Abstract
Constipation affects up to a quarter of the population in developed countries and is associated with poor quality of life and significant economic burden. Many patients with chronic constipation are dissatisfied with current therapy due to lack of long-term efficacy or side effects. Previous nonselective 5-hydroxytryptamine receptor 4 (5-HT4) agonists have been associated with significant interactions with other receptors (5-HT1B, 5-HT1D, and 5-HT2B for tegaserod; hERG for cisapride), leading to adverse cardiovascular events resulting in withdrawal of these drugs from the market. Prucalopride is a novel gastrointestinal prokinetic agent. It acts as a high affinity, highly-selective 5-HT4 agonist. Its efficacy in patients with chronic constipation has been demonstrated in several phase II and phase III clinical trials showing significant improvements in bowel transit, bowel function, gastrointestinal symptoms, and quality of life, with benefit maintained for up to 24 months in open label, multicenter, follow-up studies. Prucalopride’s high selectivity for the 5-HT4 receptor may explain its favorable safety and tolerability profiles, even in elderly subjects with stable cardiovascular disease. Prucalopride is a well tolerated and efficacious prokinetic medication that should enhance the treatment of chronic constipation unresponsive to first-line treatments.
Introduction
Constipation is a common, often chronic, gastrointestinal disorder with higher prevalence in women and the elderly.Citation1 The Rome III Committee on Functional GI DisordersCitation2 set criteria for the diagnosis of chronic constipation which include a description of chronicity (for the last 3 months with symptoms and an onset at least 6 months prior) and symptoms (2 or more of which must be present at least 25% of defecations). These symptoms include: fewer than 3 bowel movements per week, hard or lumpy stools, straining with defecation, a sensation of incomplete evacuation, a sensation of anorectal obstruction or blockage, and use of maneuvers to assist defecation.Citation2 It affects 10% to 15% of the population in developed countries,Citation3,Citation4 and up to 27% of North Americans.Citation5 In a survey of almost 14,000 persons, of whom 12% reported constipation, over half experienced symptoms for three or more years.Citation4 In the United States (US), constipation results in 92,000 hospitalizations and more than 2.5 million physician visits per year; these figures may be rising with time.Citation1,Citation6
Chronic constipation compromises health-related quality of life (HR-QOL) proportionately to symptom severity.Citation3,Citation7,Citation8 It is also associated with significant economic impact, directly from medical evaluation and treatment including the problem of self medication and adherence to therapy, as well as indirectly from absenteeism. Thus, chronic constipation is a significant public health problem.Citation3,Citation9
Constipation can be classified by three major categories based on pathophysiology: normal transit constipation, slow transit constipation, and defecatory disorder.Citation10
Overview of standard therapies, development of new agents
Treatment for constipation is often guided by the severity of symptoms and the underlying pathophysiology. Lifestyle changes such as increasing oral fluid intake and regular exercise do not appear efficacious in alleviating chronic constipation, except in cases of dehydration.Citation11,Citation12 Other therapeutic approaches for chronic constipation include fiber intake (20 to 25 g daily via diet or with fiber supplements), osmotic laxatives (such as polyethylene glycol, milk of magnesia, lactulose, or sorbitol), stimulant laxatives (such as bisacodyl or senna derivatives), secretory agents (such as lubiprostone),Citation13,Citation14 and prokinetic drugs (such as cisapride and tegaserod, which are no longer easily available in most countries).
The nonselective 5-hydroxytryptamine receptor 4 (5-HT4) receptor agonists, cisapride and tegaserod, promote intestinal motility and relieve constipation,Citation15–Citation17 but their lack of selectivity for the 5-HT4 receptor may account for adverse cardiovascular events, resulting in their restricted availability.Citation18 Tegaserod is an agonist at 5-HT1B and 5-HT1D receptors and an antagonist at 5-HT2B receptors within the range of concentrations used for treatment of constipation. These nonselective interactions may explain the association of tegaserod with rare instances of ischemic adverse events, including stroke and angina.Citation18 Cisapride inhibits the human ether-à-gogo related gene (hERG) potassium channel at therapeutic concentrations. This can lead to cardiac electrophysiologic derangements including QT prolongation, torsade de pointes, ventricular tachycardia, and ventricular fibrillation, particularly in patients with underlying cardiovascular diseases or the concurrent use of a medication that inhibits metabolism of cisapride.Citation18,Citation19
Biofeedback retraining is essential for outlet dysfunction resulting in chronic constipation. Intractable cases of severe slow transit constipation associated with colonic inertia may require colectomy with ileorectostomy.Citation10
Despite their widespread use, evidence of long-term clinical efficacy for laxative medications is lacking.Citation20–Citation22 Patient dissatisfaction with current laxative treatment is high, with lack of efficacy reported by 82% and concerns about side effects in 16% of constipated subjects in a US based survey.Citation8
New pharmacotherapeutic approaches for treatment of chronic constipation include guanylate cyclase C agonists (eg, linaclotide),Citation23,Citation24 neurotrophins (eg, NT-3),Citation25,Citation26 and serotonergic agents, predominantly 5-HT4 receptor agonists, with enterokinetic properties. This class of compounds includes prucalopride, velusetrag, and ATI-7505. Of these, the agent with the largest clinical trial evidence of efficacy is prucalopride.
Pharmacology and mode of action of prucalopride
Prucalopride (previously known as R093877 and R108512) is a dihydro-benzofurancarboxamide derivative, with a different structure relative to older serotonergic gastrointestinal prokinetics such as cisapride (a substituted benzamide derivative) and tegaserod (an aminoguanidine indole derivative). Prucalopride is a highly selective agonist and has high affinity for 5-HT4 receptors promoting cholinergic and nonadrenergic, noncholinergic neurotransmission by enteric neurons. Prucalopride displays high affinity binding to human 5-HT4a and 5-HT4b receptor isoforms with pKi values of 8.6 and 8.1, respectively.Citation27 Prucalopride also displays very high specificity for the 5-HT4 receptor isoforms, with a greater than 290-fold selectivity for 5-HT4 receptor isoforms than for the only three other receptors showing measurable affinity to prucalopride (human dopamine D4 receptor with pKi of 5.63, mouse 5-HT3 receptor with pKi of 5.41, and human σ1 receptor with pKi of 5.43).Citation27 Agonist binding to the G protein-coupled 5-HT4 receptor activates adenylate cyclase and increases intracellular cyclic adenosine monophosphate (AMP) levels.Citation28
Specific activation of the 5-HT4 receptors that are present in the range quantities in the gastrointestinal tract promotes gastrointestinal motility and mucosal secretion.Citation28 Gastrointestinal motility stimulation has been demonstrated in several experimental models in vitro and in vivo. In isolated guinea pig colon, prucalopride induced dose-dependent, nonadrenergic, noncholinergic contractions (pEC50 = 7.48 ± 0.06).Citation27 Selectivity for the 5-HT4 receptor was confirmed via inhibition by a selective 5-HT4 antagonist (GR113808), but lack of inhibition by 5-HT2A (ketanserin) and 5-HT3 antagonists (granisetron).Citation27 Prucalopride also stimulated contractions in the stomach (in rat and dog) and colon (in dog and human) (pEC50 = 7.50 ± 0.08), but mediated relaxation of the esophagus (in rat) (pEC50 = 7.81 ± 0.17) in a manner sensitive to 5-HT4 receptor antagonism.Citation27
Prucalopride stimulated contractions in colonic longitudinal smooth muscles by promoting acetylcholine release via activation of 5-HT4 receptors on presynaptic, cholinergic enteric neurons.Citation29 Prucalopride also induced relaxation of human colonic and canine rectal circular smooth muscles through local 5-HT4 receptor activation.Citation29,Citation30 Therefore, 5-HT4 agonists facilitate gastrointestinal motility by promoting longitudinal smooth muscle contractility while suppressing the resistance to propulsion due to circular smooth muscle contraction.
Prucalopride’s in vivo effects in the canine colon suggest a coordinated, region-specific mechanism, whereby longitudinal smooth muscles demonstrate increased contractile activity in the proximal colon, but reduced contractility in the distal colon.Citation31 In addition, circular smooth muscle relaxation is negligible in the proximal colon, but increasingly more pronounced towards the distal colon with prucalopride treatment.Citation30 In conscious, fasted dogs, prucalopride also induces colonic giant migrating contractions (GMC) that propagate along the entire length of the colon to facilitate propulsion of luminal contents.Citation31 GMC are the canine equivalent to human high amplitude propagated contractions (HAPC). A reduction of HAPC is observed in patients with idiopathic constipation.Citation32
Prucalopride, given intravenously at a dosage of 1 to 2 mg/kg in fasted rats, increased whole gut transit of activated charcoal, mainly by increasing colonic transit, and without affecting gastric or proximal small bowel transit.Citation33 On the other hand, in dogs, prucalopride dose-dependently promotes gastric contractilityCitation34 and accelerates gastric emptying.Citation35
Pharmacokinetics of prucalopride
Prucalopride is rapidly and extensively absorbed from the gastrointestinal tract after oral dosing. Peak plasma concentration of 4.34 ng/mL (mean) was achieved in 2.1 hours after a single 2 mg dose in 14 healthy adult subjects.Citation36 Absolute oral bioavailability of prucalopride exceeds 93% and is not affected by food intake.Citation36 Prucalopride displays linear pharmacokinetics with exposure to drug, increasing proportionally with increasing dosage over the dose range 1 to 20 mg daily.Citation36 Plasma protein binding is low at 28% to 33%.Citation36 Extensive distribution is reflected in a steady state volume of distribution of 567 L.Citation36
Prucalopride undergoes limited metabolism in the human body. Only small amounts of its metabolites are found in the urine and feces, with the major metabolite accounting for less than 4% of the dose.Citation37 Unchanged prucalopride accounts for about 85% of the plasma radioactivity after administration of radiolabeled drug in an oral dose study.Citation37 The drug is excreted largely unchanged, with about 60% of the administered dose excreted in urine by active secretion and passive filtration and greater than 6% appearing in the feces.Citation36 The elimination half-life of prucalopride of between 24 and 30 hours supports once daily dosing.Citation37
Age, gender, body weight, and race have no influence on pharmacokinetics. Plasma prucalopride concentrations are nearly 30% higher in the elderly due to age-related decline in renal function. Therefore, a reduction of the dosage to half the normal adult dosage is recommended for elderly patients and for patients with severe renal insufficiency (creatinine clearance less than 25 mL/min).Citation37 Given the relatively low level of metabolism by the liver, hepatic impairment is unlikely to alter prucalopride pharmacokinetics significantly. Halving the normal adult dosage is recommended for patients with severe liver dysfunction.Citation37
Prucalopride has a low potential for drug-drug interactions due to lack of significant metabolism by the cytochrome P450 system at therapeutic concentrations and due to lack of extensive binding to plasma protein.
Efficacy studies
Pharmacodynamics in humans
In healthy volunteers, placebo-controlled pharmacodynamic studies showed that prucalopride treatment for 1 week accelerated colonic transit at 0.5, 1, 2, and 4 mg/day,Citation38,Citation39 orocecal transit at 1 mg/day,Citation40 and whole gut transit at 1 and 2 mg/day.Citation40 An increase in stool frequency and a loosening of stool consistency were also documented.Citation38–Citation41 There was no rebound effect on bowel function after discontinuation of drug.Citation40 Prucalopride’s effects on gastrointestinal transit and bowel function in healthy men were comparable to those observed in healthy women in a trial that enrolled an equal number of male and female healthy volunteers.Citation39 In addition, a study conducted exclusively in males provided results similar to those of trials that enrolled mostly females.Citation40
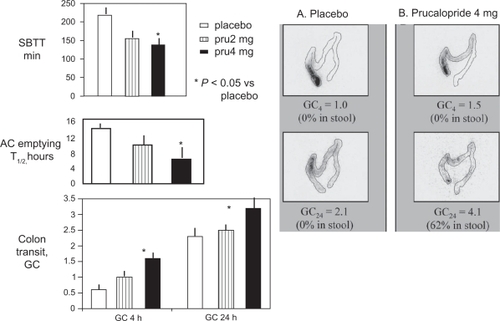
In patients with chronic constipation in whom an evacuation disorder was excluded (), prucalopride therapy at 4 mg/day for 1 weekCitation42 or at 1 mg/day for 4 weeksCitation43 accelerated gastric emptying half-time,Citation42 ascending colon emptying half-time,Citation42 overall colonic transit,Citation42 orocecal transit time,Citation42,Citation43 and whole gut transit.Citation43 Prucalopride-induced acceleration of colonic transit was also associated with increased stool frequency and loosening of stool consistency in patients with chronic constipation.Citation43,Citation45 Prucalopride exhibited dose responsiveness in effects on gastrointestinal transit and bowel functions.Citation43,Citation44
Figure 1 Effect of prucalopride on small bowel and colonic transit.
*P < 0.05 vs placebo treatment..
Reprinted from Bouras EP, Camilleri M, Burton DD, et al. Prucalopride accelerates GI and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354–360.Citation76
Abbreviations: GC, geometric center at 4 (GC 4 h) and 24 (GC 24 h) hours; SBTT, small bowel transit time; AC, ascending colon; pru, prucalopride.
Therapeutic efficacy
Phase IIB studies
Phase IIB clinical trials assessing prucalopride’s efficacy in chronic constipation were conducted for up to 4 weeks with dosage ranging from 0.5 to 4 mg/day.Citation45–Citation48 The primary endpoint of ≥3 spontaneous, complete bowel movements per week (SCBM/w) is thought to reflect clinical response, since consensus criteria show that healthy people have 3 bowel movements per week. This endpoint was achieved in ∼32% and 55% for patients on 2 mg/day and 4 mg/day, respectively.Citation46,Citation47 Another endpoint of significant clinical relevance to patients is the ability to have spontaneous bowel movements (SBM). SBM per week (SBM/w) were increased 1.8- to 3.5-fold with prucalopride relative to placebo.Citation44,Citation45,Citation49 Prucalopride also ameliorated a number of secondary endpoints: frequency of straining during bowel movements, stool consistency, subjective sense of constipation, and time to first bowel movement.Citation49 In children aged 4 to 12 years, prucalopride decreased number of days with hard stools or without stools and increased average number of days with bowel movements.Citation50
Studies conducted in subgroups of patients with secondary constipation suggest prucalopride is also efficacious:
In opioid-induced constipation, ∼36% of prucalopride-treated patients had an increase of one or more SMB/w, compared to 23% for patients on placebo.Citation51
In patients with spinal cord injury, prucalopride (2 mg/day) increased number of bowel movements per week (BM/w) and reduced colonic transit relative to placebo without affecting stool consistency.Citation52
In patients with multiple sclerosis and constipation, prucalopride (1 to 2 mg/day) decreased time to first BM and severity of constipation, and increased number of BM/w by ≥1 in 57% of patients on prucalopride compared to 25% placebo. This led to a decrease in the need for laxatives.Citation53
Phase III studies
The clinical efficacy of prucalopride is best demonstrated by the three pivotal phase III clinical trials conducted in the treatment of chronic constipation in patients not experiencing symptomatic relief with laxatives ().Citation54–Citation56 The trials had virtually identical design (2-week run-in, 12 weeks on treatment), doses (placebo, prucalopride 2 and 4 mg) and primary outcome measurements (percentage of patients achieving ≥3 SCBM/w over the 12-week treatment period) in patients with chronic constipation (based on self report of <2 SCBM/w for 6 or more months,Citation54 averaged over 12 weeks,Citation55 and for a minimum of 2 weeks,Citation56 respectively). Patients were required to have hard or lumpy stool, as well as straining on defecation and a sensation of incomplete evacuation during ≥25% of BM. Secondary constipation was excluded.
Table 1 Pivotal phase III trials of prucalopride in patients with chronic constipation who are dissatisfied with current laxative treatment
Efficacy endpoints were derived from daily diaries of bowel habits, the Patient Assessment of Constipation Symptoms (PAC-SYM),Citation57 and the Patient Assessment of Constipation Quality of Life (PAC-QOL).Citation58 Compared to 11% of the placebo group, 23.6% (2 mg/day) and 24.7% (4 mg/day) of patients achieved the primary endpoint (≥3 SCBM/w), which reflects normalization of bowel function.Citation54
Prucalopride was also effective in significantly improving secondary endpoints, such as proportion of patients achieving an increase of ≥1 SCBM/w over the 12 weeks of therapy relative to baseline, the average number of SCBM/w, stool consistency, time to first SCBM, sensation of incomplete evacuation, need for rescue medication, patient-rated satisfaction, overall PAC-SYM score, and overall treatment effectiveness.Citation54–Citation56
Efficacy of prucalopride in retreatment was shown during a second 4-week treatment period compared to the first 4-week period: retreatment was associated with improved bowel function and associated patient-reported symptoms.Citation59
There are no reported direct comparative studies of prucalopride with other colonic prokinetics or secretagogues. The data from clinical trials regarding the average increase in number of BM/w with prucalopride suggest that it may be more efficacious (+1.4–1.8 SCBM/w)Citation54 relative to the mean number of BM/w on placebo when compared to tegaserod (+1.3 BM/w) and renzapride (+0.2 BM/d), and similar to the efficacy of cisapride (+1.9 SBM/w) and bulk laxatives (+1–2 BM/w).Citation60–Citation63
In open label, long-term, follow-up studies, continuation of prucalopride for a median of about 1 year and a range of up to 24 months was associated with sustained patient satisfaction with bowel function which was documented every 3 months for up to 18 or 24 months.Citation64,Citation65 There was also overall satisfaction with treatment.
Safety and tolerability
Prucalopride’s high selectivity at therapeutic dosages minimizes interactions with other receptors that may lead to serious adverse events.Citation18 It is eliminated from the human body without extensive metabolism, thus reducing potential for drug–drug interactions with medications that affect hepatic or renal metabolism and clearance.Citation36
Cardiac safety
Since the report of 341 cases of cisapride-related, serious cardiac arrhythmias in 2000,Citation66 extensive cardiac monitoring, in particular the duration of the QTc interval, has been mandated for development of 5-HT4 receptor agonists, as there are 5-HT4 receptors in the atrium and ventricle. Prucalopride has some inotropic and chronotropic effects on the heart;Citation67–Citation69 however, studies with prucalopride in the porcine atrium suggest low cardiac risks,Citation70 consistent with the almost 300-fold difference in affinity constant for the 5-HT4 receptor and hERG.Citation18 This indicates a high safety margin and low risk of cardiac side effects with prucalopride.Citation71,Citation72 No arrhythmic activity was demonstrated in human atrial cells treated with prucalopride, even after pretreatment with β-adrenoceptor antagonists to increase the proarrhythmic potential.Citation73
In the prucalopride clinical trial cohorts (∼4000 people), there were no clinically relevant cardiac adverse events. In healthy volunteers in two phase I trials exposing healthy volunteers to up to 10 times the therapeutic dosage of prucalopride, there was higher heart rate and associated decreases in PQ and QT intervals, but not in the Fridericia-corrected QT (QTcF) interval.Citation74 Longer-term clinical trials exposing patients to up to 4 mg/day for a maximum of 24 months confirmed prucalopride’s safety.Citation64 A phase II safety study in nursing home patients, of whom >85% had a history of cardiovascular disease,Citation75 showed no detrimental change in pulse rate, blood pressure, electrocardiographic indices, or laboratory safety parameters.Citation75
General tolerability
Prucalopride is generally well tolerated; the adverse event profile after long-term treatment is similar to that of 12-week exposure. The most common adverse events (which occur in 10% or more of treated subjects) are headache, nausea, abdominal pain, and diarrhea.Citation45,Citation49,Citation54–Citation56,Citation76,Citation77 Most adverse events have been mild or of moderate severity, transient, and have occurred mainly on the first day of treatment, independent of the dosage received. Treatment-related adverse events leading to discontinuation of medication occurred in <8.3% of patients.Citation54–Citation57 Four patient deaths have been reported, with three unrelated to drug and treatment information unavailable for the fourth patient.Citation54,Citation65
Patient perspectives: quality of life, satisfaction, acceptability, and adherence
As illustrated above, prucalopride treatment was associated with improved quality of life (based on PAC-QOL) during placebo-controlled trials and satisfaction with bowel function was maintained during open label treatment with prucalopride for up to 24 months (less than 10% prucalopride cessation because of treatment emergent adverse events). Therefore, patients appear to tolerate and benefit from this treatment in the medium- and long-term. It is also worth noting that >80% patients entering the pivotal trials of 12 weeks’ duration reported lack of satisfaction with current laxatives and there was significant improvement in QOL scores as estimated by the validated PAC-QOL assessment.
Conclusion
Prucalopride is a novel compound, stimulating 5-HT4 receptors with high affinity. The lack of interaction of prucalopride with other receptors or channels at therapeutic doses is advantageous relative to available prokinetics. In patients with chronic constipation, prucalopride increases stool frequency and loosens stool consistency by stimulating gastrointestinal and colonic motility. The drug appears to be safe (with a safety window of ∼300 between efficacy in constipation and proarrhythmic potential), is well tolerated, and adverse events are mild.
In July 2009, prucalopride was approved by the European Medicines Agency for the symptomatic treatment of chronic constipation for women in whom laxatives fail to provide satisfactory relief.Citation78 Prucalopride will be the first oral medication marketed for severe chronic constipation in the European Union. Recommended dosage is 2 mg by mouth once daily, except for those over 65 years of age for whom the recommended dosage is 1 mg by mouth once daily. The dose can be subsequently increased to 2 mg daily, as tolerated.
Current data suggest that, at the least, prucalopride will prove to be a valuable addition to the therapeutic arsenal in treating chronic constipation. At present, prucalopride should be regarded as a second-line treatment after supplementation of fiber, and osmotic, or over-the-counter laxatives.
Acknowledgements
We thank Mrs Cindy Stanislav for excellent secretarial assistance.
Disclosures
Dr Camilleri has a confidentiality disclosure agreement with European specialty pharmaceutical company, Movetis, related to access of data on prucalopride. He has received no financial compensation for this activity or for any authorship of papers on prucalopride.
References
- ChoungRSLockeGR3rdSchleckCDZinsmeisterARTalleyNJCumulative incidence of chronic constipation: a population-based study 1988–2003Aliment Pharmacol Ther2007261521152817919271
- DrossmanDAThe functional gastrointestinal disorders and the Rome III processGastroenterology20061301377139016678553
- DennisonCPrasadMLloydABhattacharyyaSKDhawanRCoyneKThe health-related quality of life and economic burden of constipationPharmacoeconomics20052346147615896098
- StewartWFLibermanJNSandlerRSEpidemiology of constipation (EPOC) study in the United States: relation of clinical subtypes to sociodemographic featuresAm J Gastroenterol1999943530354010606315
- HigginsPDJohansonJFEpidemiology of constipation in North America: a systematic reviewAm J Gastroenterol20049975075915089911
- SonnenbergAKochTRPhysician visits in the United States for constipation: 1958 to 1986Dig Dis Sci1989346066112784759
- IrvineEJFerrazziSParePThompsonWGRanceLHealth-related quality of life in functional GI disorders: focus on constipation and resource utilizationAm J Gastroenterol2002971986199312190165
- JohansonJFKralsteinJChronic constipation: a survey of the patient perspectiveAliment Pharmacol Ther20072559960817305761
- JohansonJFReview of the treatment options for chronic constipationMed Gen Med2007925
- LemboACamilleriMChronic constipationN Engl J Med20033491360136814523145
- MeshkinpourHSelodSMovahediHNamiNJamesNWilsonAEffects of regular exercise in management of chronic idiopathic constipationDig Dis Sci199843237923839824122
- YoungRJBeermanLEVanderhoofJAIncreasing oral fluids in chronic constipation in childrenGastroenterol Nurs1998211561619849179
- JohansonJFMortonDGeenenJUenoRMulticenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipationAm J Gastroenterol200810317017717916109
- SweetserSBusciglioIACamilleriMEffect of a chloride channel activator, lubiprostone, on colonic sensory and motor functions in healthy subjectsAm J Physiol Gastrointest Liver Physiol2009296G295G30119033530
- FinkSChaudhuriTKPalmerJDCisapride accelerates colonic transit in constipated patients with colonic inertiaAm J Gastroenterol1990852162172301347
- PratherCMCamilleriMZinsmeisterARMcKinzieSThomfordeGTegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndromeGastroenterology200011846346810702196
- TackJMuller-LissnerSBytzerPA randomised controlled trial assessing the efficacy and safety of repeated tegaserod therapy in women with irritable bowel syndrome with constipationGut2005541707171316020489
- De MaeyerJHLefebvreRASchuurkesJA5-HT4 receptor agonists: similar but not the sameNeurogastroenterol Motil2008209911218199093
- WysowskiDKCorkenAGallo-TorresHTalaricoLRodriguezEMPostmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actionsAm J Gastroenterol2001961698170311419817
- JonesMPTalleyNJNuytsGDuboisDLack of objective evidence of efficacy of laxatives in chronic constipationDig Dis Sci2002472222223012395895
- PetticrewMRodgersMBoothAEffectiveness of laxatives in adultsQual Health Care20011026827311743157
- TramonteSMBrandMBMulrowCDAmatoMGO’KeefeMERamirezGThe treatment of chronic constipation in adults. A systematic reviewJ Gen Intern Med19971215249034942
- AndresenVCamilleriMBusciglioIAEffect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndromeGastroenterology200713376176817854590
- LemboAJKurtzCBMacdougallJELinaclotide is effective for patients with chronic constipationGastroenterology2009
- CoulieBSzarkaLACamilleriMRecombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humansGastroenterology2000119415010889153
- ParkmanHPRaoSSReynoldsJCNeurotrophin-3 improves functional constipationAm J Gastroenterol2003981338134712818279
- BriejerMRBosmansJPVan DaelePThe in vitro pharmacological profile of prucalopride, a novel enterokinetic compoundEur J Pharmacol2001423718311438309
- HegdeSSEglenRMPeripheral 5-HT4 receptorsFaseb J199610139814078903510
- PrinsNHAkkermansLMLefebvreRASchuurkesJA5-HT(4) receptors on cholinergic nerves involved in contractility of canine and human large intestine longitudinal muscleBr J Pharmacol200013192793211053213
- PrinsNHVan HaselenJFLefebvreRABriejerMRAkkermansLMSchuurkesJAPharmacological characterization of 5-HT4 receptors mediating relaxation of canine isolated rectum circular smooth muscleBr J Pharmacol19991271431143710455293
- BriejerMRPrinsNHSchuurkesJAEffects of the enterokinetic prucalopride (R093877) on colonic motility in fasted dogsNeurogastroenterol Motil20011346547211696108
- BassottiGChiarioniGVantiniIAnorectal manometric abnormalities and colonic propulsive impairment in patients with severe chronic idiopathic constipationDig Dis Sci199439155815648026270
- QiHBLuoJYLiuXEffect of enterokinetic prucalopride on intestinal motility in fast ratsWorld J Gastroenterol200392065206712970907
- PrinsNHvan Der GrijnALefebvreRAAkkermansLMSchuurkesJA5-HT(4) receptors mediating enhancement of contractility in canine stomach; an in vitro and in vivo studyBr J Pharmacol20011321941194711309267
- BriejerMMeulemansAWellensASchuurkensJR093877 dose-dependently accelerates gastric emptying in conscious dogsGastroenterology1997112A705
- Van de VeldeVAusmaJVandeplasscheLPharmacokinetics of prucalopride (Resolor®) in manGut200857A282
- Investigator’s brochure on prucalopride. Movetis NV, Turnhout, Belgium.
- BourasEPCamilleriMBurtonDDMcKinzieSSelective stimulation of colonic transit by the benzofuran 5HT4 agonist, prucalopride, in healthy humansGut19994468268610205205
- PoenACFelt-BersmaRJVan DongenPAMeuwissenSGEffect of prucalopride, a new enterokinetic agent, on gastrointestinal transit and anorectal function in healthy volunteersAliment Pharmacol Ther1999131493149710571606
- EmmanuelAVKammMARoyAJAntonelliKEffect of a novel prokinetic drug, R093877, on gastrointestinal transit in healthy volunteersGut1998425115169616313
- De SchryverAMAndriesseGISamsomMSmoutAJGooszenHGAkkermansLMThe effects of the specific 5HT(4) receptor agonist, prucalopride, on colonic motility in healthy volunteersAliment Pharmacol Ther20021660361211876716
- BourasEPCamilleriMBurtonDDThomfordeGMcKinzieSZinsmeisterARPrucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorderGastroenterology200112035436011159875
- EmmanuelAVRoyAJNichollsTJKammMAPrucalopride, a systemic enterokinetic, for the treatment of constipationAliment Pharmacol Ther2002161347135612144586
- SlootsCEPoenACKerstensREffects of prucalopride on colonic transit, anorectal function and bowel habits in patients with chronic constipationAliment Pharmacol Ther20021675976711929394
- CoremansGKerstensRDe PauwMStevensMPrucalopride is effective in patients with severe chronic constipation in whom laxatives fail to provide adequate relief. Results of a double-blind, placebo-controlled clinical trialDigestion200367828912743445
- NicholsTBeyensGAusmaJA double-blind, placebo-controlled, dose-finding trial to evaluate the efficacy and safety of prucalopride in patients with chronic constipation UEGW. Vienna2008P0894
- MinerPJNicholsTSilversDThe efficacy and safety of prucalopride in patients with chronic constipationGastroenterology1999116A1043
- Felt-BersmaRBouchouchaMWurzerHEffects of a new enterokinetic drug, prucalopride, on symptoms of patients with chronic constipation: a double-blind, placebo-controlled, multicenter study in EuropeGastroenterology1999116A1043
- EmmanuelAVRoyAJNichollsTJKammMAPrucalopride, a systemic enterokinetic, for the treatment of constipationAliment Pharmacol Ther2002161347135612144586
- WinterHAusmaJVandeplasscheLAn open label follow-up study of prucalopride solution in pediatric subjects with functional fecal retentionGastroenterology2009136A129
- MoulinDRykxAKerstensRVandeplasscheLRandomized, double-blind, placebo-controlled trial to evaluate efficacy and safety of prucalopride (Resolor®) in patients with opioid-induced constipationGastroenterology2008134A92
- KroghKJensenMBGandrupPEfficacy and tolerability of prucalopride in patients with constipation due to spinal cord injuryScand J Gastroenterol20023743143611989834
- D’HoogheBGuillaumDMedaerRTreatment of constipation in multiple sclerosis patients: pilot study with the novel enterokinetic prucaloprideNeurogastroenterol Motil199911A256
- CamilleriMKerstensRRykxAVandeplasscheLA placebo-controlled trial of prucalopride for severe chronic constipationN Engl J Med20083582344235418509121
- QuigleyEMVandeplasscheLKerstensRAusmaJClinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation – a 12-week, randomized, double-blind, placebo-controlled studyAliment Pharmacol Ther20092931532819035970
- TackJvan OutryveMBeyensGPrucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxativesGut20095835736518987031
- FrankLKleinmanLFarupCPsychometric validation of a constipation symptom assessment questionnaireScand J Gastroenterol19993487087710522604
- MarquisPDe La LogeCDuboisDDevelopment and validation of the Patient Assessment of Constipation Quality of Life questionnaireScand J Gastroenterol20054054055116036506
- GalandiukSRykxAAusmaJA two-period, double-blind, placebo-controlled study to evaluate the effects of re-treatment of prucalopride (Resolor) on efficacy and safety in patients with chronic constipationGut200857Suppl IIA86
- JohansonJFWaldATougasGEffect of tegaserod in chronic constipation: a randomized, double-blind, controlled trialClin Gastroenterol Hepatol2004279680515354280
- TackJMiddletonSHorneMPilot study of the efficacy of renzapride on GI motility and symptoms in patients with constipation-predominant irritable bowel syndromeAliment Pharmacol Ther2006231655166516696817
- Müller-LissnerSTreatment of chronic constipation with cisapride and placeboGut198728103310383311905
- PetticrewMRodgersMBoothAEffectiveness of laxatives in adultsQual Health Care20011026827311743157
- CamilleriMBeyensGKerstensRVandeplasscheLLong-term follow-up safety and satisfaction with bowel function in response to oral prucalopride in patients with chronic constipationGastroenterology2009136A31
- Van OutryveMJBeyensGKerstensRLong term follow-up study of oral prucalopride (Resolor) administered to patients with chronic constipation [abstract #T1400]Gastroenterology20081344 Suppl 1A547
- Propulsid (cisapride) Dear Healthcare Professional Letter. FDA, Safety Information. Washington DC: FDA/Safety, 2000. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm175000.htm Accessed Sep 27, 2009
- KrobertKABrattelidTLevyFOKaumannAJPrucalopride is a partial agonist through human and porcine atrial 5-HT4 receptors: comparison with recombinant human 5-HT4 splice variantsNaunyn Schmiedebergs Arch Pharmacol200537147347916012870
- De MaeyerJStraetemansRSchuurkesJLefebvreRPorcine left atrial and sinoatrial 5-HT(4) receptor-induced responses: fading of the response and influence of developmentBr J Pharmacol200614714015716331294
- BachTSyversveenTKvingedalA5HT4(a) and 5-HT4(b) receptors have nearly identical pharmacology and are both expressed in human atrium and ventricleNaunyn Schmiedebergs Arch Pharmacol200136314616011218067
- De MaeyerJHSchuurkesJALefebvreRASelective desensitization of the 5-HT4 receptor-mediated response in pig atrium but not in stomachBr J Pharmacol200915636237619154432
- PotetFBouyssouTEscandeDBaroIGI prokinetic drugs have different affinity for the human cardiac human ether-a-gogo K(+) channelJ Pharmacol Exp Ther20012991007101211714889
- ChapmanHPasternackMThe action of the novel GI prokinetic prucalopride on the HERG K+ channel and the common T897 polymorphEur J Pharmacol20075549810517109852
- PauDWorkmanAJKaneKARankinACElectrophysiological effects of prucalopride, a novel enterokinetic agent, on isolated atrial myocytes from patients treated with beta-adrenoceptor antagonistsJ Pharmacol Exp Ther200531314615315644433
- BoyceMKerstensRBeyensGCardiovascular safety of prucalopride in healthy subjects: results from two randomized, double-blind, placebo-controlled, cross-over trailsGastroenterology2009136T1265
- CamilleriMBeyensGKerstensRSafety assessment of prucalopride in elderly patients with constipation: a double-blind, placebocontrolled studyNeurogastroenterol Motil Epub200999
- BourasEPCamilleriMBurtonDDPrucalopride accelerates GI and colonic transit in patients with constipation without a rectal evacuation disorderGastroenterology200112035436011159875
- SlootsCEPoenACKerstensREffects of prucalopride on colonic transit, anorectal function and bowel habits in patients with chronic constipationAliment Pharmacol Ther20021675976711929394
- Committee for medicinal products for human use: summary of positive opinion for Resolor. EMEA Committee for medicinal products for human use. London: EMEA/Pre-Authorisation Evaluation of Medicines for Human Use, 2009. Available at: http://www.emea.europa.eu/pdfs/human/opinion/Resolor_44905009en.pdf Accessed Sep 27, 2009.
- CamilleriMDeiterenAInvited Review. Prucalopride for constipationExp Opin Pharmacother201011451461