Abstract
Atopic dermatitis (AD) is a common disease with worldwide prevalence, affecting up to 20% of children and 3% of adults. Recent evidence regarding pathogenesis has implicated epidermal barrier defects deriving from filagrin mutations with resulting secondary inflammation. In this report, the authors comprehensively review the literature on atopic dermatitis therapy, including topical and systemic options. Most cases of AD will benefit from emollients to enhance the barrier function of skin. Topical corticosteroids are first-line therapy for most cases of AD. Topical calcineurin inhibitors (tacrolimus ointment, pimecrolimus cream) are considered second line therapy. Several novel barrier-enhancing prescription creams are also available. Moderate to severe cases inadequately controlled with topical therapy may require phototherapy or systemic therapy. The most commonly employed phototherapy modalites are narrow-band UVB, broadband UVB, and UVA1. Traditional systemic therapies include short-term corticosteroids, cyclosporine (considered to be the gold standard), methotrexate, azathioprine, mycophenolate mofetil, and most recently leflunamide. Biologic therapies include recombinant monoclonal antibodies acting on the immunoglobulin E / interleukin-5 pathway (omalizumab, mepolizumab), acting as tumor necrosis factor-α inhibitors (infliximab, etanercept, adalimumab), and acting as T-cell (alefacept) and B-cell (rituxumab) inhibitors, as well as interferon γ and intravenous immunoglobulin. Efficacy, safety, and tolerability are reviewed for each medication.
Introduction
Atopic dermatitis (AD; synonym: atopic eczema or eczema) is a common disease with worldwide prevalence. AD affects up to 20% of children and 3% of adults.Citation1,Citation2 It is associated with the development of atopic respiratory disorders such as allergic rhinitis and asthma (40%–60% of cases) and persists beyond childhood in 40%–60% of cases.Citation2 A preponderance of data indicates that AD is a genetic disease with variable expression that is highly influenced by immunologic and environmental factors.Citation3 Originally, atopic dermatitis was thought to primarily be due to an abnormality in adaptive immunity due to dysregulation of Th1 and Th2 lymphocyte mediated immunity with inappropriate Th2-mediated inflammation leading to skin barrier dysfunction and pruritus. However, recent evidence points to atopic dermatitis being due to a primary barrier defect with resulting secondary inflammation. The barrier defect is due to null mutatons in the epidermal protein filagrin which is involved in normal cornification of the epidermis as well as acting as a natural moisturizing factor in the stratum corneum.Citation4 On a population-based scale, 11%–15% of all cases of atopic dermatitis can be attributed to filaggrin null mutations.Citation5
Methods
The PubMed® database was comprehensively searched for English-language publications containing the keywords atopic dermatitis or atopic eczema. The database was last accessed on March 1, 2010. Articles discussing therapy of AD were selected for further review, with a focus upon recent articles (published within the last 5 years) and those detailing novel therapies. Randomized, double-blind placebo-controlled trials and meta-analyses of the literature were particularly sought, though other types of studies (including open-label trials and case series) were also reviewed.
Clinical presentation
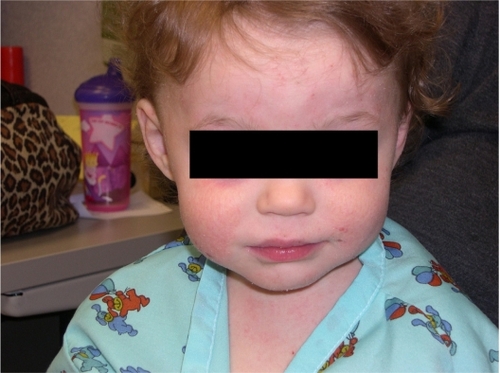
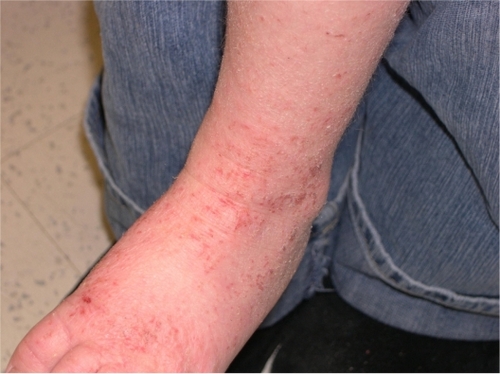
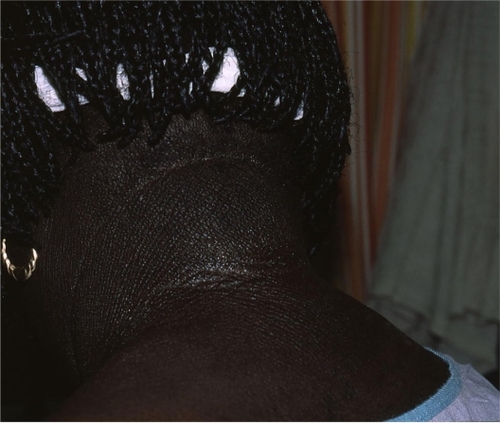
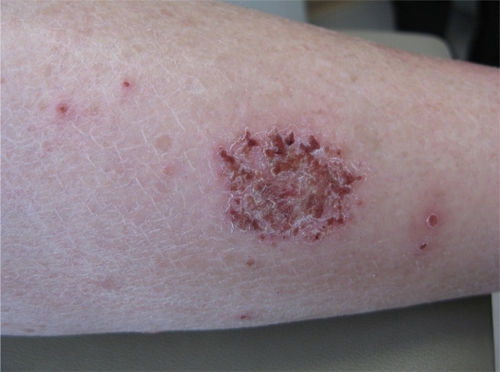
AD is a chronic, relapsing dermatitis with pruritus as a major feature. Diagnostic features are reviewed in . Clinically, eczematous patches and plaques are seen, which favor the face and extensor surfaces in young children () and flexor surfaces (including the antecubital and popliteal fossae, ankles, and neck) in older children () and adults. Lichenification from chronic scratching is common (). Nummular lesions commonly occur on the extremities ().
Table 1 Diagnostic features of atopic dermatitisCitation3,Citation7,Citation13
The course and severity of AD varies widely. Patients may enjoy prolonged periods of remission, though periodic flares of disease activity are common. Cases may be graded as mild, moderate, or severe, depending the extent of skin disease. The majority of cases develop before the age of 5 years.Citation3 Resolution by adolescence occurs in many cases, though some patients will have persistence of disease into adulthood.Citation2 The signs and symptoms of AD are associated with a significant detrimental impact on quality of life, with significant physical discomfort in addition to negative psychosocial consequences and impaired social development.Citation2,Citation3,Citation6
Therapy of atopic dermatitis: overview
The most effective therapy of AD will involve a flexible plan that includes short-term treatment of flares and a long-term maintenance approach to skin care designed to prevent or minimize flares.Citation7 Management of almost every case of AD will include topical therapy. For patients with mild to moderate eczema, topical therapy may be entirely sufficient to control disease activity. Patients with more severe disease may require more advanced therapy including phototherapy or systemic therapy. An overview of treatment options is provided in .
Table 2 Treatment overview of AD
Topical therapy
Topical therapy is integral to the management of chronic eczema. Patients with eczema have been objectively shown to have impaired skin barrier function compared to normal controls using clinical measures such as skin hydration (reduced in eczema) and transepidermal water loss (increased in eczema).Citation8 The majority of cases of eczema will be adequately managed with topical therapy. Flexible treatment strategies that include patient-centered plans for long-term maintenance as well as management of acute disease flares, result in the best chance for effective eczema control.
Daily interventions
Most patients with eczema have sensitive skin that is prone to xerosis and irritation. Using hypoallergenic skin care products is generally recommended. As seasonal flares are common, the use of a humidifier in the home, especially during low-humidity winter months, can have a positive impact in preventing eczema flares. Optimizing the bathing method can be helpful in promoting the integrity of the skin’s barrier function. Limiting bathing to ten minutes per day, using warm (rather than hot) water, and using mild soap or body wash, will help to minimize irritancy. After gently toweling dry, emollients applied to slightly damp skin will help to minimize xerosis.
Emollients
Emollients should be considered as first-line therapy for mild disease. Emollients may be applied multiple times daily, and especially after bathing. Continued use of emollients during periods of disease quiescence can reduce the tendency for eczema flares. In a recent study of 44 patients with eczema, half were randomized to emollient therapy and half were randomized to no treatment. The 22 patients not using emollient experienced a disease flare after a median 30 days; the 22 patients using emollient did not relapse during the 180 day follow-up period of the study.Citation9 In a similar study of 52 children with eczema treated with mid potency topical steroid to lesional skin for two weeks, subsequent daily application of emollient significantly improved xerosis and pruritus compared to no application of emollient.Citation10 Proper emollient use also leads to a reduction in topical corticosteroid use as demonstrated in 51 children with atopic dermatitis followed for one year.Citation11
Patients are generally instructed to apply emollients “liberally,” though the clinical meaning of this term is subjective. A recent of study of 67 pediatric patients (48 with eczema, 19 controls) found that 130 g/m2/week of emollient was adequate for 95.8% of patients.Citation8 However, the study did not detect differences in clinical response.
Medical therapy
Topical corticosteroids (TCs) are the cornerstone of therapy for AD flares. During the past decade, topical calcineurin inhibitors (TCIs) have gained a prominent and often complementary role in AD management. Both TCs and TCIs may be regarded as immunomodulating medications. While TCs broadly inhibit the inflammatory pathway, TCIs inhibit the immune response in a more targeted fashion.Citation12
Topical corticosteroids
TCs are considered first-line therapy for AD flares.Citation13 These agents work by activation of nuclear glucocorticoid receptors to alter expression of cytokines involved in the inflammatory response. These medications are divided into seven classes based on potency as determined by vasoconstrictor assays, with I being the strongest, and VII the weakest. Commonly used options include low potency (class VII and VI; eg, hydrocortisone, desonide), mid-potency (class III–V; eg, triamcinolone, mometasone, fluticasone) to high-potency (class I–II; eg, fluocinonide, desoximetasone, betamethasone dipropionate, clobetasol, halobetasol) corticosteroids.Citation3 Available vehicles include ointments, creams, gels, lotions, liquids, and foams. These agents have a relatively low risk of cutaneous side effects when used twice daily in two-week cycles followed by at least a one week rest from use. Occlusion under plastic wrap can enhance the effectiveness. Ointments and creams will generally be the most effective in treating AD as these vehicles tend to be more moisturizing.
For managing flares, it is generally recommended to use mid-to-high potency TCs twice daily for up to two weeks on the trunk and extremities, and lower potency steroids on the face, intertriginous areas, and in young children. Once control is gained, topical steroids should be used intermittently. While conventional wisdom dictates the use of the lowest-potency compound that brings relief, flares may be controlled more rapidly with shorter term use of higher potency preparations.
Topical corticostroids: efficacy
A multicenter study of 174 children with mild-moderate atopic eczema showed no significant difference in disease severity, symptom control, or quality of life between low potency (1% hydrocortisone) applied twice daily for 7 days, compared to higher potency (betamethasone valerate) TCs applied twice daily for 3 days followed by emollient alone for 4 days.Citation14 In a study of 111 patients with chronic dermatitis including AD, treatment with halobetasol propionate cream (superhigh potency) was associated with significant improvement in skin disease compared to vehicle, without reported systemic effects or skin atrophy.Citation15 In a similar report detailing two vehicle-controlled trials of 124 and 100 adults with chronic dermatoses, halobetasol propionate cream was significantly more effective than vehicle (83% vs 28% of patients on active treatment and vehicle achieving “clear” to “markedly improved,” respectively.Citation16
In another study of 55 children (aged 4 months to 12 years) with AD, treatment with prednicarbate emollient cream 0.1% (mid-potency corticosteroid) daily for three weeks resulted in global evaluation improvement without evidence of hypothalamic–pituitary–adrenal (HPA) axis suppression.Citation17 Multiple trials have established that there is no difference in efficacy of once daily application over twice daily application of TCS in the treatment of atopic dermatitis.Citation18
Topical corticosteroids: safety
Adverse effects of TCs are generally reported only after long-term use and/or use of inappropriately potent preparations to given body sites. Cutaneous adverse effects can include formation of striae, telangiectasia, and atrophy. Systemic absorption of TCs is reported, potentially resulting in suppression of the HPA axis, but systemic adverse effects appear to be rare.
In a systemic review of the literature through 2005, Callen et al concluded that the literature supports good overall safety of topical medications used to treat AD.Citation19 Systemic exposure may occur with topical application of medications, but physiologic consequences are uncommon and systemic complications are rare and have only been reported for topical corticosteroids.Citation19
In a trial of children (aged 6 months–6 years) with moderate to severe AD (mean body surface area involvement of 51%), treated with desonide hydrogel 0.05% (a low potency, class VI corticosteroid) twice daily for four weeks, none of 34 patients who properly completed the study protocol showed evidence of adrenal suppression.Citation20 However, a case of Cushing’s syndrome was reported in an 11-month-old infant associated with continuous use of moderate- to high-potency topical steroids for AD.Citation21
In a multicenter, open-label trial in children aged 6 months to 18 years with moderate-severe AD (>20% body surface area involved), once daily treatment with fluocinonide 0.1% cream (class I/superhigh potency steroid) was not associated with HPA suppression (as determined by intravenous cosyntropin challenge) in any of 63 subjects. However, twice daily treatment was associated with HPA suppression in 3/63 subjects.Citation22 The risk of HPA suppression was no higher in infants and young children compared to older children and adolescents.Citation22 In a study of 51 children (aged 3 months to 6 years) with extensive eczema (≥35% BSA), fluticasone propionate (a mid potency steroid) applied twice daily for 3 to 4 weeks was associated with no cutaneous adverse effects and only mild HPA suppression in 2/43 children.Citation23 Similarly, no evidence of HPA suppression was seen in children with atopic dermatitis treated with either desonide 0.05% ointment or hydrocortisone 2.5% ointment for four weeks.Citation24 In a study of 29 adults with chronic AD, lumbar bone mineral density scores were lower in a subset of patients with severe disease requiring long-term use of topical corticosteroids of potency higher than hydrocortisone.Citation25 In a study of 125 patients with moderate to severe AD, over a third had osteoporosis or osteopenia, which was independent of oral or topical corticosteroid use within the prior five years. The authors speculated that the high rate of low bone mineral density may relate to the underlying inflammatory disease or long-term effects of remote exposure to corticosteroids.Citation26
Topical corticosteroids: issues in patient education
In a questionnaire of 200 dermatology outpatients with atopic eczema (aged 4 months to 67 years), nearly three-quarters were worried about side effects of topical corticosteroids, with a third expressing worry about skin atrophy and 10% expressing worry about systemic absorption. A quarter of patients had chosen not to use prescribed TCs due to their perception of risks, which the authors termed as “phobias,” out of proportion to any evidence of harm.Citation27 Recent evidence suggests that over 40% of caregivers of children with eczema have tried alternative or nontraditional therapies, with fear about TC side effects cited as the most common reason.Citation28 This highlights the need for appropriate education of patients and caregivers to allay their concerns regarding the role of topical steroids in the ongoing therapy of chronic eczema.
Topical calcineurin inhibitors
TCIs, including tacrolimus and pimecolimus have been available for nearly 10 years and have been extensively studied in the management of AD. These agents work by inhibiting the phosphatase activity of calcineurin to block expression of cytokines.Citation7 They thus act “downstream” in the glucocorticoid receptor pathway, and thus are thought to represent a more targeted way to limit inflammation and avoid many of the possible adverse effects of topical corticosteroids.
The labeled indication of TCIs is for application twice daily for up to 6 weeks as second line therapy for patients showing an inadequate response or adverse effects to topical corticosteroids. Pimecrolimus 1% cream and tacrolimus 0.03% ointment are approved for patients ≥ two years old, while tacrolimus 0.1% ointment is approved for patients ≥ 16 years old.Citation7
TCIs may be used either as monotherapy or as combination or sequential therapy. A cost-utility comparison found that TCs are generally less expensive and more effective than TCIs, though individual clinical situations will arise in which TCIs are preferred (eg, topical corticosteroids ineffective or associated with actual or feared adverse effects).Citation29
Topical calcineurin inhibitors: efficacy
Multiple studies have confirmed the efficacy of TCIs, including randomized, vehicle-controlled clinical trials, open-label trials, and trials comparing TCIs to topical steroids (reviewed by Beck).Citation30 In a recent meta-analysis of studies published from 1997–2006, 19 reports including 7378 patients with AD treated with tacrolimus or pimecrolimus were reviewed. The authors concluded that both TCIs are more effective than placebo, that tacrolimus is more effective than low-potency topical steroids or pimecrolimus, and that tacrolimus is comparably effective as mid-potency topical steroids.Citation31
In three 12-week, randomized vehicle-controlled trials, including nearly 1000 patients (children and adults) with moderate to severe AD, 28%–41% of patients achieved ≥90% improvement with tacrolimus ointment (0.03% or 0.1%) compared to 7% with vehicle. In addition, 62%–78% achieved ≥50% improvement compared to 20%–27% treated with vehicle.Citation32–Citation34 In a study of 617 children and adults with mild to moderate AD, treatment with tacrolimus ointment (0.03%) for 6 weeks was associated with significant improvement of skin disease (47.9% “clear” or “almost clear”) vs vehicle (29%; P < 0.001) by the end of the study, with a significant difference seen in degree of improvement by the fourth day of therapy.Citation35
In a six-week study of 200 children with mild–moderate facial AD, pimecrolimus cream was significantly more effective than vehicle (clear/almost clear 74.5% vs 51%).Citation36 In paired studies of children (aged 2–17 years), treatment with pimecrolimus 1% cream twice daily for 26 weeks resulted in significant improvement in global assessment compared to vehicle, with 34.8% of patients “clear” or “almost clear” compared to 18.4% of vehicle-treated patients (P < 0.001). Pimecrolimus showed significantly greater efficacy in treatment of the face and neck compared to the rest of the body (P < 0.0001).Citation37
Open-label 12 month studies in over 500 patients have confirmed these findings and shown ongoing efficacy.Citation38,Citation39 In a multicenter European study, 116 adults (aged ≥ 18 years) with moderate to severe AD were treated with tacrolimus 0.1% ointment for 12 months; 86% of patients showed “marked” to “excellent” improvement/clearance at the end of the study.Citation39
Studies comparing TCIs with TCs have shown that similar improvement can be expected with both topical medications. In a trial of 570 adults with moderate to severe AD, 36% clinical improvement (as determined by the eczema area and severity index) was seen for both tacrolimus 0.1% ointment and hydrocortisone butyrate 0.1% ointment (mid potency steroid), and both were superior to tacrolimus 0.03% ointment.Citation40 Similar trials showed tacrolimus ointment (0.03% and 0.1%) to be clinically superior to a lower potency steroid (hydrocortisone acetate 1%) in children and adults.Citation41,Citation42
In a randomized, double-blind study of patients with facial eczema, tacrolimus 0.1% ointment (n = 288) was superior to fluticasone 0.005% ointment (n = 280) when applied twice daily for three weeks, with more patients in the tacrolimus group showing ≥60% improvement compared to fluticasone (93% vs 88%; P = 0.026).Citation43 In a similar trial of 73 patients (aged 2–49 years) with eczema unresponsive to topical steroid, pimecrolimus 1% cream was associated with clinical improvement, especially in the head/neck areas.Citation44
In a randomized, double-blind multicenter European study, 658 adults with moderate to severe AD used either pimecrolimus or TCs (triamcinolone 0.1% and/or 1% hydrocortisone creams) twice daily to all affected areas until clear or for up to one year, with most patients using the medications continuously. Both therapies were effective, but pimecrolimus was associated with fewer adverse effects, including fewer skin infections and no striae formation (seen in three patients treated with TCs). 42% of patients were maintained on pimecolimus as monotherapy.Citation45 The study is particularly interesting in that use of the medications was unrestricted as to duration of application (eg, 2–3 weeks for TCs and six weeks for TCIs); the incidence of striae in steroid-treated patients is lower than might be expected under these circumstances.
In a two-phase study of 152 children (aged 2–15 years) with moderate to severe AD, twice-daily application of TCs (aclometasome ointment 0.05%) resulted in more rapid improvement of active eczema than tacrolimus 0.03% ointment. However, once the dermatitis was stabilized, tacrolimus applied three-times weekly to previously affected skin for up to 40 weeks was significantly more effective than vehicle in maintaining disease stabilization.Citation46 A similar study of 125 children and adults with stabilized AD found that application of tacrolimus ointment (0.03% or 0.1%) three times weekly as maintenance therapy for 40 weeks, was associated with more flare-free days (177 vs 134; P = 0.003) and a longer time to first relapse (169 days vs 43 days, P = 0.037) compared to vehicle.Citation47
At least one large trial has compared the two TCIs to each other. In a study of adults with moderate AD (mean body surface area ∼16%), 98 were treated with tacrolimus 0.1% ointment and 90 were treated with pimecrolimus 1% cream. Tacrolimus ointment was associated with a significantly greater improvement in the eczema severity index (59%) compared to pimecrolimus (43%; P = 0.01). Adverse effects were minor and did not differ between the treatment groups.Citation48
Topical calcineurin inhibitors: safety
In clinical practice, the most common side effects of TCIs are application site-irritation reactions, including pruritus and perceived burning sensation, particularly upon initiation of treatment.Citation39 The burning sensation is thought to be due to transient local nerve fiber release of substance P and calcitonin gene-related peptide.Citation49
In a study of adolescents or adults with moderate to severe AD, treatment with pimecrolimus for three weeks applied either twice daily (n = 24) or four times daily (n = 25) resulted in clinical improvement in both groups. No significant differences in adverse effects or serum levels of drug were seen between the groups, with 46/49 patients showing undetectable serum levels of the drug and three patients (one in the four times daily and two in the twice daily) showed detectable but low serum levels of the drug. Seven patients reported mild adverse effects (typically transient burning sensation at the application site).Citation50
In an in vitro study, pimecrolimus was found to have significantly lower permeation through skin compared to tacrolimus and high-potency TCs, likely owing to the molecule’s higher lipophilicity.Citation51 This may relate to studies suggesting lower relative efficacy and greater perceived safety of pimecrolimus.
In January 2006, the US Food and Drug Administration (FDA) changed the product label of both tacrolimus ointment and pimecrolimus cream to include a black-box warning regarding risk of cancer and lymphoproliferative disease, based largely on case reports of cancer in patients using TCIs, animal studies involving high-dose oral calcineurin inhibitors, and mechanism of action-based theoretical risks.Citation7 The blackbox warning based on inferred causality created controversy in the field of dermatology and increased the complexity of continuing to use these effective medications in clinical practice.
In a 2006 report by an American Academy of Dermatology (AAD) Task Force, a review of available information led the authors to conclude that no causal proof existed that TCIs cause lymphoma or skin cancer.Citation52 Since that time, a retrospective cohort observational study involving over 950,000 patients with AD was performed.Citation53 Sixteen cases of T-cell lymphoma were identified in patients treated with either topical tacrolimus or pimecrolimus between 2001 and 2004. When charts were reviewed, at least four of these cases were suspected prior to exposure to TCIs and were excluded from further analysis. The odds ratio for T-cell lymphoma was determined to be 5.4 for tacrolimus (95% confidence interval: 2.5–11.8, P < 0.001) and was not significant for pimecrolimus. No other subtypes of cancer were seen at increased incidence in the study population.Citation53 Despite the small number of cases of lymphoma and the possibility that this was a pre-existing condition (as cutaneous lymphoma may clinically mimic dermatitis), this study represents the best evidence to date linking TCIs (tacrolimus at least) to cutaneous lymphoma. As suggested by the AAD Task Force, it is important for dermatologists and their patients to remain informed, to be aware of treatment indications and guidelines, and to be cognizant of the risks and benefits of any therapy.Citation52
Prescription barrier creams
As discussed above, concerns about safety with ongoing use of both TCs and TCIs have spurred interest in the development of novel prescription emollients and barrier creams for use as ancillary or primary therapy of chronic eczema. At least four nonsteroid barrier creams have become available in recent years. These products may improve the signs and symptoms of AD by addressing the damaged skin barrier and providing anti-inflammatory action.Citation7 Approved as “medical devices” rather than drugs by the FDA, these products are recognized to serve a structural role in cutaneous barrier function rather than exerting chemical or receptor-based effects.Citation7 Moreover, clinical efficacy data for approval is less stringent for medical devices than for drugs.
Industry-sponsored studies have supported the efficacy of Tetrix®,Citation54 Mimyx®,Citation55 Atopiclair®,Citation56–Citation59 and Epiceram®.Citation60 All of these agents are marketed only under their registered tradenames. Of these agents, Atopiclair is perhaps the most studied. The putative active ingredients of this compound include hyaluronic acid, telmesteine, Vitis vinifera and glycyrrhetinic acid, which have moisturizing, anti-inflammatory, and antioxidant properties.Citation56 Two studies in 248 adults treated for 35–50 days showed found significant improvements in clinical parameters (surface area affected, severity index, and itch score) with Atopiclair compared to control.Citation56,Citation57 Similar results were seen in 202 pediatric patients (aged 6 months to 17 years) with AD treated for 22 or 43 days, with Atopiclair showing clinical superiority to vehicle.Citation58,Citation59
Mimyx contains lipids as well as N-palmitoylethanolamine which may negatively regulate the inflammatory response through agonist activity on mast cell cannabinoid receptors.Citation7 A multicenter observational uncontrolled study of 2456 patients (aged 2–70 years), reported that use of Mimyx was associated with clinical improvement (including pruritus, erythema, excoriation, dryness, lichenification, scaling, and sleep quality) and reduction of topical steroid usage.Citation61 EpiCeram is triple-lipid barrier repair cream containing ceramides, cholesterol, and free fatty acids. In a multicenter randomized trial of 121 of patients (aged 6 months to 18 years) with moderate to severe AD, Epiceram was associated with similar clinical improvement (SCORAD severity index, pruritus, and sleep score) at 28 days compared to mid-potency topical steroid (fluticasone), though the topical steroid was associated with more rapid improvement.Citation60
Combination and sequential topical therapy
The most effective therapeutic approach is to combine therapies in a fashion that is tailored to the individual patient. Combining treatments offers the advantage of gaining benefit from medications with different and complimentary mechanisms of action while limiting concerns regarding overuse of a single agent.Citation62,Citation63
In a 16 week study of 221 patients with chronic eczema, use of TCs twice weekly in addition to emollient resulted in a 3.5-fold reduction in disease relapse compared to use of emollient alone.Citation64 In a three-week study of 57 patients with AD, patients treated for 3 weeks concomitantly with a mid-potency steroid (clocortolone pivalate) and tacrolimus 0.1% ointment showed significant improvements in a variety of clinical parameters (including excoriation, induration, erythema, crusting, and lichenification) compared to monotherapy with either medication.Citation65
A recent study in 31 pediatric patients showed clinical improvement in eczema severity with a combination approach, using TCIs, TCs, and emollients. During the induction phase, children with active eczema (2, 25, and 4 with mild, moderate, or severe disease, respectively) were treated for two weeks with tacrolimus ointment (0.03%) in the morning and a topical steroid (variable potency) in the evening, followed by a two-week period of tacrolimus twice daily on weekdays and on weekend mornings with TCs on weekend evenings. This was followed by a two-week period without TCs in which tacrolimus was applied twice daily, then a six week period with application of emollient alone with tacrolimus used when necessary. Improvements in disease severity indices, pruritus, and sleep disturbance were seen.Citation66 This study illustrates that combined treatment is beneficial at improving outcomes while limiting potential adverse effects that may result from overuse of an individual medication. It also illustrates that combined therapy regimens are potentially complex and highlights that the treatment plan should be tailored to the individual situation to avoid confusion and maximize adherence.
Use of wet-wrap therapy (WWT) may enhance the efficacy of topical treatments. This involves applying topical medication then occluding the body area with a damp dressing. Advantages include rapid response and effective relief of symptoms. Disadvantages include higher cost, inconvenience, a need for specialized training, and an increased potential for adverse effects from occluded corticosteroids (including systemic absorption, atrophy, and striae), and increased incidence of skin infection requiring antibiotics.Citation67–Citation69 Short-term WWT with diluted TCs was found, in a study of 8 prepubertal children, not to influence bone turnover or short-term growth.Citation70
Adherence to topical therapy
Lack of adherence to therapy is a barrier to effective treatment, and this may be particularly true as relates to topical therapy. In a study of 37 children with AD prescribed triamcinolone 0.1% cream, usage of medication was monitored using electronic devices in the tubes and by measuring the weight of the tube after 4 weeks. Mean adherence to therapy throughout the study was only 32%, with higher adherence on the days surrounding the office visits.Citation71 The authors concluded that better adherence, which might be achieved by more frequent follow-up visits, might be associated with better clinical outcomes and less need for systemic therapy.
Phototherapy
Phototherapy exerts beneficial effects on chronic skin diseases such as AD through several mechanisms, including reduction of Langerhans cells, induction of immunomodulatory cytokines, and promoting apoptosis of infiltrating T lymphocytes.Citation72 When chronic AD is not controlled adequately with topical therapy, phototherapy should be considered as a second-line treatment due to its efficacy and and favorable risk–benefit profile compared to most systemic agents. Phototherapy will often be a part of a multitherapeutic approach involving topical treatments and perhaps systemic treatments.
Ultraviolet A and ultraviolet B
Early phototherapy trials for patients with AD compared combined UVA and UVB treatment against either modality alone, with variable results. In two half-sided studies involving 43 patients with AD, combined UVA and UVB was significantly more effective than either UVA or UVB alone.Citation73
Advancement of phototherapy technology brought clinical studies of narrow-band UVA and medium dose UVA1 (50 J/cm2). In a trial of ten patients with severe generalized AD, exposure of one side of the body to high-dose UVA1 and exposure of the contralateral side to half that dose (medium dose UVA1) five times weekly for three weeks, resulted in significant improvement over baseline (29%–38%) which was comparable for both sides.Citation74 In a study of 32 patients with severe eczema, treatment with medium-dose UVA1 therapy (50 J/cm2 5 times per week for 3 weeks), resulted in significant improvement which persisted one month after completion of therapy. However, disease severity returned to baseline by the third month post-treatment.Citation75
In a pilot study, all five patients with severe atopic eczema treated with narrow-band UVB showed improvement of the disease after three weeks of therapy.Citation76 In a study of 73 adults with moderate to severe atopic eczema treated with phototherapy twice weekly for 12 weeks, NB-UVB was more effective than UVA in reducing disease severity.Citation77 A half-sided study of 12 patients with severe chronic AD showed equivalent disease improvement (64%–65%) after phototherapy with PUVA or NB-UVB administered three times weekly for 6 weeks.Citation78 Recent informative studies have compared medium-dose UVA1 to NB-UVB. In a study in which 13 adults (aged 20–56 years) with chronic AD received half-sided phototherapy three times weekly for 8 weeks, NB UVA and medium dose UVA1 were both equally effective in reducing disease severity.Citation72 In a comparative crossover study of phototherapy modalities, 28 patients completed separate 6 week courses of both UVA1 and NB-UVB phototherapy. Both therapies were equally effective in significantly decreasing scores for pruritus and clinical severity.Citation79 A few studies have directly compared phototherapy to TC treatment. In a multicenter study of 53 patients with AD, high-dose UVA1 was significantly more effective than treatment with either fluocortolone or combined UVA-UVB therapy.Citation80 In a study of 21 adults with severe AD, UVB photo-therapy three times weekly for 12 weeks was associated with a 68% reduction in disease severity and an 88% reduction in TC use. 15/24 continued to show benefit 24 weeks after discontinuing UVB.Citation81
Extracorporeal photopheresis
Extracorporeal photopheresis (ECP) involves the irradiation of blood fractions in the presence of psoralen and is thought to suppress pathogenic clones of T lymphocytes. In a study of seven patients (median age 47 years) with severe refractory atopic eczema, extracorporeal photopheresis (consisting of two treatments on successive days every two weeks for 12–20 weeks) was associated with a mean 28% decrease in disease severity score.Citation82 Although associated with low toxicity, a primary drawback to ECP is that it is generally only available at tertiary care centers.
Phototherapy: summary
In a systematic review of the literature regarding phototherapy and AD, nine studies meeting inclusion criteria were analyzed. The authors concluded that UVA1 may be most effective at controlling acute flares of AD, while NB-UVB may be most effective in managing chronic AD.Citation83 In another literature survey of phototherapy for a variety of nonpsoriatic skin conditions including AD, vitiligo, cutaneous lymphoma, and chronic urticaria, 28 articles were reviewed. Most patients in these studies had a primary diagnosis of either AD (n = 719) or generalized vitiligo (n = 305).Citation84 Other common diagnoses included cutaneous lymphoma, chronic urticaria, and polymorphic light eruption. The authors concluded that based on its excellent safety profile and equivalent to superior efficacy, NB-UVB should be considered as the first-line phototherapy modality for these conditions.Citation84
Phototherapy will not be beneficial for all patients with AD. Some will not tolerate the associated heat and sweating, though many phototherapy units are now equipped with filtering and cooling systems. A small number of patients with AD will have photosensitivity or co-existing polymorphous light eruption.Citation85 While an increased risk of skin cancer is seen with prolonged PUVA therapy, a ten-year study found no evidence of an increased skin cancer risk in 195 psoriasis patients receiving broadband or NB-UVB.Citation86
Systemic therapies: overview
For particularly severe cases of eczema, systemic therapy may be required for management of acute flares or to suppress the activity of chronic disease. These therapies may be broadly grouped into traditional medications and biologic agents (targeted monoclonal antibodies). A systematic review of the literature found 37 studies totalling 979 patients with severe atopic eczema treated with systemic therapy. Eleven studies showed cyclosporine to be effective. IFN and azathioprine were shown effective in randomized, controlled trials, mycophenylate mofetil was effective in two small studies. Systemic steroids were not adequately studied to recommend; IVIG and infliximab were not supported.Citation87 Since this review was published, several trials of both traditional agents and newer biologic options have become available to guide clinical decisions.
Systemic therapies: traditional
Corticosteroids
Corticosteroids act through binding cytoplasmic receptors which are then translocated to the nucleus to regulate the transcription of multiple genes involved in the inflammatory cascade. Though often highly effective in eliminating the skin inflammation underlying the dermatitis of AD, systemic corticosteroids are not recommended for chronic therapy for AD owing to a high likelihood of significant adverse effects.Citation88 Well-known complications of systemic corticosteroids include suppression of the HPA axis, hyperglycemia, osteoporosis, avascular necrosis of the hip, hypertension, ocular changes (posterior subcapsular cataracts, glaucoma), altered immune function, and altered body habitus. These risks may be both dose-dependent and dose-independent and are generally more likely with prolonged treatment. Children on prolonged corticosteroid therapy are particularly at risk for growth suppression and posterior subcapsular cataracts.Citation88
In clinical practice however, systemic corticosteroids are not uncommonly used as short-term “rescue-therapy” for severe flares of disease.Citation89 Often, oral prednisone (1 mg/kg/day, or equivalent) tapered over a 2-week period will quell a flare and allow ongoing topical maintenance therapy. Somewhat surprisingly, there have been few clinical studies to formally evaluate this therapy method.
In an Italian study of seven children with severe AD unresponsive to standard therapy, an intravenous bolus of methylprednisolone (20 mg/kg/day) for three days resulted in clinical improvement for several months in 5/7 patients, with transient lymphopenia being the only reported side-effect.Citation90 Cases of rebound flaring of AD after oral corticosteroids have been reported,Citation91 and this highlights the importance using topical therapy both as a mainstay of therapy and particularly during severe flares which may require more intensive systemic therapy.
Cyclosporine
Cyclosporine is the best-studied treatment for severe chronic eczema and is considered by many authors to represent the “gold standard” for systemic treatment of the severe manifestations of this disease. Cyclosporine’s cellular mechanism of action involves binding cyclophilin; this complex then inhibits the phosphatase activity of calcineurin, thereby blocking the activation of transcription factor NF-AT (nuclear factor of activated T cells).Citation88 In a multicenter, double-blind, placebo-controlled crossover study involving 33 patients with severe chronic atopic dermatitis, therapy with cyclosporine for 8 weeks was associated with highly significant improvement of quality of life parameters by multiple clinical indices.Citation92
In a randomized double-blind trial of 38 adults with severe eczema, cyclosporine (2.7–4 mg/kg/day for 6 weeks) was significantly more effective than prednisolone (0.5–0.8 mg/kg/day for 2 weeks) in achieving a stable remission, with 6/17 patients on cyclosporine remaining clear 12 weeks after active treatment compared to 1/21 patients on prednisolone.Citation93 Cyclosporine therapy is generally associated with a rapid response. In a meta-analysis of 15 studies including 602 patients with severe eczema, treatment with cyclosporine was associated with a pooled mean decrease in disease severity of 22% (3 mg/kg/day) to 40% (≥4 mg/kg/day) after 2 weeks of therapy. The relative effectiveness was 55% after 6–8 weeks of therapy.Citation94 Cyclosporine can be used in both adults and children. The authors of a meta-analysis suggest that cyclosporine may be better tolerated in children compared to adults.Citation94 However; cyclosporine use in children has been linked to lower bone mineral density.Citation95
Cyclosporine is reported to have efficacy lasting long beyond the active therapy interval. In a follow-up study of patients with severe eczema treated with cyclosporine (5 mg/kg/day for 1–2 treatment periods of 6 weeks), 35 of 37 remained in remission two years after their last dose.Citation96 Due to predictable dose related adverse effects (primarily renal impairment and hypertension, but also gingival hyperplasia, hypercholesterolemia, hypertrichosis, tremor, and fatigue), treatment of atopic dermatitis with cyclosporine is generally short-term (under 6 months). In a study of 73 patients (mean age 33.8 years) with severe atopic dermatitis treated with cyclosporine for at least 6 months (mean duration of therapy 1.3 years), 56 (77%) responded to therapy. 10%–15% of patients experienced renal impairment (serum creatinine >30% baseline) or hypertension. 33/73 (45%) of patients experienced remained in remission for at least three months. 8% of these patients experienced a rebound of disease shortly after discontinuation of cyclosporine.Citation97
Methotrexate
Methotrexate (MTX) inhibits DNA synthesis by substrate competition with dihydrofolate reductase. It is generally dosed weekly with oral delivery more common than intramuscular or intravenous. MTX (mean dose 15 mg/week) was shown to be effective in controlling chronic eczema symptoms; 11/12 patients completed a 24-week trial (one discontinued due to adverse effects) mean disease activity (measured by the six area six sign atopic dermatitis score) improved by 52% in subjects completing the trial, with 8/9 patients showing continued improvement 12 weeks after stopping methotrexate.Citation98 In a retrospective study of 20 patients with chronic – eczema on weekly MTX (7.5–25 mg/week), 75% of patients showed improvement at three months, with 65% of patients showing an improvement in global assessment score >70%. Ten percent of patients discontinued the medication due to nausea and increased liver enzymes.Citation99
MTX therapy (dosed 7.5–25 mg administered orally or intramuscularly) was associated with a ∼45% improvement in quality of life measures in a study of 20 adults with chronic moderate-to-severe atopic dermatitis. A quarter of patients in this study had nausea and increased liver enzymes, leading to discontinuation in therapy for three. An additional patient developed peripheral neuropathy which resolved when the medication was discontinued.Citation100 In another study, MTX (10–20 mg weekly) was effective in 9/9 adult patients with chronic eczema, with 6/9 patients achieving complete remission after 3 months.Citation101
In a case series, 3 of 4 elderly patients with chronic atopic eczema (aged 71–83 years), treatment with low-dose MTX, dosed initially up to 7.5 mg, was effective in controlling the skin disease. After several months of therapy, doses for the three responders were decreased to 2.5 mg weekly for 2 patients and discontinued entirely for one patient, with ongoing remission.Citation102
The onset of improvement with MTX is generally slower than with cyclosporine, with onset 4–8 weeks for methotrexate compared to 2 weeks for cyclosporine.Citation99 MTX requires careful clinical and laboratory monitoring. Gastrointestinal disturbance, anorexia, fatigue, stomatitis, and alopecia are the most common adverse effects.Citation88 Hepatotoxicity (including cirrhosis) and bone marrow suppression (including pancytopenia) are the most serious potential adverse effects. Renal impairment and pulmonary toxicity (interstitial pneumonitis) are also reported. Patients with liver disease or significant ethanol intake, as well as patients with renal dysfunction, should not be treated with MTX.
Azathioprine
Azathioprine is a thiopurine prodrug which is activated to 6-thioguanine, which is further activated to several effectors which block purine synthesis. With proper laboratory monitoring, azathioprine is a relatively safe medication. Adverse effects include myelosuppression, gastrointestinal disturbance (nausea, vomiting, diarrhea, hepatitis, pancreatitis), and risk of infection and malignancy (particularly hematologic). Selecting the dose of azathioprine based on the activity of thiopurine methyltransferase (TPMT), which is a key factor in azathioprine-induced myelotoxicity, may limit adverse effects.Citation103
In a 12 week, placebo controlled trial of 63 patients with chronic eczema, azathioprine was associated with a significant improvement in disease activity compared to placebo (37% vs 20%). Nine patients (7 on azathioprine, 2 on placebo) withdrew from the study.Citation103 In a retrospective review of 37 patients with chronic atopic eczema treated with azathioprine over an 18 year period, 15/37 (40.5%) achieved remission in a median period of 5 months; ten patients did not respond adequately, and five had adverse reactions requiring discontinuation of the drug.Citation104 Azathioprine has also been studied in children with severe eczema. In a retrospective study, 28/48 children had an excellent response to azathioprine; 13/48 had a good response, and 7/48 had a poor response.Citation105 The authors recommended dosing the medication at 2.5–3.5 mg/kg in patients with a normal TPMT level.Citation105
Mycophenolate mofetil
Mycophenolate mofetil (MMF) is derived from mycophenolic acid, which acts as an inhibitor of de novo purine biosynthesis, by inhibition of inosine monophosphate dehydrogenase, the enzyme which produces guanosine-5-phosphate from inosine- and xanthine-5-phosphate. Lymphocytes are particularly targeted by this cytotoxic effect as these cells lack a highly active purine salvage pathway. MMF is generally well-tolerated. Adverse effects include gastrointestinal disturbance and rare bone marrow suppression.Citation88 In a study of ten patients with severe AD, treatment with oral MMF (1 g twice daily for the first week then 2 g twice daily for 11 weeks) was associated with a 68% improvement in disease severity (SCORAD index), without any reported adverse effects.Citation106 In an open-label pilot study, 10 patients were treated with MMF 1 g twice daily for 4 weeks, with a 55% reduction in disease severity (SCORAD index) by week 4. Of the 7 patients completing the 20 week follow-up period, reduction of disease severity was 74%; 6/7 had no relapse of disease. One patient dropped out during therapy due to herpes keratitis.Citation107
In a retrospective review of 20 patients with severe AD treated with MMF, 17/20 patients responded, within 4 weeks of beginning therapy. 10 patients experienced remission and discontinued MMF; 7 continued MMF as maintenance therapy.Citation108 Infectious complications were relatively high in this study, with 5/20 developing viral infections (zoster in 4, herpes simplex in one) and 2/20 developing Staphylococcus aureus skin infections. As the study was uncontrolled, attributing the infections to MMF is conjecture.
A retrospective review of 14 patients with severe childhood atopic dermatitis treated with MMF (dosed at 30–40 mg/kg daily in adolescents and 40–50 mg/kg daily in younger children), 8/14 patients obtained 90%–100% improvement and an additional 5/14 experienced 60%–90% improvement, with one nonresponder. Initial responses were seen at a mean of 4 weeks and peaked at a mean of 9 weeks. No adverse effects were reported.Citation109
Other nonbiologic systemic agents
Leflunamide is an immunosuppressant which blocks de novo pyrimidine synthesis and is approved for treatment of rheumatoid arthritis and psoriatic arthritis.Citation110 In published case reports, two patients with severe, near-erythrodermic atopic dermatitis achieved long-term (20 months) improvement with leflunamide (20 mg daily after three days of 100 mg as loading dose) as monotherapy.Citation111 A third with chronic eczema showed only partial response on leflunamide and continued to require systemic corticosteroid.Citation110 Significant adverse effects were not reported in these three patients.
In a recent case report, everolimus (a rapamycin-derived immunosuppressive used in organ transplant patients) was not found to be effective in two patients with severe AD concurrently on either prednisone or cyclosporine.Citation112
Systemic therapy: biologic agents
Biologic agents are produced by living systems and are protein-based therapies including soluble receptors, monoclonal antibodies, or cytokines designed to modulate the immune response.Citation113 Generally indicated for treatment of autoimmune inflammatory diseases such as rheumatoid arthritis, Crohn disease, psoriatic arthritis and moderate-to-severe plaque psoriasis, injected “biologic” agents have been tested off-label as a potential therapy for severe AD. These agents may represent a more targeted and less toxic option. Of the agents discussed here, interferon has been available for the longest time and has the most clinical data supporting its use.
Recombinant interferon
As AD has been shown to be associated with low interferon (IFN)-γ levels leading to elevated interleukin-4 levels and upregulation in immunoglobulin E levels, IFN was identified as a potential immunologic therapy for severe AD. In an early trial of IFN-α administered 3 times weekly, an adequate response was seen in 5/13 subjects.Citation114 In a subsequent randomized trial of 83 patients with moderate to severe AD, treatment with recombinant IFN-γ (as 50 μg/m2 daily subcutaneous injection for 12 weeks) was superior to placebo in both patient-assessed and physician-assessed response, with 45% of the IFN-group achieving >50% improvement compared to 21% of the placebo group (P = 0.016). Headache, myalgia, and chills occurred in 30%–60% of IFN-γ treated patients.Citation115 A similar study found recombinant IFN-γ to induce significant improvement in 8 of 14 (57%) of patients treated for 6 weeks, with half of responders showing ongoing improvement three months after therapy was discontinued.Citation116
Treatment with IFN-γ (50 micrograms/m2 daily or every other day) in 15 patients for at least 22 months was associated with significant reduction in body surface area (61.6% baseline involvement vs 18.5% involvement at 24 months) and overall clinical improvement, with no significant adverse effects reported.Citation117 In a randomized, placebo-controlled study of 51 patients with severe AD treated with IFN-γ administered subcutaneously three times weekly for 12 weeks, both low dose (500,000 U/m2) and high dose (1.5 million U/m2) were associated with reduced disease severity compared to placebo, with the high-dose group showing a faster initial response.Citation118
In contrast, IFN-α 2b (9–15 million U/week for 4–6 weeks) was judged ineffective in treating AD in 8 subjects, with 4/8 patients showing exacerbation of their disease.Citation119 IFN-α 2a administered daily for 3 weeks was associated with only short-lived (<3 weeks) improvement in severe AD in 8/9 treated patients.Citation120
While IFN-γ has been shown to be highly effective in a subset of patients, its use is limited by tolerability (high incidence of flu-like syndrome), a relatively low response rate, and high cost. Baseline serum IgE < 1500 IU/ml and serum eosinophils <9% may be predictive of a favorable clinical outcome.Citation121
Intravenous immunoglobulin
Intravenous immunoglobulin (IVIG) is pooled purified immunoglobulin obtained from the serum of multiple donors. It is used as therapy for primary immunodeficiency, Kawasaki disease, idiopathic thrombocytopenic purpura, and has been tried in other inflammatory diseases including AD. IVIG is relatively safe but remains expensive. Infusion reactions, including headache, fever, chills, mylagia, and fatigue, occur in up to 6% of patients. Other adverse events including hemolysis, acute renal failure, and transmission of viruses, are rare.Citation88
In a study of nine patients with severe AD (and one with hypereosinophilic syndrome) administered IVIG (2 mg/kg monthly × 6 months), six showed slight clinical improvement. No significant change was seen in serum IgE levels. The authors concluded that IVIG was associated with no benefit.Citation122 In a case series, three patients with severe AD requiring systemic corticosteroid were treated with monthly IVIG (2 mg/kg). Each showed clinical improvement and were able to decrease their steroid dose.Citation123
In a comparative trial of 12 infants with severe AD, five infants (aged 7–12 months) received IVIG 2 mg/kg monthly for three months. An age-matched control group of seven infants received topical steroid therapy only. After three months, the SCORAD index was significantly (P = 0.01) improved in the group treated with IVIG.Citation124
When these and other initial studies and other case reports in the English literature (up to the year 2001) were reviewed, Jolles identified 32 patients with severe AD treated with IVIG and determined that improvement was seen in 61%, with children showing a better response rate (90%) compared to adults (48%), with a longer duration of response.Citation125 Children generally improved with IVIG as monotherapy, while adults were generally administered IVIG concurrently with other systemic agents.Citation125
In a small trial, ten adults with recalcitrant AD were treated with IVIG given as 1 mg/kg daily for two days. When assessed at day 30, no significant improvement was observed by either SCORAD index or global severity measures.Citation126 In an open-label study, six adults with severe AD were treated with six monthly cycles of IVIG (2 mg/kg) then followed for three additional months. Four of the six patients showed significant clinical improvement and demonstrated reduction in pathogenic T cell levels.Citation127 In a comparative study of 14 patients with severe atopic dermatitis, treatment with intravenous immunoglobulin (2 g/kg as a single infusion) was associated with significantly lower efficacy compared to treatment with cyclosporine (4 mg/kg/day for three months).Citation128
TNF-inhibitors
As TNF-α and TNF-dependent cytokines are involved in the immune-based inflammatory etiology of AD, blockade of this effector molecule is a plausible therapy target for chronic eczema. Currently available TNF-inhibitors include infliximab, etanercept, and adalimumab. To date, published reports have detailed experience with infliximab and etanercept, but not adalimumab, for chronic eczema.
In a prospective trial of 9 patients with AD, infliximab (5 mg/kg administered by intravenous infusion at week 0, 2, 6, then every 8 weeks for 4 additional doses) was associated with clinical improvement during induction (53% improvement at week 2) but not maintenance, with only 2/9 patients showing sustained improvement by the end of the study.Citation129 In a case report, two adults with chronic AD experienced complete resolution on etanercept (50 mg injected subcutaneously twice a week) for 8–11 months of therapy and remained in remission for 26–31 months after discontinuation.Citation130 In contrast, etanercept was associated with no improvement in two pediatric patients with AD.Citation131
Adverse effects of TNF therapy
As a class, TNF therapy is associated with increased risks of infection and malignancy (particularly hematologic). Of 12 patients with AD treated with TNF inhibitors, one developed an infliximab-infusion reaction, one developed an urticarial reaction to etanercept, and one developed a methicillin-resistant Staphylococcus aureus infection while on etanercept.Citation113
TNF-inhibitors are also associated with triggering an eczema-like drug eruption. In a prospective study of 92 patients treated for indications other than skin disease/psoriasis, 15 (16%) developed eczema during treatment with infliximab.Citation132 A personal history of atopy was predictive of this medication-response.Citation132
Alefacept
Alefacept is a fully human fusion protein derived from immunoglobulin G1 and lymphocyte function-associated (LFA)-3 that selectively inhibits T-cell activation and reduces memory T cells. In a 16 week study, nine adults with severe AD were administered alefacept as a weekly intramuscular injection. Two of nine subjects achieved an Eczema Area Severity Index score of 50%; three of nine subjects achieved a Physician Global assessment score of mild to almost clear. Importantly, an equal number of patients (3/9) showed a worsening of their disease as showed a significant improvement.Citation133
IgE/IL-5 pathway inhibition: omalizumab
Omalizumab is a recombinant humanized monoclonal antibody to immunoglobulin E, with specificity to selectively bind to free IgE and membrane-bound IgE on B cells. The role played by IgE in AD is unclear, with limited data to support this molecule as a major pathogenic effector.Citation113 In a literature review, Flohr et al estimated that only a third of patients with AD show true IgE sensitization.Citation134 However, given the central role of IgE in atopy and the efficacy of anti-IgE therapy in allergic asthma and allergic rhinitis, a small number of studies have been conducted to study the effect of omalizumab on chronic severe AD. In four case series and one case report, 30/35 patients (86%) with AD have shown improvement with omalizumab therapy.
In a prospective study of 21 patients (aged 14–64 years) with both AD and moderate-to-severe persistent allergic asthma, all showed statistically significant improvement of their skin disease, regardless of baseline serum IgE levels.Citation135 In a retrospective study, 5 of 7 patients treated with omalizumab for their asthma, had improvement in their concurrent AD by the third month of therapy.Citation136 In another retrospective report, 3/3 patients (aged 10–13 years) treated with omalizumab (every two weeks, doses up to 400 mg) for their severe eczema, showed improvement after 2–12 weeks.Citation137
A case report detailed improvement in the pruritus associated with chronic eczema in a 41 year old man treated with omalizumab 375 mg every 2 weeks for three months.Citation138 In contrast, none of three patients with severe AD (aged 34–48 years) treated with omalizumab (450 mg administered subcutaneously every other week) showed any improvement after 4 months of therapy, though concurrent asthma improved in one patient.Citation139
No significant adverse effects have been reported in the above studies. Surveillance of 39,510 patients taking omalizumab for all indications revealed a 0.09% rate of anaphylaxis, triggering a black-box warning on the label of this medication.Citation113
IgE/IL-5 pathway inhibition: mepolizumab
Mepolizumab is a humanized monoclonal anti-IL-5 antibody that has been studied for treatment of eosinophil-mediated disease such as asthma, AD, eosinophilic gastrointestinal disease, and hypereosinophilic syndrome. IL-5 enhances production and maturation of eosinophils, and blockade of IL-5 with mepolizumab inhibited infiltration of eosinophils into allergen-injected skin in 24 subjects with AD.Citation140 In a prospective trial, patients with severe AD were administered two doses of mepolizumab (750 mg intravenous, one week between doses, 18 subjects) or placebo (22 subjects).Citation141 Despite inducing a significant decrease in peripheral eosinophil levels, mepolizamab was associated with significant improvement in symptoms (by physican global assessment) in only 4/18 patients (22%) compared with 1/22 patients (4.6%) treated with placebo. Modest (<50%) improvement was seen in significantly more patients treated with mepolizumab (13/18, 72%) compared to placebo (9/22, 41%).Citation141 Only mild and temporary adverse effects were noted. It is possible that a longer trial would show increased benefit.
Other agents
Rituximab is a chimeric monoclonal antibody against CD20, a surface antigen on B lymphocytes. Binding of rituximab to its ligand induces cell lysis. It has been proposed that the complex immune dysregulation of AD may involve B cells directly or indirectly.Citation113 Six patients with severe atopic eczema received rituximab (1 g intravenous, two doses separated by two weeks) and all showed clinical improvement within 4 to 8 weeks.Citation142
Biologic therapy for AD: summary
In a 2009-published review of the available literature on the safety and efficacy of biologic agents for AD, Bremmer et al. identified no reported type-I immediate hypersensitivity reactions among 261 patients.Citation113 Regarding efficacy, the authors concluded that interferon-γ remains the agent with the best-proven efficacy, though its significant drawbacks (low percentage of responders, daily subcutaneous dosing, and high cost) preclude its widespread use.Citation113
Two notable events have occurred since that review. First, a biologic agent, efalizumab, which had been studied for possible efficacy in severe AD, has been removed from the market for safety concerns. Second, an additional randomized controlled trial showing efficacy of omalizumab in severe asthma-associated AD was published.Citation135 It is thus possible that this agent, or perhaps another with a similar (or different) mechanism of action, will find a place in the therapeutic armamentarium. Clearly, biologic agents are a part of a rapidly changing therapeutic landscape. As severe AD remains a disease without ideal management options, further trials of these medications are needed and warranted.
Ancillary therapies
Oral antihistamines are commonly used to manage the pruritus and sleeplessness associated with AD.Citation143,Citation144 Evidence supporting these medications as a primary treatment for AD is generally lacking, though they are widely used for symptom control and relief of underlying allergic conditions and urticaria.Citation144 Randomized controlled studies have supported the antipruritic effect of cetirizine, levocetirizine, fexofenadine, loratadine, and hydroxyzine in helping to control these symptoms in patients with AD.Citation145–Citation149 In contrast, chlorpheniramine and terfenadine were found to be ineffective at improving nocturnal itching and scratching behavior.Citation150,Citation151 In addition to antihistamine therapy, the leukeotriene receptor antagonist montelukast was found to be effective at relieving symptoms of AD in two trials.Citation152,Citation153
Secondary infections (often with Staphylococcus aureus) are common in patients with AD and may be associated with disease flares.Citation154 Treatment of secondary infection with measures including oral antibiotics (eg, cephalexin), topical antibiotics (mupirocin) and dilute bleach baths have been shown to decrease the clinical severity of eczema in patients with signs of secondary infection.Citation155
Novel therapies
A pilot study of 12 children with localized chronic atopic eczema demonstrated improvement in eczema severity after a single treatment with pulsed dye (595 nm) laser. The authors suggest that dermal vasculature (targeted by the laser energy at this wavelength) interacts with cutaneous immunity to impact eczema activity.Citation156
Conclusion
Chronic AD is a prevalent disease that presents with a wide range of severity and a tendency for periodic disease flares. As evidenced by this review, a broad number of treatment options are available. All cases will benefit from a gentle skin care regimen including mild cleansers and emollients. Most cases of AD will be adequately managed with topical therapy. Topical corticosteroids of the appropriate potency and duration remain front-line treatment, though many patients will benefit from intermittent use of topical calcineurin inhibitors. Persistent or severe cases may require periods of systemic therapy. It is important to carefully weigh the risks and benefits of any therapy. Optimal management will be tailored to the individual and will often involve multimodal strategies.
Disclosure
The authors report no conflicts of interest in this work.
References
- AbramovitsWAtopic dermatitisJ Am Acad Dermatol200553S86S9315968268
- WüthrichBClinical aspects, epidemiology, and prognosis of atopic dermatitisAnn Allergy Asthma Immunol19998346447010582732
- KangKPolsterAMNedorostSTStevensSRCooperKDAtopic DermatitisBologniaJJorizzoJRapiniRDermatologyPhiladelphiaMosby2003199214
- PalmerCNIrvineADTerron-KwiatkowskiACommon loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitisNat Genet20063844144616550169
- BrownSJIrvineADAtopic eczema and the filaggrin storySemin Cutan Med Surg20082712813718620134
- BrenninkmeijerEELegierseCMSillevis SmittJHLastBFGrootenhuisMABosJDThe course of life of patients with childhood atopic dermatitisPediatr Dermatol200926142219250399
- SimpsonELAtopic dermatitis: a review of topical treatment optionsCurr Med Res Opin20102663364020070141
- HonKLChingGKLeungTFChoiCYLeeKKNgPCEstimating emollient usage in patients with eczemaClin Exp Dermatol201035222619489850
- WirénKNohlgårdCNybergFTreatment with a barrier-strengthening moisturizing cream delays relapse of atopic dermatitis: a prospective and randomized controlled clinical trialJ Eur Acad Dermatol Venereol2009231267127219508310
- SzczepanowskaJReichASzepietowskiJCEmollients improve treatment results with topical corticosteroids in childhood atopic dermatitis: a randomized comparative studyPediatr Allergy Immunol20081961461818208463
- CorkMJBrittonJButlerLYoungSMurphyRKeohaneSGComparison of parent knowledge, therapy utilization and severity of atopic eczema before and after explanation and demonstration of topical therapies by a specialist nurseBr J Dermatol200314958258914510993
- SpergelJMImmunology and treatment of atopic dermatitisAm J Clin Dermatol2008923324418572974
- HanifinJMCooperKDHoVCGuidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (AAD)/American Academy of Dermatology Association “Administrative Regulations for Evidence-Based Clinical Practice Guidelines”J Am Acad Dermatol20045039140414988682
- ThomasKSArmstrongSAveryARandomised controlled trial of short bursts of a potent topical corticosteroid versus prolonged use of a mild preparation for children with mild or moderate atopic eczemaBMJ200232476811923161
- KantorICookPRCullenSIWillisIGibsonJRStanfieldJWDouble-blind bilateral paired comparison of 0.05% halobetasol propionate cream and its vehicle in patients with chronic atopic dermatitis and other eczematous dermatosesJ Am Acad Dermatol199125118411861757615
- GuzzoCAWeissJSMogaveroHSA review of two controlled multicenter trials comparing 0.05% halobetasol propionate ointment to its vehicle in the treatment of chronic eczematous dermatosesJ Am Acad Dermatol199125117911831757614
- MoshangTPrednicarbate emollient cream 0.1% in pediatric patients with atopic dermatitisCutis200168636911480151
- WilliamsHCEstablished corticosteroid creams should be applied only once daily in patients with atopic dermatitisBMJ2007334127217569936
- CallenJChamlinSEichenfieldLFA systematic review of the safety of topical therapies for atopic dermatitisBr J Dermatol200715620322117223859
- EichenfieldLFBasuSCalvareseBTrancikRJEffect of desonide hydrogel 0.05% on the hypothalamic-pituitary-adrenal axis in pediatric subjects with moderate to severe atopic dermatitisPediatr Dermatol20072428929517542883
- CoureauBBussièresJFTremblaySCushing’s syndrome induced by misuse of moderate- to high-potency topical corticosteroidsAnn Pharmacother2008421903190719017822
- SchlessingerJMillerBGilbertRDPlottRTVanos Study GroupAn open-label adrenal suppression study of 0.1% fluocinonide cream in pediatric patients with atopic dermatitisArch Dermatol20061421568157217178982
- FriedlanderSFHebertAAAllenDBFluticasone Pediatrics Safety Study GroupSafety of fluticasone propionate cream 0.05% for the treatment of severe and extensive atopic dermatitis in children as young as 3 monthsJ Am Acad Dermatol20024638739311862174
- LuckyAWGroteGDWilliamsJLEffect of desonide ointment, 0.05%, on the hypothalamic-pituitary-adrenal axis of children with atopic dermatitisCutis1997591511539071556
- Aalto-KorteKTurpeinenMBone mineral density in patients with atopic dermatitisBr J Dermatol19971361721759068726
- HaeckIMHamdyNATimmer-de MikLLow bone mineral density in adult patients with moderate to severe atopic dermatitisBr J Dermatol20091611248125419673879
- CharmanCRMorrisADWilliamsHCTopical corticosteroid phobia in patients with atopic eczemaBr J Dermatol200014293193610809850
- AndersonPCDinulosJGAtopic dermatitis and alternative management strategiesCurr Opin Pediatr2009221131138
- PittMGarsideRSteinKA cost-utility analysis of pimecrolimus vs topical corticosteroids and emollients for the treatment of mild and moderate atopic eczemaBr J Dermatol20061541137114616704646
- BeckLAThe efficacy and safety of tacrolimus ointment: a clinical reviewJ Am Acad Dermatol2005532 Suppl 2S165S17016021171
- El-BatawyMMBosseilaMAMashalyHMHafezVSTopical calcineurin inhibitors in atopic dermatitis: a systematic review and meta-analysisJ Dermatol Sci200954768719303745
- PallerAEichenfieldLFLeungDYStewartDAppellMA 12-week study of tacrolimus ointment for the treatment of atopic dermatitis in pediatric patientsJ Am Acad Dermatol200144S4744S57
- HanifinJMLingMRLangleyRBrenemanDRafalETacrolimus ointment for the treatment of atopic dermatitis in adult patients: part I, efficacyJ Am Acad Dermatol2001144S28S3811423833
- SoterNAFleischerABJrWebsterGFMonroeELawrenceITacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safetyJ Am Acad Dermatol2001144S39S4611423833
- ChapmanMSSchachnerLABrenemanDUS Tacrolimus Ointment Study GroupTacrolimus ointment 0.03% shows efficacy and safety in pediatric and adult patients with mild to moderate atopic dermatitisJ Am Acad Dermatol200553S177S18516021173
- HoegerPHLeeKHJautovaJThe treatment of facial atopic dermatitis in children who are intolerant of, or dependent on, topical corticosteroids: a randomized, controlled clinical trialBr J Dermatol200916041542219067708
- LangleyRGEichenfieldLFLuckyAWBoguniewiczMBarbierNCherillRSustained efficacy and safety of pimecrolimus cream 1% when used long-term (up to 26 weeks) to treat children with atopic dermatitisPediatr Dermatol20082530130718577032
- KangSLuckyAWPariserDLawrenceIHanifinJMLong-term safety and efficacy of tacrolimus ointment for the treatment of atopic dermatitis in childrenJ Am Acad Dermatol200144Suppl 1S58S6411145796
- ReitamoSWollenbergASchöpfESafety and efficacy of 1 year of tacrolimus ointment monotherapy in adults with atopic dermatitis. The European Tacrolimus Ointment Study GroupArch Dermatol2000136999100610926735
- ReitamoSRustinMRuzickaTEuropean Tacrolimus Ointment Study GroupEfficacy and safety of tacrolimus ointment compared with that of hydrocortisone butyrate ointment in adult patients with atopic dermatitisJ Allergy Clin Immunol200210954755511898005
- ReitamoSVan LeentEJHoVEuropean/Canadian Tacrolimus Ointment Study GroupEfficacy and safety of tacrolimus ointment compared with that of hydrocortisone acetate ointment in children with atopic dermatitisJ Allergy Clin Immunol200210953954611898004
- ReitamoSHarperJBosJDEuropean Tacrolimus Ointment Group0.03% Tacrolimus ointment applied once or twice daily is more efficacious than 1% hydrocortisone acetate in children with moderate to severe atopic dermatitis: results of a randomized double-blind controlled trialBr J Dermatol200415055456215030341
- DossNReitamoSDubertretLSuperiority of tacrolimus 0.1% ointment compared with fluticasone 0.005% in adults with moderate to severe atopic dermatitis of the face: results from a randomized, double-blind trialBr J Dermatol200916142743419416227
- LeungDYHanifinJMPariserDMEffects of pimecrolimus cream 1% in the treatment of patients with atopic dermatitis who demonstrate a clinical insensitivity to topical corticosteroids: a randomized, multicentre vehicle-controlled trialBr J Dermatol2009161435443 Epub 2009 Mar 30.19416245
- LugerTALahfaMFölster-HolstRLong-term safety and tolerability of pimecrolimus cream 1% and topical corticosteroids in adults with moderate to severe atopic dermatitisJ Dermatolog Treat20041516917815204150
- PallerASEichenfieldLFKirsnerRSShullTJaraczESimpsonELUS Tacrolimus Ointment Study GroupThree times weekly tacrolimus ointment reduces relapse in stabilized atopic dermatitis: a new paradigm for usePediatrics2008122e1210e121819015204
- BrenemanDFleischerABJrAbramovitsWTacrolimus Ointment Study GroupIntermittent therapy for flare prevention and long-term disease control in stabilized atopic dermatitis: a randomized comparison of 3-times-weekly applications of tacrolimus ointment versus vehicleJ Am Acad Dermatol20085899099918359127
- AbramovitsWFleischerABJrJaraczEBrenemanDAdult patients with moderate atopic dermatitis: tacrolimus ointment versus pimecrolimus creamJ Drugs Dermatol200871153115819137769
- StanderSStanderHSeelingerSLugerTASteinhoffMTopical pimecrolimus and tacrolimus transiently induce neuropeptide release and mast cell degranulation in murine skinBr J Dermatol20071561020102617388925
- LingMGottliebAPariserDA randomized study of the safety, absorption and efficacy of pimecrolimus cream 1% applied twice or four times daily in patients with atopic dermatitisJ Dermatolog Treat20051614214816096179
- BillichAAschauerHAszódiAStuetzAPercutaneous absorption of drugs used in atopic eczema: pimecrolimus permeates less through skin than corticosteroids and tacrolimusInt J Pharm2004269293514698574
- BergerTGDuvicMVan VoorheesASVanBeekMJFriedenIJThe use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task ForceJ Am Acad Dermatol20065481882316635663
- HuiRLLideWChanJSchottingerJYoshinagaMMillaresMAssociation between exposure to topical tacrolimus or pimecrolimus and cancersAnn Pharmacother2009431956196319903860
- SladeHBFowlerJDraelosZDReeceBTCargillDIClinical efficacy evaluation of a novel barrier protection creamCutis20081082212819202673
- AbramovitsWPerlmutterAMimyX creamSkinmed20065293016522980
- BelloniGPinelliSVeraldiSA randomised, double-blind, vehicle-controlled study to evaluate the efficacy and safety of MAS063D (Atopiclair) in the treatment of mild to moderate atopic dermatitisEur J Dermatol200515313615701590
- AbramovitsWBoguniewiczMAdult Atopiclair Study GroupA multicenter, randomized, vehicle-controlled clinical study to examine the efficacy and safety of MAS063DP (Atopiclair) in the management of mild to moderate atopic dermatitis in adultsJ Drugs Dermatol2006523624416573256
- PatriziACapitanioBNeriIA double-blind, randomized, vehicle-controlled clinical study to evaluate the efficacy and safety of MAS063DP (ATOPICLAIR) in the management of atopic dermatitis in paediatric patientsPediatr Allergy Immunol20081961962518298424
- BoguniewiczMZeichnerJAEichenfieldLFMAS063DP is effective monotherapy for mild to moderate atopic dermatitis in infants and children: a multicenter, randomized, vehicle-controlled studyJ Pediatr200815285485918492531
- SugarmanJLParishLCEfficacy of a lipid-based barrier repair formulation in moderate-to-severe pediatric atopic dermatitisJ Drugs Dermatol200981106111120027938
- EberleinBEickeCReinhardtHWRingJAdjuvant treatment of atopic eczema: assessment of an emollient containing N-palmitoylethanolamine (ATOPA study)J Eur Acad Dermatol Venereol2008122738218705632
- CaproniMTorchiaDAntigaEThe comparative effects of tacrolimus and hydrocortisone in adult atopic dermatitis: an immunohistochemical studyBr J Dermatol20072156312319
- NorrisDAMechanisms of action of topical therapies and the rationale for combination therapyJ Am Acad Dermatol200553S17S2515968260
- PesericoAStädtlerGSebastianMFernandezRSVickKBieberTReduction of relapses of atopic dermatitis with methylprednisolone aceponate cream twice weekly in addition to maintenance treatment with emollient: a multicentre, randomized, double-blind, controlled studyBr J Dermatol200815880180718284403
- TorokHMMaas-IrslingerRSlaytonRMClocortolone pivalate cream 0.1% used concomitantly with tacrolimus ointment 0.1% in atopic dermatitisCutis20037216116612953943
- KubotaYYonedaKNakaiKEffect of sequential applications of topical tacrolimus and topical corticosteroids in the treatment of pediatric atopic dermatitis: an open-label pilot studyJ Am Acad Dermatol20096021221719027990
- OranjeAPDevillersACKunzBTreatment of patients with atopic dermatitis using wet-wrap dressings with diluted steroids and/or emollients. An expert panel’s opinion and review of the literatureJ Eur Acad Dermatol Venereol2006201277128617062046
- HindleyDGallowayGMurrayJGardenerLA randomised study of “wet wraps” versus conventional treatment for atopic eczemaArch Dis Child20069116416816308411
- PeiAYChanHHHoKMThe effectiveness of wet wrap dressings using 0.1% mometasone furoate and 0.005% fluticasone proprionate ointments in the treatment of moderate to severe atopic dermatitis in childrenPediatr Dermatol20011834334811576413
- McGowanRTuckerPJosephDShort-term growth and bone turnover in children undergoing occlusive steroid (‘Wet-Wrap’) dressings for treatment of atopic eczemaJ Dermatolog Treat20031414915214522624
- Krejci-ManwaringJTusaMGCarrollCStealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitisJ Am Acad Dermatol20075621121617224366
- MajoieIMOldhoffJMvan WeeldenHNarrowband ultraviolet B and medium-dose ultraviolet A1 are equally effective in the treatment of moderate to severe atopic dermatitisJ Am Acad Dermatol200960778419103360
- JeklerJLarköOPhototherapy for atopic dermatitis with ultraviolet A (UVA), low-dose UVB and combined UVA and UVB: two paired-comparison studiesPhotodermatol Photoimmunol Photomed199181511561814425
- TzanevaSSeeberASchwaigerMHönigsmannHTanewAHigh-dose versus medium-dose UVA1 phototherapy for patients with severe generalized atopic dermatitisJ Am Acad Dermatol20014550350711568738
- AbeckDSchmidtTFesqHLong-term efficacy of medium-dose UVA1 phototherapy in atopic dermatitisJ Am Acad Dermatol2000422 Pt 125425710642681
- Grundmann-KollmannMBehrensSPoddaMPeterRUKaufmannRKerscherMPhototherapy for atopic eczema with narrow-band UVBJ Am Acad Dermatol19994099599710365933
- ReynoldsNJFranklinVGrayJCDiffeyBLFarrPMNarrow-band ultraviolet B and broad-band ultraviolet A phototherapy in adult atopic eczema: a randomised controlled trialLancet20013572012201611438134
- Der-PetrossianMSeeberAHönigsmannHTanewAHalf-side comparison study on the efficacy of 8-methoxypsoralen bath-PUVA versus narrow-band ultraviolet B phototherapy in patients with severe chronic atopic dermatitisBr J Dermatol2000142394310651692
- GambichlerTOthlinghausNTomiNSMedium-dose ultraviolet (UV) A1 vs narrowband UVB phototherapy in atopic eczema: a randomized crossover studyBr J Dermatol200916065265819120333
- KrutmannJDiepgenTLLugerTAHigh-dose UVA1 therapy for atopic dermatitis: results of a multicenter trialJ Am Acad Dermatol1998385895939555799
- GeorgeSABilslandDJJohnsonBEFergusonJNarrow-band (TL-01) UVB air-conditioned phototherapy for chronic severe adult atopic dermatitisBr J Dermatol199312849568427822
- SandMBecharaFGSandDExtracorporeal photopheresis as a treatment for patients with severe, refractory atopic dermatitisDermatology200721513413817684376
- MeduriNBVandergriffTRasmussenHJacobeHPhototherapy in the management of atopic dermatitis: a systematic reviewPhotodermatol Photoimmunol Photomed20072310611217598862
- GambichlerTBreuckmannFBomsSAltmeyerPKreuterANarrowband UVB phototherapy in skin conditions beyond psoriasisJ Am Acad Dermatol20055266067015793518
- ten BergeOvan WeeldenHBruijnzeel-KoomenCAde Bruin-WellerMSSigurdssonVThrowing a light on photosensitivity in atopic dermatitis: a retrospective studyAm J Clin Dermatol20091011912319222251
- WeischerMBlumAEberhardFRöckenMBerneburgMNo evidence for increased skin cancer risk in psoriasis patients treated with broadband or narrowband UVB phototherapy: a first retrospective studyActa Derm Venereol20048437037415370703
- SchmittJSchäkelKSchmittNMeurerMSystemic treatment of severe atopic eczema: a systematic reviewActa Derm Venereol20078710011117340015
- WallingHWGeramiPSontheimerRDJuvenile-onset clinically amyopathic dermatomyositis: an overview of recent progress in diagnosis and managementPaediatr Drugs201012233420034339
- RicciGDondiAPatriziAMasiMSystemic therapy of atopic dermatitis in childrenDrugs20096929730619275273
- GalliEChiniLMoscheseVMethylprednisolone bolus: a novel therapy for severe atopic dermatitisActa Paediatr1994833153178038536
- ForteWCSumitaJMRodriguesAGLiusonDTanakaERebound phenomenon to systemic corticosteroid in atopic dermatitisAllergol Immunopathol (Madr)20053330731116371217
- SalekMSFinlayAYLuscombeDKCyclosporin greatly improves the quality of life of adults with severe atopic dermatitis. A randomized, double-blind, placebo-controlled trialBr J Dermatol19931294224308217757
- SchmittJSchäkelKFölster-HolstRPrednisolone vs ciclosporin for severe adult eczema. An investigator-initiated double-blind placebo-controlled multicentre trialBr J Dermatol20091026 (Epub)
- SchmittJSchmittNMeurerMCyclosporin in the treatment of patients with atopic eczema – a systematic review and meta-analysisJ Eur Acad Dermatol Venereol20072160661917447974
- PedreiraCCKingEJonesGOral cyclosporin plus topical corticosteroid therapy diminishes bone mass in children with eczemaPediatr Dermatol20072461362018035982
- GranlundHErkkoPReitamoSLong-term follow-up of eczema patients treated with cyclosporineActa Derm Venereol19987840439498025
- HijnenDJten BergeOTimmer-de MikLBruijnzeel-KoomenCAde Bruin-WellerMSEfficacy and safety of long-term treatment with cyclosporin A for atopic dermatitisJ Eur Acad Dermatol Venereol200721858917207173
- WeatherheadSCWahieSReynoldsNJMeggittSJAn open-label, dose-ranging study of methotrexate for moderate-to-severe adult atopic eczemaBr J Dermatol200715634635117223876
- GoujonCBérardFDahelKMethotrexate for the treatment of adult atopic dermatitisEur J Dermatol20061615515816581567
- LyakhovitskyABarzilaiAHeymanRLow-dose methotrexate treatment for moderate-to-severe atopic dermatitis in adultsJ Eur Acad Dermatol Venereol201024434919552716
- ZollerLRamonMBergmanRLow dose methotrexate therapy is effective in late-onset atopic dermatitis and idiopathic eczemaIsr Med Assoc J20081041341418669134
- ShaffraliFCColverGBMessengerAGGawkrodgerDJExperience with low-dose methotrexate for the treatment of eczema in the elderlyJ Am Acad Dermatol20034841741912637922
- MeggittSJGrayJCReynoldsNJAzathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trialLancet200636783984616530578
- HughesRCollinsPRogersSFurther experience of using azathioprine in the treatment of severe atopic dermatitisClin Exp Dermatol2008337101118681884
- MurphyLAAthertonDA retrospective evaluation of azathioprine in severe childhood atopic eczema, using thiopurine methyltransferase levels to exclude patients at high risk of myelosuppressionBr J Dermatol200214730831512174104
- NeuberKSchwartzIItschertGDieckATTreatment of atopic eczema with oral mycophenolate mofetilBr J Dermatol200014338539110951150
- Grundmann-KollmannMPoddaMOchsendorfFBoehnckeWHKaufmannRZollnerTMMycophenolate mofetil is effective in the treatment of atopic dermatitisArch Dermatol200113787087311453805
- MurrayMLCohenJBMycophenolate mofetil therapy for moderate to severe atopic dermatitisClin Exp Dermatol200732232717059445
- HellerMShinHTOrlowSJSchafferJVMycophenolate mofetil for severe childhood atopic dermatitis: experience in 14 patientsBr J Dermatol200715712713217489974
- WozelGVitézLPfeifferCSevere atopic dermatitis and leflunomide: first clinical experience and highlights of pertinent experimental dataDermatol Online J200633012616638420
- SchmittJWozelGPfeifferCLeflunomide as a novel treatment option in severe atopic dermatitisBr J Dermatol20041501182118515214907
- Van VelsenSGHaeckIMBruijnzeel-KoomenCASevere atopic dermatitis treated with everolimusJ Dermatolog Treat20092036536719954394
- BremmerMSBremmerSFBaig-LewisSSimpsonELAre biologics safe in the treatment of atopic dermatitis? A review with a focus on immediate hypersensitivity reactionsJ Am Acad Dermatol200961666676 Epub 2009 Jul 31. PMID: 1964677919646779
- TorreloAHartoASendagortaECzarnetzkiBMLedoAInterferon-alpha therapy in atopic dermatitisActa Derm Venereol1992723703721361287
- HanifinJMSchneiderLCLeungDYRecombinant interferon gamma therapy for atopic dermatitisJ Am Acad Dermatol1993281891978432915
- ReinholdUKukelSBrzoskaJKreyselHWSystemic interferon gamma treatment in severe atopic dermatitisJ Am Acad Dermatol19932958638315079
- SchneiderLCBazZZarconeCZurakowskiDLong-term therapy with recombinant interferon-gamma (rIFN-gamma) for atopic dermatitisAnn Allergy Asthma Immunol1998802632689532976
- JangIGYangJKLeeHJClinical improvement and immunohistochemical findings in severe atopic dermatitis treated with interferon gammaJ Am Acad Dermatol2000421033104010827410
- JullienDNicolasJFFrappazAThivoletJAlpha interferon treatment in atopic dermatitisActa Derm Venereol1993731301328103259
- NielsenBWReimertCMHammerRSchiøtzPOThestrup-PedersenKInterferon therapy for atopic dermatitis reduces basophil histamine release, but does not reduce serum IgE or eosinophilic proteinsAllergy1994491201287513506
- NohGWLeeKYBlood eosinophils and serum IgE as predictors for prognosis of interferon-gamma therapy in atopic dermatitisAllergy199853120212079930598
- WakimMAlazardMYajimaASpeightsDSaxonAStiehmERHigh dose intravenous immunoglobulin in atopic dermatitis and hyper-IgE syndromeAnn Allergy Asthma Immunol1998811531589723561
- JollesSHughesJRustinMThe treatment of atopic dermatitis with adjunctive high-dose intravenous immunoglobulin: a report of three patients and review of the literatureBr J Dermatol200014255155410735971
- HuangJLLeeWYChenLCKuoMLHsiehKHChanges of serum levels of interleukin-2, intercellular adhesion molecule-1, endothelial leukocyte adhesion molecule-1 and Th1 and Th2 cell in severe atopic dermatitis after intravenous immunoglobulin therapyAnn Allergy Asthma Immunol20008434535210752921
- JollesSA review of high-dose intravenous immunoglobulin treatment for atopic dermatitisClin Exp Dermatol20021273711952664
- PaulCLahfaMBachelezHChevretSDubertretLA randomized controlled evaluator-blinded trial of intravenous immunoglobulin in adults with severe atopic dermatitisBr J Dermatol200214751852212207594
- JollesSSewellCWebsterDAdjunctive high-dose intravenous immunoglobulin treatment for resistant atopic dermatitis: efficacy and effects on intracellular cytokine levels and CD4 countsActa Derm Venereol20038343343714690338
- BemanianMHMovahediMFarhoudiAHigh doses intravenous immunoglobulin versus oral cyclosporine in the treatment of severe atopic dermatitisIran J Allergy Asthma Immunol2005413914317301437
- JacobiAAntoniCMangerBSchulerGHertlMInfliximab in the treatment of moderate to severe atopic dermatitisJ Am Acad Dermatol20055252252615761436
- RullanPMuraseJTwo cases of chronic atopic dermatitis treated with soluble tumor necrosis factor receptor therapyJ Drugs Dermatol2009887387619746681
- BukaRLReshBRobertsBCunninghamBBFriedlanderSEtanercept is minimally effective in 2 children with atopic dermatitisJ Am Acad Dermatol20055335835916021144
- EsmailzadehAYousefiPFarhiDPredictive factors of eczema-like eruptions among patients without cutaneous psoriasis receiving infliximab: a cohort study of 92 patientsDermatology200921926326719684381
- MoulDKRouthouskaSBRobinsonMRKormanNJAlefacept for moderate to severe atopic dermatitis: a pilot study in adultsJ Am Acad Dermatol20085898498918395294
- FlohrCJohanssonSGWahlgrenCFWilliamsHHow atopic is atopic dermatitis?J Allergy Clin Immunol200411415015815241359
- SheinkopfLERafiAWDoLTKatzRMKlaustermeyerWBEfficacy of omalizumab in the treatment of atopic dermatitis: a pilot studyAllergy Asthma Proc20082953053718926061
- VigoPGGirgisKRPfuetzeBLCritchlowMEFisherJHussainIEfficacy of anti-IgE therapy in patients with atopic dermatitisJ Am Acad Dermatol20065516817016781320
- LaneJECheyneyJMLaneTNKentDECohenDJTreatment of recalcitrant atopic dermatitis with omalizumabJ Am Acad Dermatol2006546872 Epub 2005 Nov 28.16384758
- FormanSBGarrettABSuccess of omalizumab as monotherapy in adult atopic dermatitis: case report and discussion of the high-affinity immunoglobulin E receptor, FcepsilonRICutis200780384017725062
- KrathenRAHsuSFailure of omalizumab for treatment of severe adult atopic dermatitisJ Am Acad Dermatol20055333834016021135
- PhippsSFlood-PagePMenzies-GowAOngYEKayABIntravenous anti-IL-5 monoclonal antibody reduces eosinophils and tenascin deposition in allergen-challenged human atopic skinJ Invest Dermatol20041221406141215175031
- OldhoffJMDarsowUWerfelTAnti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitisAllergy20056069369615813818
- SimonDHösliSKostylinaGYawalkarNSimonHUAnti-CD20 (rituximab) treatment improves atopic eczemaJ Allergy Clin Immunol200812112212818206507
- BorchardKLOrchardDSystemic therapy of paediatric atopic dermatitis: an updateAustralas J Dermatol20084912313418638218
- HermanSMVenderRBAntihistamines in the treatment of dermatitisJ Cutan Med Surg2003746747315926215
- SimonsFEProspective, long-term safety evaluation of the H1-receptor antagonist cetirizine in very young children with atopic dermatitis. ETAC Study Group. Early Treatment of the Atopic ChildJ Allergy Clin Immunol19991042 Pt 143344010452767
- SimonsFEEarly Prevention of Asthma in Atopic Children (EPAAC) Study GroupSafety of levocetirizine treatment in young atopic children: An 18-month studyPediatr Allergy Immunol20071853554217561929
- MonroeEWRelative efficacy and safety of loratadine, hydroxyzine, and placebo in chronic idiopathic urticaria and atopic dermatitisClin Ther19921417211349509
- KawashimaMTangoTNoguchiTInagiMNakagawaHHaradaSAddition of fexofenadine to a topical corticosteroid reduces the pruritus associated with atopic dermatitis in a 1-week randomized, multicentre, double-blind, placebo-controlled, parallel-group studyBr J Dermatol20031481212122112828751
- DiepgenTLEarly Treatment of the Atopic Child Study Group. Long-term treatment with cetirizine of infants with atopic dermatitis: a multi-country, double-blind, randomized, placebo-controlled trial (the ETAC trial) over 18 monthsPediatr Allergy Immunol20021327828612390444
- MundayJBloomfieldRGoldmanMChlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching componentDermatology2002205404512145433
- Berth-JonesJGraham-BrownRAFailure of terfenadine in relieving the pruritus of atopic dermatitisBr J Dermatol19891216356372574594
- EhlayelMSBenerASabbahAMontelukast treatment in children with moderately severe atopic dermatitisEur Ann Allergy Clin Immunol20073923223618236999
- CapellaGLGrigerioEAltomareGA randomized trial of leukotriene receptor antagonist montelukast in moderate-to-severe atopic dermatitis of adultsEur J Dermatol20011120921311358726
- GongJQLinLLinTSkin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trialBr J Dermatol200615568068716965415
- HuangJTAbramsMTlouganBRademakerAPallerASTreatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severityPediatrics2009123e808e81419403473
- SyedSWeibelLKennedyHHarperJIA pilot study showing pulsed-dye laser treatment improves localized areas of chronic atopic dermatitisClin Exp Dermatol20083324324818201257