occur ().
There are several botulinum toxin type A products available worldwide. In North America, onabotulinumtoxinA, known as Botox® (Allergan, Inc., Irvine, CA, USA) and abobotulinumtoxinA, known as Dysport™ (Ipsen Ltd, Wrexham, UK and Medicis Aesthetics Inc., Scottsdale, AZ) are FDA approved products for aesthetic treatments. Botox® was approved in 1989 for certain medical conditions, but it was not until 2002 that this product received FDA approval for temporary improvement in the appearance of moderate to severe glabellar lines in adults aged 65 or younger. It was just several months ago that Dysport™ gained FDA approval for cosmetic indications although it has been used widely in Europe for several years. The list of ingredients for each of these products is listed in . Two other botulinum toxin type A products are available outside the United States for treatment of blepharospasm and torticollis: Xeomin® (Mertz, Frankfurt, Germany) and Neuronox® (Medy-Tox Inc., South Korea).
Table I List of ingredients for Botox® and Dysport™
Botox®, Dysport™, and any other botulinum toxin type A products are distinctive and not interchangeable. Thus, the generic term, botulinum toxin type A, will be used when a distinction does not need to be made, but otherwise the specific product will be named throughout the review.
Safety and efficacy of botulinum toxin type A
The safety and efficacy of botulinum toxin type A has been investigated in both small, single-center trials,Citation4–Citation12 as well as, large, multicenter, randomized, double-blind studies.Citation13–Citation17 Recently, several trials have addressed the concern of long-term safety and efficacy of botulinum toxin type A and these found sustained safety and efficacy after repeated administration of the neurotoxin.Citation15,Citation18–Citation23 There is unequivocal evidence from two decades of research that botulinum toxin type A is safe and effective for the treatment of glabellar lines.
Two interesting clinical pearls have been discovered during the course of the research trials. First, while the data is limited and not statistically significant, it appears that there is a significant decrease in efficacy seen in subjects older than 65 years of age.Citation16,Citation24,Citation25 Second, Carruthers et al found that the effects on patient appearance at rest appear to be sustained longer than the effect at maximal frown, thus suggesting that botulinum toxin type A may have a persistent benefit even once the paralysis has reversed. The authors hypothesize that this may occur for several reasons including dermal remodeling, slight muscle atrophy, and behavior modification.Citation13–Citation14
Dosing
Many different doses have been evaluated for the treatment of glabellar rhytids, although the current suggested dose is 20 Units (U) for Botox®Citation24 and 50 U for Dysport™Citation25,Citation26 It is recommended that there should be at least a three month period between botulinum toxin injections. Carruthers et al compared 10, 20, 30, and 40 U doses of Botox® for the improvement in glabellar rhytids in females and found that dosing with 20–40 U of botulinum toxin type A was significantly more effective at reducing glabellar lines than 10 U alone.Citation2 A study evaluating 20 U compared to 30 U in African American females found that either dose was safe and effective.Citation27 Larger doses of botulinum toxin type A are needed to improve the glabellar rhytids in men. In one dose-comparing study, it was shown that 20 U of Botox® was ineffective and greater treatment outcomes were seen with the use of 40, 60, and 80 U doses.Citation28
In June of 2009, Carruthers et al published a dose-comparing study with Botox® for the improvement in upper facial rhytids (crows feet, forehead, and glabella) and found similar efficacy and safety with 32, 64, and 96 U. Due to the dose-dependent response observed and a higher incidence of brow ptosis with 96 U, the authors concluded it is best to use a more moderate 64 U.Citation29
There is less published literature evaluating dosing for Dysport™. After a Phase II dose-ranging trial,Citation26 most subsequent trials evaluated a 50 U dose compared to placebo and found it to be safe and effective.Citation15,Citation17 Another dose-ranging trial comparing 20, 50, 75 U, or placebo found 50 U of Dysport™ to be the optimal dose.Citation30 Comparatively, another trial found that both 30 U and 50 U were safe and effective, although there was a slight improvement in treatment outcomes with the 50 U dose.Citation31 Kane et al propose that a standardized dose is not optimal since gender and muscle mass affect the dose required for efficacy.Citation16 This group carried out a randomized, double-blind, placebo-controlled trial that evaluated variable doses of Dysport™ based on gender and muscle mass. After grading the mass of the procerus and corrugator muscles (small, medium, large) the investigators established the dosing for each subject with options including; 50, 60, or 70 U in women and 60, 70, or 80 U in men. The investigators found the variable doses to be efficacious compared to placebo and did not report an increase in adverse events in the higher treatment doses.
As mentioned, Botox®, Dysport™, and any other botulinum toxin type A product have different potencies and the doses are not interchangeable. The biologic activity of the toxin is measured in mouse units (LD50), the median intraperitoneal lethal dose in mice. Despite the fact that both neurotoxins are supplied in units of biologic activity (LD50), the units are not transposable due to differences in assays used to determine the units. There have been a few studies attempting to create dose ratios between Botox® and Dysport™ to compare efficacy. Lowe et al found 2.5:1 dose ratio (Dysport™: Botox®) to be comparable in terms of tolerability. The authors reported prolonged efficacy and higher patient satisfaction in the Botox® treatment arm at the week 12 timepoint.Citation32 Others suggest that at 4:1 dose ratio (Dysport™: Botox®) provides similar clinical efficacy.Citation33,Citation34 Wohlfarth et al recently carried out a systematic review of preclinical and clinical dose ratio studies of botulinum toxin type A used for various therapeutic indications. The authors found a range of Dysport™: Botox® ratios from 2:1 to 11:1 described in the literature. Their literature review established that randomized, controlled clinical trials indicate that a 3:1 dose ratio is more appropriate than 4:1, and there is no evidence for a ratio greater than 4:1. However, the studies do not prove that a 3:1 dose ratio is equivalent clinically.Citation35 A previous study by Wohlfarth et al used statistical modeling with compound muscle action potential amplitude of the extensor digitorum brevis to confirm the use of a 3:1 dose ratio.Citation36
Dilution
There is disagreement over the effect of dilution volume on treatment efficacy and there is limited clinical trial data to support either argument. Dilutions range between 100 U/cc to 10 U/cc, with most choosing to dilute 100 U of botulinum toxin with 1–3 cc of saline. Using a more concentrated solution, such as 1 U/0.1 cc, may allow for more accurate placement, limit pain, restrict diffusion, and thus decrease risk of side effects. Administering less concentrated doses, such as 4 U/1cc, may be easier to work with. The increased potential for diffusion with lower concentration, higher volume doses can be advantageous since it allows fewer units to cover a greater area. Dilution may just be a matter of preference, as one comparative study for the treatment of blepharospasm showed no difference in efficacy or incidence of patient-reported complications (eg, bruising, redness, complications of injection) with two different dilutions (10 U/cc vs. 100 U/cc) injected into either ocular area.Citation37 Similarly, other studies found that concentrations varying from 10 to 100 U/cc had no difference in efficacy and the adverse events experienced were similar across all dilution arms. The more dilute treatment arms did have more subjects with swelling and ptosis although the data was not statistically significant.Citation38,Citation39 Hankins et al found no difference in efficacy or adverse events in concentrations ranging from 50 to 200 U/cc.Citation7 However, a study conducted by Hsu et al determined that dilution did impact treatment efficacy, as the larger volume injections led to greater diffusion and increased the area affected.Citation40
With the more concentrated dilutions, it is efficient to inject with a 30-gauge needle and a 0.3 cc insulin syringe. The insulin syringe does not have a dead space within the needle hub and this serves to decrease the waste of botulinum toxin within the needle. With more dilute concentrations, it is best to use a traditional 30–32 gauge needle.
Diffusion
With injections into the small targets of the glabella and knowledge of the delicate surrounding areas, there is concern of product diffusion leading to muscle paralysis outside of the target site. There is debate about the mechanism of diffusion, as well as, how and if diffusion differs among botulinum toxin type A products.
Some believe the complex size of the botulinum toxin affects the diffusion potential and that larger proteins have less diffusion potential. If this is the case, Botox® (uniform 900 kDa complexes) would be less likely to diffuse outside the target tissue compared to Dysport™ (heterogeneous mixture of 500–900 kDa complexes). The molecular weight of each product is reported and refuted in the literature and hence the validity of this data is debatable.Citation41
There are several studies that have found no significant difference in diffusion between Botox® and Dysport™.Citation36,Citation42,Citation43 Furthermore, investigators suggest the complex protein size is irrelevant since dissociation of the complex occurs immediately after injection, releasing uniform-sized botulinum toxin. These researchers believe diffusion simply depends on the concentration and volume of product used. In other words, the higher the concentration and the greater the volume, the greater the diffusion potential.Citation36,Citation41
Reconstitution and storage
Previously, it was thought that botulinum toxin should be used within 4 hours of reconstitution.Citation44 The Botox® package insert instructs to use the product within 24 hours of reconstitution,Citation24 while the Dysport™ package insert instructs use within 4 hours.Citation25 The concern is decreased efficacy and increased bacterial growth the longer the reconstituted neurotoxin remains unused. Many different studies have evaluated longer reconstitution periods including 15 days, 42 days, and 49 days without lessening efficacy or producing evidence of bacterial contamination.Citation45–Citation47 Both preservative-free and preserved saline were used in these studies and both were shown to be safe after prolonged reconstitution. Once reconstituted, botulinum toxin must be kept at a refrigerated temperature between 2–8°C.
Complications
The most common side effects reported with botulinum toxin type A injections include pain, swelling, erythema, ecchymosis, respiratory infection, headache, nasopharyngitis, sinusitis, flu-like symptoms, nausea, and limited hypesthesia. In clinical trials with Botox®, the incidence of headache, nausea, and flu-like symptoms in the treatment arm was the same as seen in the placebo arm.Citation13,Citation14,Citation24
Upper eyelid ptosis is also a complication in treatment of the glabellar region. Understanding anatomic landmarks and proper technique will decrease the incidence of ptosis. This complication arises when the neurotoxin diffuses through the orbital septum and affects the levator palpebrae superioris muscle. The rate of ptosis appears to be determined by the skill and experience of the injector. In studies with repeated administration of botulinum toxin type A, the rate of ptosis decreased over successive cycles indicating improved technique with experience.Citation18 Reported rates of ptosis from large clinical trials are similar between Botox® and Dysport™ and range from 0.8% to 5.4%.Citation13–Citation15,Citation17–Citation19,Citation26 Ptosis can be treated with α-adrenergic agonist (apraclonidine 0.5% or phenylephrine hydrochloride 2.5%) ophthalmic drops twice a day to the affected side.Citation48
The complications associated with botulinum toxin injection are most often mild and self-limited. Most complications are related to technique, hence complications decrease with proper training of facial anatomy, dosing, and injection technique.
Administration technique
Proper technique for injection of the glabella is debatable, but variations of procedure will still lead to successful treatment outcomes. The patient should be seated in the upright position and all injections should be aimed away from the eye. The neurotoxin should be injected into the muscle belly of the procerus and corrugator supercilli muscles. Administration of the neurotoxin into the forehead should be superficial, aiming for the subcutis. The neurotoxin will spread down to the frontalis muscle. The toxin may spread up to 3 cm from the injection site and this should be considered during administration. There is increased likelihood of eyelid ptosis if the toxin is injected too inferior on the forehead. Medial corrugator injections should be placed 1 cm above the bony supraorbital ridge to avoid ptosis.
Massage after injection is another debated practice among physicians. Massaging horizontally may facilitate smoothing of the lump after injection, aid with diffusion, and prevent inferior spread of the botulinum toxin.Citation48 Massage becomes problematic, however, if the botulinum toxin diffuses to other muscles. There are currently no studies to support either argument. Digital pressure after the injection may also decrease the diffusion of the toxin.Citation48 After the treatment, patients may be instructed to stay upright for at least 4 hours to prevent the diffusion of the product in the wrong direction, although there is no data to confirm the necessity of this action. Some physicians also instruct patients to contract the treated muscles for the first couple hours to distribute the toxin to the entire muscle, but, again, this may be unnecessary.
Several of the complications discussed above can potentially be prevented. For instance, to prevent inducing ecchymosis with superficial injection the patient should be advised to avoid aspirin, nonsteroidal anti-inflammatory drugs (NSAIDS), and high-dose vitamin ECitation49 for at least a week prior to injection. Likewise, removing makeup prior to injection will allow for better visualization of the treatment area and avoidance of small, superficial vessels.Citation50 It has been deemed helpful to hold direct pressure and application of ice to the injection site.Citation48,Citation50 To decrease the amount of discomfort for the patient, use of a topical lidocaine prior to the procedure, and slow injection with small amounts of concentrated botulinum toxin type A using a 30–34 gauge needle is recommended.Citation48,Citation51,Citation52
Furthermore, it has been found that patients complain of less pain when the botulinum toxin is reconstituted with preserved saline, rather than sterile, non-preserved saline as directed on the package insert since the preservative in the saline, benzyl alcohol, acts as an anesthetic. This is supported by a finding in a randomized clinical trial where not only did the subjects report less pain with botulinum toxin type A reconstituted with preserved saline, but it was found to be as safe and effective as that reconstituted with non-preserved saline.Citation52 With preserved saline, there does not appear to be an increased risk of bacterial contamination after prolonged reconstitution and repeated extractions from the bottle.Citation53 Preparing patients for the possibility of headache after injection and instruction to use over the counter (OTC) analgesics can prevent unnecessary patient panic and worry.
Contraindications to use
There are several contraindications for the use of botulinum toxin type A, including active infection at the injection site or history of a hypersensitivity reaction to any of the ingredients (ie, human albumin, lactose, saline, botulinum toxin type A). Dysport™ may contain trace amounts of cow’s milk protein and patients known to be allergic to this should not be treated with Dysport™. However, it is safe for patients with lactose intolerance to receive Dysport™. Low doses of botulinum toxin may induce a neuromuscular crisis. Patients with a history of neuromuscular disorders including myasthenia gravis, Eaton–Lambert syndrome, and amyotrophic lateral sclerosis should not receive botulinum toxin injections. Patients taking aminoglycoside antibiotics including, but not limited to, amikacin, neomycin, streptomycin, tobramycin, or gentamicin should avoid botulinum toxin because this combination may potentiate the effect of the neurotoxin. Patients should also avoid botulinum toxin injections if they are taking other drugs that interfere with neuromuscular transmission such as magnesium sulfate, succinylcholine, penicillamine, tetracyclines, calcium channel blockers, lincosamides, polymyxins, or anticholinesterases. Despite the lack of evidence of teratogenicity with Botox® or Dysport™, both are category C drugs and should not be administered during pregnancy. It is not known whether the neurotoxin is excreted in human milk and therefore should not be used in nursing patients. Botulinum toxin type A should not be used in subjects with a bleeding disorder and used with care in patients taking medication that affects clotting. Relative contraindications to use include patients with unrealistic goals and patients with psychiatric disease.Citation24,Citation25
Botulinum toxin and resistance
Botulinum toxin is a potentially immunogenic protein that can cause neutralizing antibody formation with repeated injections. Antibody formation may lead to decreased effectiveness of the neurotoxin. There appears to be a heightened risk of antibody formation with increased doses and frequency of administration. With the first generation of Botox®, it was recommended that injections take place at greater than 1 month intervals and that no more than 100 Units be used in a patient at one time. The protein load of the newer generation Botox® has decreased 5-fold consequently reducing the antigenic potential.Citation48 Several researchers in the fields of neurology and dermatology have looked into the issue of resistance and established a variety of findings. These findings must be evaluated while keeping in mind that observed incidence of antibody positivity in an assay may be affected by methodology and several other factors. In a four year trial for cervical dystonia, 4 of 326 (1.2%) subjects tested positive for antibodies. Three of these subjects stopped responding clinically to botulinum toxin.Citation54 Lange et alCitation55 report that while they had a large number of subjects with neutralizing antibodies, many of them still responded clinically to treatment. Secondary non-responders with neutralizing antibodies were seen in higher dose indications (eg, focal spasticity and spasmodic torticollis) and with shorter injection intervals. Also, neutralizing antibody development was independent of the commercial preparation used.Citation55 Other trials within the scope of cosmetic treatments found no antibody formation with repeated botulinum toxin type A injections.Citation15,Citation22 It is possible that the dosage of botulinum toxin type A for cosmetic indications is not significant enough to induce antibody formation.
Evaluating treatment outcomes
In clinical trials, treatment outcomes are measured with investigator global assessments and patient satisfaction rating scales. These traditional global assessments do not evaluate the specific outcomes that are significant to patients. Outside of clinical trials to determine efficacy, patient reported outcomes are truly the most import measure of treatment success. Carruthers et al have proposed two new patient-reported outcome measures: the facial line outcome questionnaire (FLO) and the self-perception of age (SPA). The FLO uses scales to allow patients to rate the extent to which their facial lines impact their self-perceptions. The goal of the SPA is to assess the patient’s current perception of his or her age of appearance.Citation56–Citation58 The FLO and SPA assessments provide evidence that botulinum toxin type A injections are improving patients’ perception of themselves, which is the real determinant of treatment success with cosmetic procedures.Citation59
Improving treatment outcomes
As discussed above, patient satisfaction is crucial and there are several ways to improve treatment outcomes in this area. The initial visit should consist of a detailed consultation discussing the goals and expectations of each patient. It is advisable to use a mirror to allow the patient to point out what they consider to be the problem area while gently pointing out facial asymmetry, as well. If the patient has grand visions, attempts should be made to reestablish realistic expectations prior to any cosmetic procedures. Providing sufficient pain management should not be overlooked as patients will reflect upon the entire process when determining treatment success. At follow-up it is recommended to demonstrate muscle immobility with a mirror and to utilize before and after photos.
In addition to determining patient-perceived efficacy, the FLO assessment can be used to ascertain treatment goals for a patient. The FLO assessment may help physicians gain a better understanding of a patient’s objectives for treatment success compared to general global assessments. The SPA can be used to show patients the value of treatment.Citation57 Patient satisfaction appears to improve when multiple facial areas are treated in conjunction since this may lead to a more natural look.Citation56,Citation59 Treatments with botulinum toxin type A need to be customized to each individual patient depending on physician assessment of the patient’s needs and the patient’s own treatment expectations.
Emerging science: Topical botulinum toxin and new injectables
There has been increasing discussion about topical preparations of botulinum toxin type A, but there is concern over adequate percutaneous penetration and drug delivery to the muscle since neurotoxin is a large molecule. Consequently, the appropriate vehicle will be crucial to the success of a topical version. A topical product would be a useful, painless option, especially for treating needle-phobic patients. It seems unlikely that there will be as great an effect with the topical compared to injection treatment though.
Azzalure® (Galderma, USA) is a topical product adapted from Dysport® that has been approved in several European countries. Azzalure® is indicated for the temporary improvement in the appearance of moderate to severe glabellar lines in adult patients under 65 years, when the severity of these lines has an important psychological impact on the patient.Citation60 Revance Therapeutics (Newark, CA, USA) is currently conducting clinical trials with RT001, a botulinum toxin type A topical gel for the reduction of crow’s feet wrinkles.Citation61 Phase I studies found RT001 to be safe and tolerable when applied to the forearms of 41 healthy subjects.Citation62 Early Phase II studies found it was safe and effective for improvement in the appearance of lateral canthal lines. Adverse events experienced included mild skin erythema, ocular erythema, and ocular burning/stinging sensation.Citation63 Currently, RT001 is in a US Phase II B clinical study. Chajchir et al conducted a small, single-center study comparing a topical botulinum toxin cream (CosmeTox) versus placebo cream on upper face wrinkles and found the topical neurotoxin to be effective in terms of subject-perception.Citation64 In this study, the botulinum toxin type A was homogenized with a novel vehicle (InParT) which is thought to aid in appropriate penetration and delivery of the toxin to the muscle.Citation65
Also in pre-clinical trials, is the next generation botulinum toxin type A injection, RT002 (Revance Therapeutics). The manufacturer hypothesizes that the botulinum toxin type A molecule plus the patented TransMTS™ peptide technology could improve onset of action, increase duration of effect, and limit diffusion away from treatment site.Citation66 Large, double-blind, randomized clinical trials need to be carried out to confirm safety and efficacy in each of these new products.
Conclusion
When properly used, botulinum toxin type A is effective for the improvement of glabellar rhytids and with little incidence of complications. There are multiple botulinum toxin type A products that are commercially available, although only two are FDA approved. Each product is distinct and, as mentioned above, they are not interchangeable. Hence, clinical research must be carried out for each specific product and conclusions from clinical trials cannot necessarily be applied to any other botulinum toxin type A product. There is still room for growth and research to be done within the discipline of aesthetic use of botulinum toxin type A.
Disclosures
The authors report no conflicts of interest in this work.
References
- The American Society for Aesthetic Plastic SurgeryCosmetic surgery national data bank Available at http://www.surgery.org/sites/default/files/2008stats.pdf (Accessed October 8, 2009).
- CarruthersACarruthersJSaidSDose-ranging study of botulinum toxin type A in the treatment of glabellar rhytids in femalesDermatol Surg200531441442215871316
- FagienSCarruthersJDA comprehensive review of patient-reported satisfaction with botulinum toxin type a for aesthetic proceduresPlast Reconstr Surg200812261915192519050545
- CarruthersJDCarruthersJATreatment of glabellar frown lines with C. botulinum-A exotoxinJ Dermatol Surg Oncol199218117211740562
- LoweNJMaxwellAHarperHBotulinum A exotoxin for glabellar folds: a double-blind, placebo-controlled study with an electromyographic injection techniqueJ Am Acad Dermatol19963545695728859286
- KeenMBlitzerAAvivJBotulinum toxin A for hyperkinetic facial lines: results of a double-blind, placebo-controlled studyPlast Reconstr Surg199494194998016257
- HankinsCStrimlingRRogersGBotulinum A toxin for glabellar wrinkles. Dose and responseDermatol Surg19982411118111839834736
- OlverJMBotulinum toxin A treatment of overactive corrugator supercilii in thyroid eye diseaseBr Med J199882528533
- ErianAIonescuNECombination treatment of glabellar rhytidsInt J Cosmet Surg199971417
- FellerGBayerlCJungERzanyBTreatment of dynamic facial wrinkles with botulinum toxin type A (Dysport™)-a pilot studyAkt Dermatol2000266569
- Le LouarnCBotulinum toxin and facial wrinkles: a new injection procedureAnn Chir Plast Esthet1998435265339882892
- AscherBKlapPMarionMHChanteloubFBotulinum toxin in the treatment of frontoglabellar and periorbital wrinkles: an initial studyAnn Chir Plast Esthet19954067767668808
- CarruthersJLoweNMenterMGibsonJEadieNBotox Glabellar Lines II Study GroupDouble-blind, placebo-controlled study of the safety and efficacy of botulinum toxin type A for patients with glabellar linesPlast Reconstr Surg200311241089109812973229
- CarruthersJLoweNMenterMA multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar linesJ Am Acad Dermatol200246684084912063480
- MonheitGDCohenJLReloxin Investigational GroupLong-term safety of repeated administrations of a new formulation of botulinum toxin type A in the treatment of glabellar lines: interim analysis from an open-label extension studyJ Am Acad Dermatol200961342142519577326
- KaneMABrandtFRohrichRJNarinsRSMonheitGDHuberMBEvaluation of variable-dose treatment with a new U.S. botulinum toxin type a (Dysport) for correction of moderate to severe glabellar lines: results from a phase 3 randomized, double-blind, placebo controlled studyPlast Reconstr Surg200912451619162919584772
- BrandtFSwansonNBaumannLHuberBRandomized, placebo-controlled study of a new botulinum toxin type A for treatment of glabellar lines: efficacy and safetyDermatol Surg2009351919018816
- MoyRMaasCMonheitGHuberMBReloxin Investigational GroupLong-term safety and efficacy of a new botulinum toxin type A in treating glabellar linesArch Facial Plast Surg2009112778319289677
- RubinMGDoverJGlogauRGGoldbergDJGoldmanMPSchlessingerJThe efficacy and safety of a new U.S. Botulinum toxin type A in the retreatment of glabellar lines following open-label treatmentJ Drugs Dermatol20098543944419537366
- RzanyBDill-MüllerDGrablowitzDHeckmannMCairdDGerman-Austrian Retrospective Study GroupRepeated botulinum toxin A injections for the treatment of lines in the upper face: a retrospective study of 4,103 treatments in 945 patientsDermatol Surg2007331 Spec NoS18S2517241409
- BulstrodeNWGrobbelaarAOLong-term prospective follow-up of botulinum toxin treatment for facial rhytidesAesthetic Plast Surg200226535635912432474
- KawashimaMHariiKAn open-label, randomized, 64-week study repeating 10- and 20-U doses of botulinum toxin type A for treatment of glabellar lines in Japanese subjectsInt J Dermatol200948776877619490208
- NaumannMJankovicJSafety of botulinum toxin type A: a systematic review and meta-analysisCurr Med Res Opin200420798199015265242
- Botox® Cosmetic [Product information package insert] (72284US10A)Irvine, CAAllergan Inc2009
- Dysport™ [Product information package insert]Scottsdale, AZMedicis Aesthetics Inc2009
- MonheitGCarruthersABrandtFRandRA randomized, double-blind, placebo controlled study of botulinum toxin type A for the treatment of glabellar lines: determination of optimal doseDermatol Surg2007331 Spec NoS51S5917241415
- GrimesPEShabazzDA four-month randomized, double-blind evaluation of the efficacy of botulinum toxin type A for the treatment of glabellar lines in women with skin types V and VIDermatol Surg200935342943519250310
- CarruthersACarruthersJProspective, double-blind, randomized, parallel-group, dose-ranging study of botulinum toxin type A in men with glabellar rhytidsDermatol Surg200531101297130316188182
- CarruthersACarruthersJA single-center, dose-comparison, pilot study of botulinum neurotoxin type A in female patients with upper facial rhytids: safety and efficacyJ Am Acad Dermatol200960697297919467368
- AscherBZakineBKestemontPBaspeyrasMBougaraASantiniJA multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin A in the treatment of glabellar linesJ Am Acad Dermatol200451222323315280841
- RzanyBAscherBFratilaAMonheitGDTalaricoSSterryWEfficacy and safety of 3- and 5-injection patterns (30 and 50 U) of botulinum toxin A (Dysport) for the treatment of wrinkles in the glabella and the central forehead regionArch Dermatol2006142332032616549707
- LowePPatnaikRLoweNComparison of two formulations of botulinum toxin type A for the treatment of glabellar lines: a double-blind, randomized studyJ Am Acad Dermatol200655697598017097394
- LoweNJBotulinum toxin type A for facial rejuvenation: United States and United Kingdom perspectivesDermatol Surg199824121612189834741
- LewHYunYSLeeSYKimSJEffect of botulinum toxin A on facial wrinkle lines in KoreansOphthalmologica20022161505411901289
- WohlfarthKSychaTRanouxDNaverHCairdDDose equivalence of two commercial preparations of botulinum neurotoxin type A: time for a reassessment?Curr Med Res Opin20092571573158419463043
- WohlfarthKSchwandtIWegnerFBiological activity of two botulinum toxin type A complexes (Dysport and Botox) in volunteers: a double-blind, randomized, dose-ranging studyJ Neurol2008255121932193918854916
- BoyleMMcGwinGJrFlanaganCVicinanzoMLongJHigh versus low concentration botulinum toxin A for benign essential blepharospasm: does dilution make a difference?Ophthal Plast Reconstr Surg20092528184
- CarruthersACarruthersJCohenJDilution volume of botulinum toxin type A for the treatment of glabellar rhytides: does it matter?Dermatol Surg2007331 Spec NoS97S10417241422
- CarruthersABogleMCarruthersJA randomized, evaluator-blinded, two-center study of the safety and effect of volume on the diffusion and efficacy of botulinum toxin type a in the treatment of lateral orbial rhytidesDermatol Surg200733556757117451579
- HsuTSDoverJSArndtKAEffect of volume and concentration on the diffusion of botulinum exotoxin AArch Dermatol2004140111351135415545544
- PickettADysport: pharmacological properties and factors that influence toxin actionToxicon200954568368919332087
- CarliLMontecuccoCRossettoOAssay of diffusion of different botulinum neurotoxin type a formulations injected in the mouse legMuscle Nerve200940337438019618426
- KranzGHaubenbergerDVollerBRespective potencies of Botox and Dysport in a human skin model: a randomized, double-blind studyMov Disord200924223123618951439
- ZechmeisterMDe Oliveira Dal’FornoTConservation, dilution, and storage after dilutionHexelDTrinidade de AlmeidaACosmetic Use of Botulinum ToxinPorto AlegreAGE Editoria20024344
- AlamMYooSSWroneDAWhiteLEKimJYSterility assessment of multiple use botulinum A exotoxin vials: A prospective simulationJ Am Acad Dermatol200655227227516844511
- HexselDMDe AlmeidaATRutowitschMMulticenter, double-blind study of the efficacy of injections with botulinum toxin type A reconstituted up to six consecutive weeks before applicationDermatol Surg200329552352912752522
- HexselDRutowitschMSde CastroLCdo PradoDZLimaMMBlind multicenter study of the efficacy and safety of injections of a commercial preparation of botulinum toxin type A reconstituted up to 15 days before injectionDermatol Surg200935693393919397645
- KleinAComplications with the use of botulinum toxinDermatol Clin200422219720515222580
- HexselDMazzucoZechmeisterMComplications and adverse effects: diagnosis and treatmentHexelDTrinidade de AlmeidaACosmetic Use of Botulinum ToxinPorto AlegreAGE Editoria2002233239
- PenaMAAlamMYooSSComplications with the use of botulinum toxin type A for cosmetic applications and hyperhidrosisSemin Cutan Med Surg2007261293317349560
- WollinaUKonradHManaging adverse events associated with botulinum toxin type AAm J Clin Dermatol20056314115015943491
- AlamMDoverJSArndtKAPain associated with injection of botulinum A exotoxin reconstituted using isotonic sodium chloride with and without preservative: A double-blind, randomized controlled trialArch Dermatol2002138451051411939813
- AlamMYooSSWroneDAWhiteLEKimJYSterility assessment of multiple use botulinum A exotoxin vials: a prospective simulationJ Am Acad Dermatol200655227227516844511
- BrinMFComellaCLJankovicJLaiFNaumannMCD-017 BoNTA Study GroupLong-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assayMov Disord200823101353136018546321
- LangeOBigalkeHDenglerRWegnerFdeGrootMWohlfarthKNeutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: much ado about nothing?Clin Neuropharmacol200932421321819620852
- CarruthersJCarruthersABotulinum toxin type A treatment of multiple upper facial sites: patient-reported outcomesDermatol Surg2007331 Spec NoS10S1717241408
- CarruthersACarruthersJPatient-reported outcomes with botulinum neurotoxin type AJ Cosmet Laser Ther20079Suppl 1323717885884
- CarruthersACarruthersJA single-center dose-comparison study of botulinum neurotoxin type A in females with upper facial rhytids: Assessing patients’ perception of treamtent outcomesJ Drugs Dermatol200981092492919852121
- FagienSCoxSEFinnJCWerschlerWPKowalskiJWPatient-reported outcomes with botulinum toxin type A treatment of glabellar rhytids: a double-blind, randomized, placebo-controlled studyDermatol Surg2007331 Spec NoS2S917241410
- Galderma. IncGalderma 2009 News Available at: http://www.galderma.com/News.asp?rub=40&Id=97. Accessed on November 12, 2009.
- Revance Therapeutics, IncRevance Announces Efficacy of Topical Botulinum Toxin Type A for the Treatment of Facial Wrinkles7102009 Available at: http://www.revance.com/401-news-publications?side. Accessed on November 5, 2009.
- JonesTScottJTranowskiDJoshiTSafety and Tolerability of Topical Botulinum Toxin Type A in Healthy AdultsMontreal, CanadaPoster session presented at 69th Annual Meeting of the Society for Investigative Dermatology2009 May 6–9
- AtamorosFPTopical botulinum toxin type A for the treatment of moderate to severe lateral canthal lines: Preliminary safety and efficacy results of a blinded, randomized, placebo controlled trialBoston, MAPoster session presented at Summer Academy Meeting of the American Academy of Dermatology2009
- ChajchirIModiPChajchirANovel topical BoNTA (CosmeTox, toxin type A) cream used to treat hyperfunctional wrinkles of the face, mouth, and neckAesthetic Plast Surg200832571572218491179
- ModiPTechnical overview of topical botulinum toxin Available at: http://www.transdermalcorp.com/images/TransdermalCorp-TopicalToxin-Business.ppt. Accessed on November 19, 2009.
- Revance Therapeutics, IncMedicis and Revance announce agreement for development of next-gen neurotoxin7282009 Available at: http://www.revance.com/401-news-publications?side. Accessed on November 5, 2009.