Abstract
Although proton pump inhibitors (PPI) have a record of remarkable effectiveness and safety in the management of gastroesophageal reflux disease (GERD), several treatment challenges with PPI have emerged. Dexlansoprazole MR is the (R)-enantiomer of lansoprazole contained in a formulation that produces two distinct releases of drug and significantly extends the duration of active plasma concentrations and % time pH > 4 beyond that of conventional single-release PPI. Dexlansoprazole MR can be administered without regard to meals or the timing of meals in most patients. Dexlansoprazole MR 60 mg demonstrated similar efficacy for healing of erosive esophagitis at 8 weeks compared with lansoprazole 30 mg, and dexlansoprazole MR 30 mg was superior to placebo for maintenance of healed erosive esophagitis at 6 months with 99% of nights and 96% of days heartburn-free over 6 months in patients taking dexlansoprazole MR 30 mg. Superior relief of heartburn occurred in patients taking dexlansoprazole MR 30 mg (55% heartburn-free 24-hour periods) vs placebo (14%) for symptomatic nonerosive GERD. The safety profile of dexlansoprazole MR is similar to that of lansoprazole. The extended pharmacodynamic effects, added convenience, and efficacy and safety of dexlansoprazole MR offer a novel approach to gastric pH control in patients with acid-related disorders.
Gastroesophageal reflux disease (GERD) is a clinical condition characterized by persistent retrograde movement of gastric contents into the esophagus that typically manifests as burning retrosternal pain and/or regurgitation. Atypical symptoms of GERD have been described and include chronic cough, vocal hoarseness, globus, waterbrash, and throat pain.Citation1 Pharmacologic treatment options for GERD have been directed at suppression of gastric acid production in order to reduce both volume and acidity of gastric contents. Antisecretory agents employed for the treatment of GERD include the histamine-2 receptor antagonists (H2RA) and proton pump inhibitors (PPI). H2RA possess a rapid onset of symptom control and effectively inhibit acid production; however, their use is limited by their brief duration of action and tachyphylaxis possibly owing to histamine-2 receptor up-regulation and enhanced gastrin secretion in the presence of histamine blockade.Citation2 In contrast, PPI block the terminal step of acid production via covalent and irreversible binding of the protonated moiety of the PPI to cysteine residues on the proton pump, thereby rendering it nonfunctional, and its activity cannot be replaced until a new proton pump is synthesized. Only active proton pumps are available to be inhibited by PPI, and activation is most commonly achieved after ingestion of food. Pentagastrin has also been used experimentally to induce proton pump activation.Citation3
A model of proton pump inhibition advanced by Sachs proposed that two-thirds of activated proton pumps are inhibited by PPI which leaves up to one-third of pumps uninhibited and able to secrete acid.Citation4 In addition, not all proton pumps are activated by a meal (approximately 75%), and it is believed that subsequent food intake permits activation of dormant pumps which also contributes to acid production. Since all PPI share the same mechanism of action and have inherently brief half-lives (approximately 1–2 hours), the potential for activation of proton pumps and acid secretion exists after their plasma concentrations diminish to subtherapeutic levels. It is important to also note that proton pumps are continuously being regenerated and the entire population of pumps within the parietal cell will typically experience turnover every 48 hours.Citation4,Citation5 Because food is the primary stimulus for proton pump activation, administration of PPI is commonly recommended a short time (<60 minutes) before the morning meal, thereby ensuring subsequent daytime reduction in basal and meal-stimulated acid production.
While PPI have a nearly 20-year record of remarkable effectiveness and safety in the management of GERD, several treatment challenges with PPI have emerged. Symptoms of GERD have been reported to persist in between 25 and 40% of patients who take PPI for the treatment of erosive esophagitis.Citation6 In particular, nocturnal symptoms may predominate in such patients due to persistent or de novo proton pump activity. The effectiveness of PPI for the treatment of moderate-to-severe erosive esophagitis (LA Classification C and D) is less than complete for up to 25% of patients.Citation7 Even in those who experience complete healing of erosive esophagitis, disease relapse rates of up to 26% have been described in patients who continue PPI therapy.Citation8,Citation9 More than three-fourths of patients with recurrent erosive esophagitis are asymptomatic.Citation9 Relapse is more precipitous for more severe grades of erosive disease (occurring as rapidly as 1 month post-discontinuation of medication), but all grades tend to have similarly limited durability of healing maintenance at 6 months.Citation8 This finding has led the Cochrane Group to recommend full healing doses of PPI for maintenance of erosive esophagitis healing.Citation10 Twice daily off-label administration of PPI is used to remedy the inadequacy of PPI effectiveness in nearly one-third of patients with GERD,Citation11 especially in order to improve overall symptom control and relief of nocturnal heartburn. However, the consequence of this practice is increased cost of treatment and decreased compliance. Good compliance with PPI (defined as ≥80% fill rate of prescriptions written for PPI) resulted in significantly decreased use of the health care system and lower health care costs in GERD patients.Citation12 As a result, perhaps one of the most important drivers of PPI effectiveness is patient adherence to therapy regimens. Several barriers to full adherence have been reported. Long-term adherence to once-daily PPI has been shown to decrease rapidly over time to approximately 50% of patients reporting low or moderate adherence within 3 months of initiation which suggests significant intermittent or as-needed use.Citation13 In addition, the need to take each dose within 60 minutes prior to food intake (preferably a full meal in the morning) is problematic for many patients who do not eat in the morning or who take their dose during or shortly after a meal. A survey of patients taking PPI who experienced suboptimal benefit revealed that 54% of this group was taking their doses incorrectly with approximately equal numbers taking the dose on an empty stomach (>60 minutes before a meal), immediately after ingestion of food, or at bedtime (presumably without subsequent food intake).Citation14
Prescribing patterns of PPI are reportedly inconsistent with the recommendations of treatment guidelines and product labeling with more than one-third of primary care providers in one survey responding that the time of administration of PPI does not matter, and as many of 29% of gastroenterologists failing to address time of administration.Citation15 The clinical shortcomings of PPI and the barriers to patient adherence to therapy have created an unmet medical need in the practice of GERD management. The ideal product to address these concerns would possess efficacy for erosive and nonerosive GERD consistent with the excellent record of other PPI, provide extended duration of active drug concentrations throughout the day to inhibit proton pumps activated by subsequent meals or that are generated later in the dosing interval, be administered once daily without regard to food intake, and maintain the safety and tolerability of the PPI class.
Dexlansoprazole MR: product review
Lansoprazole is a racemic mixture composed of equal proportions (50:50) of (R)-lansoprazole (also known as dexlansoprazole) and (S)-lansoprazole. These two enantiomers have been quantified separately in blood after ingestion of lansoprazole 30 mg in healthy volunteers and it was found that the mean maximum plasma concentration (Cmax) and area under the plasma drug concentration-time curve (AUC) values were 3- to 5-fold greater for dexlansoprazole than (S)-lansoprazole.Citation16 This suggests that the hepatic clearance of lansoprazole is stereoselective in favor of the (S) enantiomer leading to higher systemic exposure of and in vivo residence for dexlansoprazole as compared to its antipode, (S)-lansoprazole. Dexlansoprazole is highly bound to plasma proteins (96.1%–98.8% bound) and has an apparent volume of distribution of 40.3 L in subjects with GERD.Citation17 The elimination of dexlansoprazole is via the hepatic route; biotransformation to oxidative metabolites occurs via CYP2C19 and CYP3A4 with subsequent conjugation to inactive products and elimination in the urine and feces. In vitro data suggest that CYP2C19 displays more specificity for R- than S-lansoprazole, and that CYP3A4 is more specific for S-lansoprazole.Citation18 Dexlansoprazole does not appear to be eliminated unchanged in the urine.
The elimination half-life of dexlansoprazole is approximately 1–2 hours in healthy subjects and in patients with symptomatic GERD; this is similar to other PPI. The Dual Delayed Release formulation (DDR™) employed in delivering dexlansoprazole is a more significant factor in prolonging drug residence time in the body after oral administration than the inherently slower clearance of dexlansoprazole as compared to the (S)-enantiomer. The DDR formulation delivers 2 drug inputs in the proximal and more distal small intestine. Distinct pH-dependent releases of drug are designed to occur from two types of enteric-coated granules housed in a gelatin capsule. Upon dissolution of the outer capsule in the stomach, the first type of granule is designed to release quickly after the granules reach the proximal duodenum providing an initial drug release profile similar to that of lansoprazole and resulting in an initial peak in plasma dexlansoprazole concentrations within 1 to 2 hours of capsule ingestion. The second release from the remaining granules is designed to release farther along the gastrointestinal tract at the distal portion of the small intestine and creates a second drug peak in plasma dexlansoprazole concentrations within 4 to 5 hours of capsule ingestion. The purpose of the second release is to provide a greater amount of drug to be absorbed later in the dosing interval in order to provide extended duration of acid suppression. Therefore, the resulting time-concentration profile of dexlansoprazole MR reveals a two-peaked pattern that extends to approximately 12 hours after a dose is ingested ().
Figure 1 Mean time-concentration profiles of dexlansoprazole MR on Day 5. Adapted by permission from Informa Healthcare Vakily M, Zhang W, Wu J, Atkinson SN, Mulford D. Pharmacokinetics and pharmacodynamics of a known active PPI with a novel dual delayed release technology, dexlansoprazole MR: a combined analysis of randomized controlled clinical trials. Curr Med Res Opin. 2009;25(3):627–638.Citation19 Copyright © 2009.
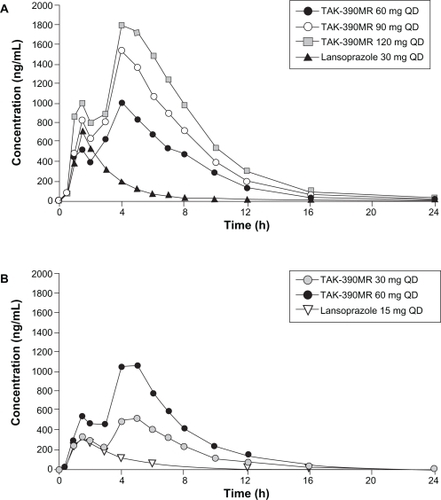
The relationship between exposure of dexlansoprazole following administration of dexlansoprazole MR and its pharmacodynamic effect measured as intragastric pH has been described using an Emax model.Citation19 A total of 83 healthy subjects met the entry criteria for 2 studies, and were included in this combined analysis. Subjects were administered 30, 60, 90, and 120 mg of dexlansoprazole MR in randomized crossover fashion in these two separate studies. The systemic exposure of dexlansoprazole measured as Cmax and AUC values was dose-proportional and time-independent. These two pharmacokinetic and pharmacodynamic studies confirmed that the DDR™ technology used in the dexlansoprazole MR formulation prolonged drug exposure; pharmacokinetic and pharmacodynamic modeling suggested that doses lower than 30 mg may result in therapeutically suboptimal intragastric pH control. Furthermore, it was demonstrated that doses higher than 90 mg would be unlikely to provide additional clinically meaningful pharmacologic response.
In a retrospective analysis using data from 2 separate but similarly designed studies the pharmacokinetic profiles of dexlansoprazole MR 60 mg and lansoprazole 60 mg were compared after 5 days of dosing in healthy volunteers, demonstrating that the tmax for both regimens occurred 1 to 2 hours after administration, and that the second peak for dexlansoprazole MR occurred 4 to 5 hours after administration.Citation20 The results from this single post-hoc analysis also showed that the mean residence time for dexlansoprazole MR was nearly double that of lansoprazole at equivalent doses of 60 mg once daily (5.5 hours vs 2.9 hours, respectively).Citation21
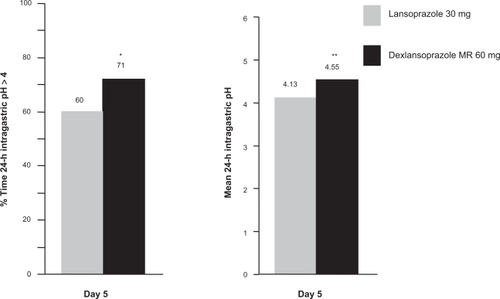
The pharmacokinetics, pharmacodynamics, and safety of three dosing regimens of dexlansoprazole MR (60, 90, and 120 mg) and lansoprazole 30 mg were assessed in an open-label, multiple-dose, single-center, four-period, crossover study in 40 subjects.Citation22 After 5 days of once daily administration dexlansoprazole MR 60 mg produced statistically significantly greater mean 24-hour intragastric pH compared to lansoprazole 30 mg (4.55 vs 4.13, respectively, P < 0.001); a statistically significant increase in % time 24-hour intragastric pH > 4 was also observed (71% vs 60%, respectively, P < 0.01) (). The 90 mg dose of dexlansoprazole MR produced 24-hour intragastric pH > 4 for 70% of the time. The pharmacodynamic effect of dexlansoprazole MR 120 mg was similar to that of the 90 mg dose. As a result, the 120 mg dose was not pursued for clinical development. The clinical significance of these differences remains unknown, but the DDR™ formulation of dexlansoprazole MR appears to provide pharmacodynamic benefit beyond that of lansoprazole most likely due to the extended duration of effective plasma concentration.
Figure 2 Mean % time intragastric pH > 4 and mean 24-hour intragastric pH with dexlansoprazole MR 60 mg vs lansoprazole 30 mg on Day 5.Citation19
*P < 0.01, **P < 0.001.
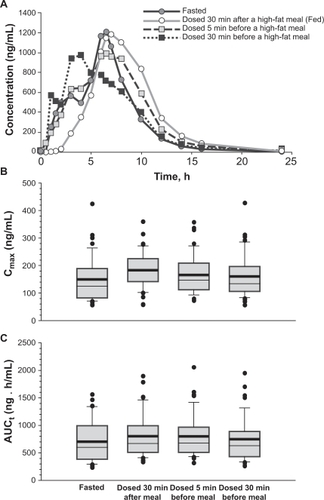
The impact of food on the pharmacokinetics and pharmacodynamics of dexlansoprazole MR was evaluated in 46 healthy subjects who completed all dosing regimens in a randomized, 4-period, open-label, crossover study.Citation23 Placebo was administered in 4 regimens: after a 10-hour fast, 30 minutes before, 5 minutes before, or 30 minutes after a high-fat breakfast on Day 1; dexlansoprazole MR 90 mg was administered in the same fashion for each crossover period on Day 3. Plasma concentrations of dexlansoprazole were measured on Day 3 and 24-hour intragastric pH was assessed on Days 1 and 3. Pharmacokinetics of dexlansoprazole in the fed conditions (administered 5 minutes before and 30 minutes after a high-fat breakfast) when compared to the fasted state displayed at least a 1.09-fold greater increase (using the point estimates) in Cmax and AUC for the fed state (). Thus, the bioavailability was increased in the fed vs fasted state. The data also showed that the systemic exposure of dexlansoprazole after dexlansoprazole MR was administered 30 minutes before a high-fat breakfast was bioequivalent to that obtained following administration of dexlansoprazole MR under fasted state. The differences in the pharmacodynamic parameters measured as mean 24-hour intragastric pH and % time 24-hour intragastric pH > 4 were not considered to be clinically meaningful between any of the periods which signified both a lack of food effect and a lack of effect of timing of food intake relative to dosing with dexlansoprazole MR on intragastric pH profile.
Figure 3 Mean dexlansoprazole plasma concentration-time profiles A), Cmax B), and AUC C) following a single dose of dexlansoprazole MR 90 mg under fasted and various fed conditions. Reproduced with permission from Lee RD, Vakily M, Mulford D, Wu J, Atkinson SN. Clinical trial: the effect and timing of food on the pharmacokinetics and pharmacodynamics of dexlansoprazole MR, a novel dual delayed release formulation of a proton pump inhibitor – evidence for dosing flexibility. Aliment Pharmacol Ther. 2009; 29(8):824–833.Citation23 Copyright © 2009 Wiley-Blackwell.
Because PPI are traditionally administered before the morning meal, it is important to determine if a PPI with extended release properties such as dexlansoprazole MR can be taken at different times during the day which may offer greater dosing flexibility. The influence of time of day of dexlansoprazole MR administration on pharmacokinetic and pharmacodynamic variables was assessed in 44 healthy subjects who completed all regimens in a 4-period, randomized, crossover fashion in which drug was administered daily for five days 30 minutes before breakfast, lunch, dinner, or a bedtime snack. Plasma drug concentrations and 24-hour intragastric pH were assessed on Day 5 of each period.Citation24 Systemic exposure of dexlansoprazole when dosed before breakfast was bioequivalent when dosed before lunch, dinner or an evening snack, and minimal but statistically significant differences were found in mean 24-hour intragastric pH between dosing at breakfast and at lunch (0.2 difference in pH) and in % time 24-hour intragastric pH > 4 between dosing at breakfast and at bedtime snack (7% difference). No other significant differences in 24-hour intragastric pH were found between breakfast and the other mealtimes. Therefore, the dosing versatility of dexlansoprazole MR appears to extend beyond the lack of an effect by food into the realm of dose timing flexibility.
The impact of dose timing on the pharmacodynamic effects of other PPI has been previously studied. Rabeprazole dose timing was studied in a crossover fashion in 20 GERD patients, and a significantly greater % time intragastric pH > 4 was observed when the dose was given once daily in the morning vs the evening.Citation25 Dosing lansoprazole in the morning produced no differences in intragastric pH (mean 24-h pH or % time pH > 4) than evening dosing in healthy subjects in one study.Citation26 However, morning dosing of lansoprazole in another study was significantly more effective than evening dosing at intragastric pH control for all time periods during the day except for overnight, when the two dosing methods were comparable.Citation27
An alternative method of dexlansoprazole administration was studied in 50 healthy subjects in a two-period, randomized, crossover study where dexlansoprazole MR 90 mg was ingested after a 10-hour fasting period as either an intact capsule with water or after the capsule was opened and the granules were sprinkled over applesauce and swallowed.Citation28 No significant differences in either AUC or Cmax were found between the two methods, and bioequivalence was established for dexlansoprazole MR regardless of whether given whole with water or sprinkled over applesauce.
Drug–drug interactions remain a potential concern for any compound that undergoes extensive hepatic metabolism, including PPI. Four separate studies were conducted in healthy subjects in which dexlansoprazole MR 90 mg was given once daily for 9 to 11 days with a single dose of a test substrate. The test substrates for the in vivo assessment of CYP enzyme activity included diazepam 5 mg (a substrate for CYP2C19 and CYP3A), phenytoin 250 mg (CYP2C9 and CYP2C19), theophylline (given as intravenous aminophylline 400 mg, CYP1A2), and warfarin 25 mg (CYP2C9).Citation29 No significant differences in Cmax or AUC of any substrate were detected when given concomitantly with dexlansoprazole MR. Furthermore, the pharmacodynamic impact of coadministration of dexlansoprazole MR with warfarin as measured by change in INR was not significant. Therefore, no significant pharmacokinetic and pharmacodynamic (for warfarin only) drug–drug interactions were found in these studies with dexlansoprazole MR. At the time of this review no studies have been conducted with dexlansoprazole and clopidogrel, so the effect of the two drugs when given together is unknown.
Due to complete metabolism in the liver to inactive metabolites and the absence of unchanged drug excreted in the urine, dexlansoprazole MR is not expected to undergo accumulation in kidney dysfunction, and no dose adjustment is required in patients with renal impairment.
Accumulation of dexlansoprazole concentrations occurred in subjects with moderate (Child Pugh Class B) hepatic impairment, but not in mild impairment (Child Pugh Class A).Citation30 Due to this finding, studies were not conducted in patients with severe hepatic impairment. Thus, the lower dexlansoprazole MR dose of 30 mg should be considered in moderate hepatic impairment, and no dosage adjustment is required in mild impairment.
Dexlansoprazole MR pharmacokinetics were not significantly altered in elderly patients,Citation31 women,Citation31 or GERD patients.Citation32
Dexlansoprazole MR: clinical studies
The clinical development program for dexlansoprazole MR was the largest for any PPI to date and comprised 6 pivotal studies in more than 4500 patients. The goals of this program were to establish the efficacy and safety of dexlansoprazole MR in the treatment and maintenance of erosive esophagitis and in the control of symptomatic nonerosive GERD.
Healing of erosive esophagitis
Two identically designed trials evaluated the efficacy and safety of dexlansoprazole MR vs lansoprazole in the healing of erosive esophagitis.Citation33 Both trials were randomized and double-blinded and compared dexlansoprazole MR 60 mg and 90 mg with lansoprazole 30 mg once daily. All doses were given once daily within 60 minutes of the morning meal to maintain blinding, and the duration of treatment was 8 weeks. All patients were adults (age >18 years) with endoscopically proven erosive esophagitis. Exclusion criteria included the presence of Helicobacter pylori infection or Barrett’s esophagus. Esophagogastroduodenoscopy (EGD) was performed at baseline (to establish the presence of esophageal erosions) and at 4 and 8 weeks. The primary endpoint was the percentage of patients with endoscopic evidence of healing at 8 weeks, and secondary endpoints included the percentage of subjects with moderate-to-severe (Los Angeles [LA] Grades C and D) erosive esophagitis who were healed at 8 weeks, and all grades healed at 4 weeks. The target proportion of patients with LA Grade C and D disease was 30% as consistent with FDA guidance that this subgroup of disease presents specific challenges to healing. The symptoms of erosive esophagitis were recorded by diary twice daily: upon awakening each morning to capture any symptoms experienced overnight and upon retiring each evening to capture any symptoms experienced while awake. The rigor of this recording method was intended to minimize the recall bias that may arise from once daily symptom recording. The primary method of analysis of the healing rate was the crude rate; this analysis method classifies any subject who does not complete the study (eg, no data for week 8 endoscopy) as a complete treatment failure. This is in contrast to life-table analysis, the statistical methodology historically used in PPI trials, in which the probability is calculated that a patient would have healed had he remained in the study and received the final EGD. As such, in life-table analysis the patient who does not complete the trial is considered a partial failure. The crude rate is an inherently more stringent analysis method, and typically yields lower healing rates than life-table. Both dexlansoprazole MR erosive esophagitis healing trials were designed to test for noninferiority; the dexlansoprazole MR doses shown to be noninferior were then tested for superiority to lansoprazole 30 mg for primary and secondary efficacy endpoints. For each study, a sample size of 520 patients per treatment group provided at least 95% power at the 0.025 level of significance to detect noninferiority between dexlansoprazole MR and lansoprazole, assuming equal healing rates of 87% at Week 8.
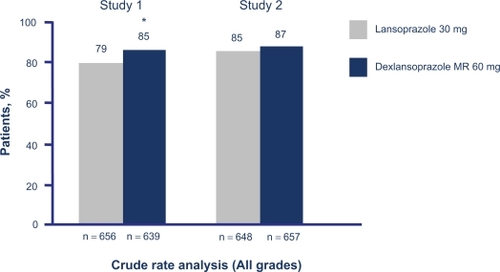
Baseline demographics were not significantly different between any of the groups in either study. Erosive esophagitis healing rates at week 8 for both dexlansoprazole MR doses were superior to lansoprazole in one study (Study 1); 60 mg of dexlansoprazole MR was noninferior and 90 mg was superior to lansoprazole in the other study (). Healing at week 4 was >64% for all groups using both crude rate and life-table analysis methods. Healing of moderate-to-severe erosive esophagitis was significantly greater with dexlansoprazole MR 60 mg than lansoprazole in Study 1 and both doses were noninferior to lansoprazole in Study 2. The median percentage of 24-hour heartburn-free days was greater than 80% in patients who received either dose of dexlansoprazole MR; this was comparable to lansoprazole.
Figure 4 Comparative 8-week crude erosive esophagitis healing rates for dexlansoprazole MR and lansoprazole.Citation33
P = 0.004 vs lansoprazole.
Maintenance of erosive esophagitis healing
Subjects who experienced healing of erosive esophagitis in either of the two healing studies mentioned previously were eligible for enrollment in one of two studies designed to evaluate the maintenance of healing over a 6-month period. One study compared dexlansoprazole MR 30 mg and 60 mg with placeboCitation34 and the other study compared 60 mg and 90 mg doses with placebo.Citation35 The placebo-controlled design was consistent with the standard comparator of other esophagitis healing maintenance studies. The final endoscopy of the previous healing study was considered the baseline assessment of healing for this maintenance study and was followed by endoscopies at 1, 3, and 6 months to document persistence of healing. The primary efficacy endpoint was the percentage of subjects who maintained healed erosive esophagitis at 6 months. Secondary efficacy endpoints included the percentage of days without daytime or nighttime heartburn and the percentage of nights without heartburn. Symptoms were recorded by subjects twice daily in a manner identical to the erosive esophagitis healing studies.
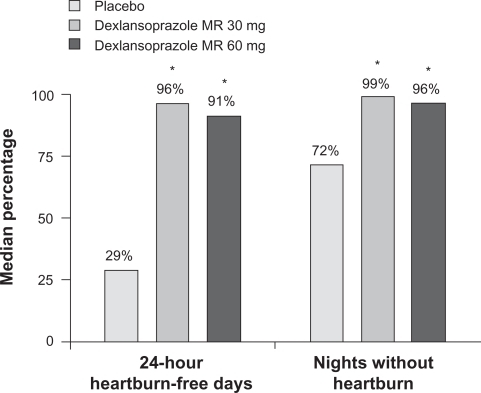
The enrollment of the study that compared dexlansoprazole MR 30 mg and 60 mg with placebo was 445 subjects, and the withdrawal rate from study medication was 83% for the placebo group and 34% for each dexlansoprazole MR group, mostly due to relapse of erosive esophagitis. Maintenance of healing rates were significantly higher for both dexlansoprazole MR doses compared to placebo, and this finding was consistent for all grades of erosive esophagitis and for moderate-to-severe disease. The median percentage of 24-hour heartburn-free days and median percentage of nights without heartburn was statistically significantly higher for all doses of dexlansoprazole MR than placebo, with 96% of 24-hour periods and 99% of nights being reported as heartburn-free over 6 months for dexlansoprazole MR 30 mg vs 29% of 24-hour periods and 72% of nights for placebo ().
Figure 5 Median percentage of 24-hour heartburn-free days and median percentage of nights without heartburn during treatment.
P < 0.0025 vs placebo (Hochberg’s procedure; Wilcoxon rank sum tests).
Reproduced with permission from Metz DC, Howden CW, Perez MC, Larsen L, O'Neil J, Atkinson SN. Clinical trial: dexlansoprazole MR, a proton pump inhibitor with dual delayed-release technology, effectively controls symptoms and prevents relapse in patients with healed erosive oesophagitis. Aliment Pharmacol Ther. 2009;29(7):742–754.Citation34 Copyright © 2009 Wiley-Blackwell.
Symptomatic relief of nonerosive GERD
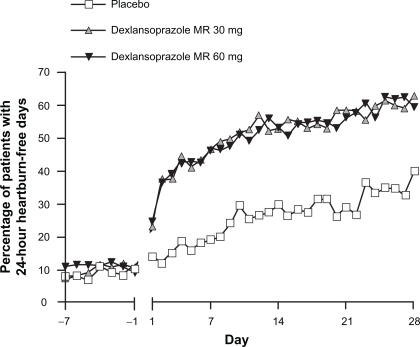
The control of nonerosive GERD symptoms remains a therapeutic challenge for practitioners, because the true etiology of the symptoms may or may not be due to acid or may be unknown. A clinical trial compared two different doses of dexlansoprazole MR (30 and 60 mg) with placebo in subjects with normal esophageal mucosa on EGD.Citation36 This study identified patients with heartburn-predominant complaints for at least 6 months and for 4 of the 7 days prior to screening for enrollment, but no minimal severity of symptoms was required. Besides EGD, no objective assessments of esophageal disease such as pH-metry were conducted and no attempts were made to identify or exclude patients with functional heartburn. Study medication was administered in a blinded fashion once daily in the morning for 28 days. Subjects recorded heartburn symptom assessments twice daily as described for the erosive esophagitis healing and maintenance studies, and investigator assessments occurred at baseline and at 2 and 4 weeks of the study. The primary endpoint was the percentage of 24-hour periods that were free of heartburn symptoms over 28 days, and the secondary endpoint was the percentage of daytime periods and nighttime periods without heartburn. The results demonstrated that a majority of the 24-hour periods were heartburn-free in the groups that received dexlansoprazole MR (median percentage 54.9% for the 30 mg group) compared with 18.5% for the placebo group (). The dexlansoprazole MR 30 mg group also experienced significantly greater nighttime periods (median percentage of nights 80.8% vs 51.7% for placebo) and daytime periods (median percentage of days 63.0% vs 26.9% for placebo) that were symptom-free. It is important to note that no nighttime symptom requirement was necessary for enrollment into the study; this may partially explain the relatively large placebo response for this endpoint. The percentage of patients during the study who experienced 24-hour heartburn-free days over the first 3 days of treatment was significantly greater for dexlansoprazole MR treatment groups than placebo. The percentage of patients with 24-hour heartburn-free days by each study day is presented in .
Figure 6 Median percentage of 24-hour heartburn-free periods with dexlansoprazole MR vs placebo in nonerosive GERD.
*P < 0.00001.
Reproduced with permission from Fass R, Chey WD, Zakko SF, et al. Clinical trial: the effects of the proton pump inhibitor dexlansoprazole MR on daytime and nighttime heartburn in patients with nonerosive reflux disease. Aliment Pharmacol Ther. 2009;29(12):1261–1272.Citation36 Copyright © 2009 Wiley-Blackwell.
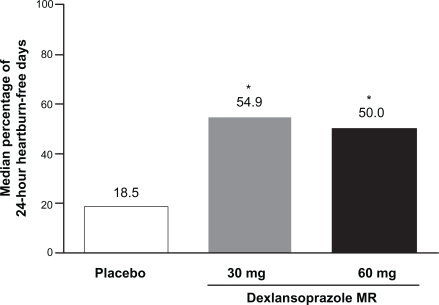
Figure 7 Percentage of patients with 24-hour heartburn-free days by each study day in nonerosive GERD.
Reproduced with permission from Fass R, Chey WD, Zakko SF, et al. Clinical trial: the effects of the proton pump inhibitor dexlansoprazole MR on daytime and nighttime heartburn in patients with nonerosive reflux disease. Aliment Pharmacol Ther. 2009;29(12):1261–1272.Citation36 Copyright © 2009 Wiley-Blackwell.
Dexlansoprazole MR: safety and tolerability
The safety and tolerability of dexlansoprazole MR was evaluated in more than 4500 patients in seven trials of the phase 3 clinical development program. Treatment-emergent adverse events were reported in which any such event that occurred after the ingestion of the first dose of study medication was recorded and analyzed. The strictness of this reporting method contrasts with reporting of treatment-related events which requires the investigator to deem an adverse event to be related to the study medication in order to be reported. The most commonly reported treatment-emergent adverse events (with a frequency of ≥2%) from all clinical studies of dexlansoprazole MR are presented in . Diarrhea was the most common adverse event leading to discontinuation form dexlansoprazole therapy in controlled clinical studies (0.7%).Citation20
Table 1 Most common adverse reactions (≥2%) that occurred at a higher incidence for dexlansoprazole MR than placebo in controlled studiesCitation17
The elevation of plasma gastrin concentrations by PPI is a well-established class effect that is due to the compensatory increase in afferent hormonal input of parietal cell acid production. The trophic effects of gastrin on the gastric mucosa and evidence of ECL-cell hyperplasia in animals have led to potential controversy about the long-term use of PPI in humans. Mean plasma gastrin AUC24 increased by approximately 3.5-fold compared with baseline values after 5 days of dosing with dexlansoprazole MR 90 mg or 120 mg; this magnitude of increase was similar to that of lansoprazole 30 mg in a crossover study in healthy subjects.Citation37 Gastrin parameters started to decline within 3 days after drug discontinuation, and returned to baseline within 7 days after the last dose of drug. Thus, the changes in gastrin associated with dexlansoprazole MR appeared to be modest, reversible, unrelated to dose, and similar to other PPI. Elevations in serum gastrin concentrations were higher in the dexlansoprazole MR groups than in the lansoprazole group in the erosive esophagitis healing studies, but were within the expected range for PPI.Citation33 Gastrin elevations also occurred in all dexlansoprazole MR groups in the maintenance and nonerosive GERD studies compared to placebo.Citation34,Citation36 These elevations were also within the range expected for patients receiving PPI.
Gastric biopsies obtained at the final visit in patients enrolled in either of the maintenance of erosive esophagitis studies revealed no findings of neuroendocrine cell proliferation or adenocarcinoma.Citation34,Citation35
Finally, no changes in the cardiac rhythm (including Q-T interval) were detected in healthy volunteers who received a single dose of dexlansoprazole MR 90 mg or 300 mg.Citation38 No consistent, clinically important changes in laboratory results, vital signs, or physical examinations were observed.
Summary and conclusions
Dexlansoprazole MR is a PPI administered by a unique delivery system that extends the duration of active plasma concentrations of drug beyond conventional PPI. It is available in two dosage strengths, 30 and 60 mg, and is currently approved for 3 clinical indications: healing of erosive esophagitis at a dose of 60 mg orally once daily for up to 8 weeks, maintenance of erosive esophagitis healing at a dose of 30 mg orally once daily for up to 6 months, and relief of symptomatic nonerosive GERD at a dose of 30 mg orally once daily for 4 weeks. In 2 large active-control studies of dexlansoprazole MR it showed healing rates of all grades of erosive esophagitis consistent with lansoprazole, and this healing was maintained for up to 6 months in nearly two-thirds of patients at either dose in another placebo-controlled study. In addition, dexlansoprazole MR provided complete relief of heartburn symptoms for a median of 55% of 24-hour periods over 28 days in patients with symptomatic nonerosive GERD. The safety profile of dexlansoprazole MR is similar to that of lansoprazole. Because dexlansoprazole MR can be taken without regard to food or time of day it is more convenient for individuals who find compliance with meal-associated dosing of medication difficult or eat at irregular times. The prolonged duration of acid suppression provided by dexlansoprazole MR addresses the short half-life of conventional PPI and offers a novel approach to extending gastric pH control in patients with selected acid-related disorders.
Disclosures
Drs Baum and Wittbrodt are employees of Takeda Pharmaceuticals, North America, Deerfield, IL, USA.
Dr Peura has served as a consultant, member of the speakers bureau, and an advisory board member for Takeda Global Research and Development Center, Inc. Deerfield, IL, USA (TAP Pharmaceutical Products Inc., Lake Forest, IL, USA is now a part of Takeda Global Research and Development Center, Inc.) as an advisory board member and consultant for Novartis Consumer Health Inc., and as a member of the speakers bureau for AstraZeneca and Santarus.
The manuscript was written by the authors.
References
- KahrilasPJShaheenNJVaeziMFAmerican Gastroenterological Association InstitiuteClinical Practice and Quality Management CommitteeAmerican Gastroenterological Association Institute technical review on the management of gastroesophageal reflux diseaseGastroenterology200813541392141318801365
- GillenDMcCollKELProblems related to acid rebound and tachyphylaxisBest Pract Res Clin Gastro2001153487495
- MetzDCFerronGMPauJProton pump activation in stimulated parietal cells is regulated by gastric acid secretory capacity: a human studyJ Clin Pharmacol200242551251912017345
- SachsGProton pump inhibitors and acid-related diseasesPharmacotherapy199717122379017763
- SachsGShinJMBrivingCWallmarkBHerseySThe pharmacology of the gastric acid pump: the H+, K+ ATPaseAnnu Rev Pharmacol Toxicol1995352773057598495
- KatzPOScheimanJMBarkunANReview article: acid-related disease – what are the unmet clinical needs?Aliment Pharmacol Ther200623Suppl 292216700899
- FennertyMBJohansenJFHwangCSostekMEfficacy of esomeprazole 40 mg vs lansoprazole 30 mg for healing moderate to severe erosive oesophagitisAliment Pharmacol Ther200521445546315709997
- LauritsenKDevièreJBigardMAEsomeprazole 20 mg and lansoprazole 15 mg in maintaining healed reflux oesophagitis: Metropole study resultsAliment Pharmacol Ther200317333334112562445
- KovacsTOFrestonJWHaberMMAtkinsonSHuntBPeuraDALong-term quality of life improvement in subjects with healed erosive esophagitis: treatment with lansoprazoleDig Dis Sci200977 [Epub ahead of print]
- DonnellanCSharmaNPrestonCMoayyediPMedical treatments for the maintenance therapy of reflux oesophagitis and endoscopic negative reflux diseaseCochrane Database Syst Rev20052CD00324515846653
- InadomiJMMcIntyreLBernardLFendrickAMStep-down from multiple- to single-dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIsAm J Gastroenterol20039891940194414499769
- GosselinALuoRLohouesHThe impact of proton pump inhibitor compliance on health-care utilization and costs in patients with gastroesophageal reflux diseaseValue Health2009121343919895371
- Van SoestEMSiersemaPDDielemanJPSturkenboomMCKuipersEJPersistence and adherence to proton pump inhibitors in daily clinical practiceAliment Pharmacol Ther200624237738516842465
- GunaratnamNTJessupTPInadomiJLascewskiDPSub-optimal proton pump inhibitor dosing is prevalent in patients with poorly controlled gastro-oesophgeal reflux diseaseAliment Pharmacol Ther200623101473147716669962
- BarrisonAFJarboeLAWeinbergBMNimmagaddaKSullivanLMWolfeMMPatterns of proton pump inhibitor use in clinical practiceAm J Med2001111646947311690573
- KatsukiHYagiHArimoriKDetermination of R(+)- and S(−)-lansoprazole using chiral stationary-phase liquid chromatography and their enantioselective pharmacokinetics in humansPharm Res19961346116158710755
- Kapidex® [prescribing information]Takeda Pharmaceutical, North AmericaDeerfield, IL2009
- KatsukiHHamadaANakamuraCArimoriKNakanoMRole of CYP3A4 and CYP2C19 in the stereoselective metabolism of lansoprazole by human liver microsomesEur J Clin Pharmacol2001571070971511829200
- VakilyMZhangWWuJAtkinsonSNMulfordDPharmacokinetics and pharmacodynamics of a known active PPI with a novel dual delayed release technology, dexlansoprazole MR: a combined analysis of randomized controlled clinical trialsCurr Med Res Opin200925362763819232037
- MayerMDVakilyMWittGMulfordDJThe pharmacokinetics of TAK-390MR 60 mg, a dual delayed release formulation of the proton pump inhibitor TAK-390, and lansoprazole 60 mg: a retrospective analysis [abstract]Gastroenterology2008 1344Suppl 1A176
- MetzMCVakilyMDixitTMulfordDReview article: dual delayed release formulation of dexlansoprazole MR, a novel approach to overcome the limitations of conventional single release proton pump inhibitor therapyAliment Pharmacol Ther200929992893719298580
- ZhangWWuJAtkinsonSPharmacokinetic (PK), pharmacodynamic (PD), and safety evaulation of single and multiple 60 mg, 90 mg, and 120 mg oral doses of modified release TAK-390 (TAK-390MR) and 30 mg oral doses of lansoprazole (Lan) in healthy subjects. [abstract]Gastroenterology20071324 Suppl 2487
- LeeRDVakilyMMulfordDWuJAtkinsonSNClinical trial: the effect and timing of food on the pharmacokinetics and pharmacodynamics of dexlansoprazole MR, a novel dual delayed release formulation of a proton pump inhibitor – evidence for dosing flexibilityAliment Pharmacol Ther200929882483319243357
- LeeRDMulfordDWuJAtkinsonSNThe effect of time-of-day dosing of TAK-390MR on the pharmacokinetics and pharmacodynamics of TAK-390: evidence for dosing flexibility with this dual delayed release proton pump inhibitor [abstract]Gastroenterology20091365 Suppl 1A-440
- PehlivanovNDOlyaeeMSarosiekIMcCallumRWComparison of morning and evening administration of rabeprazole for gastro-oesophageal reflux and nocturnal gastric acid breakthrough in patients with reflux disease: a double-blind cross-over studyAliment Pharmacol Ther200318988389014616152
- HongoMOharaSHirasawaYAbeSAsakiSToyotaTEffect of lansoprazole on intragastric pH. Comparison between morning and evening dosingDig Dis Sci19923768828901534047
- FraserAGSawyerrAMHudsonMSmithMSPounderREMorning versus evening dosing of lansoprazole 30 mg daily on twenty-four-hour intragastric acidity in healthy subjectsAliment Pharmacol Ther19961045235278853755
- CzerniakRVakilyMWuJTAK-390MR, a novel dual delayed release formulation of a PPI, is bioequivalent when administered as granules sprinkled over applesauce. [abstract]Am J Gastroenterol2008103Suppl SS4S5
- VakilyMLeeRDWuJGunawardhanaLMulfordDDrug interaction studies with dexlansoprazole modified release (TAK-390MR), a proton pump inhibitor with a dual delayed-release formulation: results of four randomized, double-blind, crossover, placebo-controlled, single-centre studiesClin Drug Invest20092913550
- LeeRDWuJVakilyMMulfordDEffect of hepatic impairment on the pharmacokinetics of TAK-390MR (modified release) [abstract]Clin Pharmacol Ther200883Suppl 1S95
- VakilyMZhangWWuJMulfordDEffect of age and gender on the pharmacokinetics of a single oral dose of TAK-390MR (modified release) [abstract]Clin Pharmacol Ther200883Suppl 1S96
- ZhangWWuJVakilyMPharmacokinetics of TAK-390MR (modified-release) 30, 60, and 90 mg in subjects with symptomatic, non-erosive gastroesophageal reflux disease (GERD) [abstract]Clin Pharmacol Ther200883Suppl 1S96
- SharmaPShaheenNJPerezMCClinical trials: healing of erosive oesophagitis with dexlansoprazole MR, a proton pump inhibitor with a novel dual delayed-release formulation – results from two randomized controlled studiesAliment Pharmacol Ther200929773174119183157
- MetzDCHowdenCWPerezMCLarsenLO’NeilJAtkinsonSNClinical trial: dexlansoprazole MR, a proton pump inhibitor with dual delayed-release technology, effectively controls symptoms and prevents relapse in patients with healed erosive oesophagitisAliment Pharmacol Ther200929774275419210298
- HowdenCLarsenLPalmerRPerezMCPlacebo-controlled trial of 2 doses of TAK-390MR, a PPI with novel dual delayed release technology, as maintenance treatment for patients with healed erosive esophagitis (EE)Aliment Pharmacol Ther200930989590719681809
- FassRCheyWDZakkoSFClinical trial: the effects of the proton pump inhibitor dexlansoprazole MR on daytime and nighttime heartburn in patients with nonerosive reflux diseaseAliment Pharmacol Ther200929121261127219392864
- ZhangWWuJAtkinsonSNEffects of dexlansoprazole MR, a novel dual delayed release formulation of a proton pump inhibitor, on plasma gastrin levels in healthy subjectsJ Clin Pharmacol200949444445419318694
- VakilyMWuJAtkinsonSEffect of single oral doses (90 and 300 mg) of TAK-390MR on QT intervals. [abstract]Clin Pharmacol Ther200781Suppl 1S26