 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective:
There has been growing interest in newer anti-epileptic drugs (AEDs) for seizure prophylaxis in the intensive care setting because of safety and monitoring issues associated with conventional AEDs like phenytoin. This analysis assessed the cost-effectiveness of levetiracetam versus phenytoin for early onset seizure prophylaxis after neurosurgery and traumatic brain injury (TBI).
Methods:
A cost-effectiveness analysis was conducted from the US hospital perspective using a decision analysis model. Probabilities of the model were taken from three studies comparing levetiracetam and phenytoin in post neurosurgery or TBI patients. The outcome measure was successful seizure prophylaxis regimen (SSPR) within 7 days, which was defined as patients who did not seize or require discontinuation of the AED due to adverse drug reactions (ADRs). One-way sensitivity analyses and probabilistic sensitivity analysis were conducted to test robustness of the base-case results.
Results:
The total direct costs for seizure prophylaxis were $8,784.63 and $8,743.78 for levetiracetam and phenytoin, respectively. The cost-effectiveness ratio of levetiracetam was $10,044.91 per SSPR compared to $11,525.63 per SSPR with phenytoin. The effectiveness probability (patients with no seizures and no ADR requiring change in therapy) was higher in the levetiracetam group (87.5%) versus the phenytoin group (75.9%). The incremental cost effectiveness ratio for levetiracetam versus phenytoin was $360.82 per additional SSPR gained.
Conclusions:
Levetiracetam has the potential to be more cost-effective than phenytoin for early onset seizure prophylaxis after neurosurgery if the payer’s willingness-to-pay is greater than $360.82 per additional SSPR gained.
Introduction
Patient who undergo neurosurgery are at increased risk of early postoperative seizure events.Citation1,Citation2 An estimated 20% to 50% of patients have at least one postoperative seizure, depending on the type of surgery.Citation3,Citation4 Early postoperative seizures, which are seizures that occur within 1 week of surgery, occur in 15% to 20% of neurosurgery patients.Citation3–Citation5 Patients who sustain traumatic brain injury (TBI) are also at increased risk, with about 6% to 10% of patients suffering early onset seizures.Citation6,Citation7 Incidence can be as high as 30% in certain groups, such as those with more severe head trauma, subdural hematomas, or penetrating head injuries.Citation6–Citation8 Seizure prophylaxis with antiepileptics has shown promise in reducing early onset seizures in both patient groups.Citation2,Citation9–Citation11 However, there is no commonly accepted treatment algorithm to provide guidance as to which anti-epileptic drug (AED) should be preferred which has led to a variety of clinical practices.Citation12
Phenytoin is the most common AED used after neuro-surgery and TBI for seizure prophylaxis.Citation9–Citation11,Citation13 In a meta-analysis that pooled early onset seizure events from five post neurosurgery trials, phenytoin was associated with decreased seizure risk in the first week by 44% (relative risk, RR: 0.56, 95% confidence interval [CI]: 0.38–0.84) compared to control.Citation13 Additionally, a review that pooled the two class I (randomized placebo controlled) studies investigating phenytoin prophylaxis after TBI showed a 63% reduction in seizures (RR: 0.37, 95% CI: 0.18–0.74).Citation9 Despite evidence that supports phenytoin use in early seizure prophylaxis, there are a number of issues that limit its use. Phenytoin requires constant laboratory monitoring which is a burden to the patient as well as time consuming for hospital staff.Citation14 Moreover, due to phenytoin’s zero-order (Michaelis-Menten) pharmacokinetics which result in a non-linear relationship between dose and subsequent serum levels, a small change in dose can result in a disproportionate increase in serum concentration.Citation15 Furthermore, rare and potentially fatal skin reactions have been reported with phenytoin, such as Stevens Johnson syndrome and purple glove syndrome.Citation16 Finally, phenytoin can act as a substrate or inducer of several of the cytochrome P450 enzyme which can potentially lead to drug interactions that may consequently require dose adjustments or discontinuation of medications.Citation14
Due to these known problems with phenytoin for early seizure prophylaxis after neurosurgery, there has been an interest in using alternative AEDs for this indication. Other medications that have been evaluated for early onset seizure prophylaxis include carbamazepine, valproate, phenobarbital, and levetiracetam.Citation9,Citation17 Shaw et al evaluated cabamazepine (CBZ) against a no treatment historical cohort after neurosurgery and reported a 39% reduction in seizure events (RR: 0.61, 95% CI: 0.29–1.29).Citation4 Early onset seizure incidence was 11% in the CBZ group (n = 106) and 19% in the no treatment historical cohort (n = 59).Citation4 Glötzner et al in a prospective placebo-control study, assessed CBZ after TBI and reported a 63% reduction in early seizure events (RR: 0.37, 95% CI: 0.18–0.78).Citation18 Seizure incidence was 10.7% in the CBZ group (n = 75) and 28.9% (n = 76) in the placebo group.Citation18 Holland et al, in a randomized double-blind study, evaluated valproate for seizure prophylaxis after craniotomy and reported a non-significant 15% reduction (RR: 0.85, 95% CI: 0.54–1.36) in early and late seizures combined.Citation19 Seizure event rates were 17.8% for patients in the valproate group (n = 152) and 20.8% for patients in the control group (n = 149).Citation19 Franceschetti et al reported that early seizures occurred in 7% of patients in a pooled phenytoin and phenobarbital group (n = 41) compared to 18% in the no treatment group (n = 22) after neurosurgery; however, this difference was not statistically significant.Citation20
Unlike the other AEDs, levetiracetam offers several advantages for early onset seizure prophylaxis in neurosurgical or TBI patients. There are no required laboratory monitoring with levetiracetam; whereas, phenytoin requires plasma level and liver function tests. Levetiracetam has a wide therapeutic window and predictable pharmacokinetics which make dosing convenient. No severe or life threatening adverse drug reactions have been reported with levetiracetam. In addition, there are very few known common drug interactions with levetiracetam. However, intravenous (iv) levetiracetam acquisition cost is much more expensive compared to phenytoin. Though prices will vary between different institutions and payers, the acquisition cost of iv levetiracetam is generally greater than the acquisition cost of iv phenytoin. Currently, there is no pharmacoeconomic analysis investigating early onset seizure prophylaxis with iv levetiracetam compared to iv phenytoin. To our knowledge, this was the first analysis to investigate the cost-effectiveness potential of levetiracetam versus phenytoin in postoperative early seizure prophylaxis.
Methods
Literature search strategy
A literature search was performed to identify clinical trials that investigated iv levetiracetam compared to iv phenytoin in post neurosurgical or TBI patients. Studies had to be a head-to-head comparison between iv levetiracetam and iv phenytoin after neurosurgery or TBI. A total of 3 studies was identified and data were abstracted by two independent reviewers.Citation21–Citation23 We focused on short-term seizure prophylaxis (within 7 days) and used the weighted mean average of seizure outcomes for our efficacy parameters in the decision analysis model. Probability parameters for ADR leading to discontinuation was taken from a single study.Citation21 This study had the largest patient population and was the only study which clearly gave discontinuation rates secondary to ADRs.Citation21
Decision analysis model
A cost-effectiveness analysis using a decision analysis model () was conducted from the United States (US) hospital perspective. The main outcome measure was a successful seizure prophylaxis regimen (SSPR). SSPR was defined as a patient receiving seizure prophylactic therapy that neither seized nor had an ADR resulting in a change in therapy. This outcome was justified by capturing seizure prevention as well ADR avoidance. Model parameters for efficacy outcomes were based on three published clinical trials ().Citation21–Citation23
Figure 1 Decision analysis model. Square nodes indicate a decision is being made. Circle nodes indicate a chance probability. Triangles nodes represent terminal nodes.
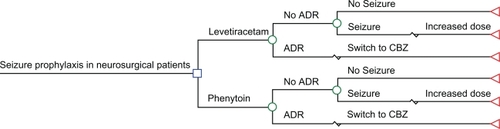
Table 1 Studies (comparing levetiracetam vs phenytoin) utilized for model probabilities
Parameters for each branch of the decision analysis model included: 1) ADR probability requiring discontinuation for each drug; and 2) seizure probabilities for the study drugs. The dosages and frequency for study drugs were assumed to be: iv levetiracetam 500 mg twice a day and iv phenytoin 100 mg 3 times a day. Patients who received iv phenytoin were also assumed to received a loading dose of fosphenytoin using 1500 mg (phenytoin equivalent) on day one of therapy.Citation24
For parameters of the model that were not available in the literature, expert opinions were solicited. Two intensive care unit (ICU) pharmacists at our institution were interviewed and asked to answer questions on treatment probability. Based on expert opinions, an average ICU stay for neurosurgical patients was defined as 5 days. For patients who had an ADR requiring a change in therapy, it was assumed that their therapy adjustment was made 2 days postoperatively, which resulted in 2 additional days in the ICU. Patients were then switched to a third agent (oral carbamazepine (CBZ) 200 mg 3 times a day), which was assumed to be effective. Moreover, patients were assumed to stay 5 additional days in the ICU after switching to CBZ. Carbamazepine was chosen as an appropriate agent to switch to after failure with either iv levetiracetam and iv phenytoin both by expert opinions and literature.Citation4,Citation9
It was assumed that seizure events would occur 2 days postoperatively, where their dose would be increased (iv levetiracetam to 1000 mg twice a day and iv phenytoin to 400 mg daily) and assumed to be effective.Citation17 The patient was then assumed to stay 5 additional days in the ICU (per expert opinions). Based on expert opinions, it was estimated that patients having either a seizure or an ADR requiring a change in therapy will need 2 extra days in the ICU. Patients on iv phenytoin were assumed to have 3 phenytoin levels drawn if duration of therapy was 5 days or less or four levels drawn if duration of therapy was more than 5 days (per expert opinions). All decision model inputs are provided in .
Table 2 Base-case parameters for the decision analysis model
Breakdowns of each individual arm in the decision model are as follows:
The patient receives levetiracetam iv 500 mg twice a day for 5 days and stays in the ICU for 5 days. The patient does not experience a seizure nor has an ADR requiring change in therapy.
The patient receives levetiracetam iv 500 mg twice a day for 2 days and seizes. The dose is increased to 1000 mg twice a day for 5 days and is assumed to be successful. Patient spends a total of 7 days in the ICU.
The patient receives levetiracetam iv 500 mg twice a day for 2 days and has an ADR requiring change in therapy. The patient is then switched to CBZ iv 200 mg 3 times a day for 5 days and is assumed to be successful. Patient spends a total of 7 days in the ICU.
The patient receives phenytoin iv 100 mg 3 times a day for 5 days and stays in the ICU for 5 days. The patient neither seizes, nor has an ADR requiring change in therapy. The patient was assumed to have three phenytoin levels drawn.
The patient receives phenytoin iv 100 mg 3 times a day for 2 days and seizes. The dose is increased to a 400 mg total daily dose for 5 days and is assumed to be successful. Patient spends a total of 7 days in the ICU. The patient was assumed to have four phenytoin levels drawn.
The patient receives phenytoin iv 100 mg 3 times a day for 2 days and has an ADR requiring change in therapy. The patient is then switched to CBZ iv 200 mg 3 times a day for 5 days, which is assumed to be successful. Patient spends a total of 7 days in the ICU. The patient was assumed to have three phenytoin levels drawn.
Economic analysis
This analysis was performed from the US hospital perspective ($US). As a result, only total direct costs were assessed. Drug acquisition costs were taken from the 2008 Red Book.Citation25 Hospital and laboratory costs were taken from the Veterans Affairs Decision Support Services (DSS) database (2008). DSS is the Veterans Affairs national database that provides costs for resource utilization directed at patient care. Discounting was not taken into account due to the short duration of the DA model.
Primary endpoint was the incremental cost-effectiveness ratio (ICER), which was calculated using the following equation:Citation26,Citation27
where C is the total direct cost for the different treatment strategies and E is the probability of SSPR. Average cost-effectiveness ratio (CER) was calculated for iv levetiracetam and iv phenytoin using the following equation:Citation26,Citation27
where C is the total direct cost of using either iv levetiracetam or iv phenytoin and E is the probability of achieving SSPR with either iv levetiracetam or iv phenytoin.
Sensitivity analysis
We evaluated the impact of parameter uncertainty by conducting one-way sensitivity analyses on all model parameters over the ranges listed in . A tornado diagram was used to illustrate the impact each of the one-way sensitivity analyses had on the incremental cost difference between iv levetiracetam and iv phenytoin. Probabilistic sensitivity analysis (second-order Monte Carlo simulation) was conducted using a cohort of 10,000 trial simulations. In probabilistic sensitivity analysis, each parameter of the model was given a reasonable range with a distribution function which was randomly drawn using the stochastic process.Citation26,Citation28 Model probabilities were sampled using a beta distribution, and cost data were sampled using a gamma distribution.Citation27 Resource utilization was sampled using a normal distribution. Random input values were then simulated through the model for a theoretical cohort of patients.Citation26 Scatter plot distribution of ICER was plotted on a cost-effectiveness plane where the incremental cost is represented along the y-axis and the incremental efficacy is represented along the x-axis.Citation29,Citation30 A dominant strategy was defined as a strategy with lower incremental cost and higher incremental benefit. A cost-effectiveness acceptability curve (CEAC) was created in order to identify which treatment would be more cost-effective at various willingness-to-pay (WTP) thresholds (cost per additional SSPR gained).Citation29,Citation30 As WTP increases on the x-axis from left to right, the probability of iv levetiracetam being a cost-effective strategy compared to iv phenytoin may change based on the observed data. Analysis was performed using TreeAge Pro Suite 2008 (TreeAge Software Inc., Williamstown, MA).
Results
In the base-case analysis, the total direct cost of iv levetiracetam and iv phenytoin were $8,784.63 and $8,742.78 per patient, respectively (). The cost-effectiveness ratios for iv levetiracetam and iv phenytoin were $10,044.91 per SSPR and $11,525.63 per SSPR, respectively. The ICER for using iv levetiracetam versus iv phenytoin was $360.82 per SSPR gained. The efficacy rate (patients with no seizures and no ADR requiring change in therapy) was higher in the iv levetiracetam group (87.5%) versus the iv phenytoin group (75.9%), an absolute difference of 11.6% favoring iv levetiracetam.
Table 3 Base-case result of the decision analysis model
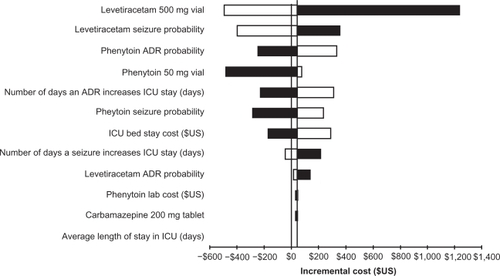
A tornado diagram illustrates the impact of each model parameter on the incremental cost difference between iv levetiracetam and iv phenytoin (). Decreasing the cost of a vial of levetiracetam 500 mg and the probability of seizures with iv levetiracetam had the most impact on the base-case results. Sensitivity was observed in 8 of the parameters used in the DA model. In several cases, iv levetiracetam was a dominant strategy compared to iv phenytoin. Increasing phenytoin’s probability of ADR, vial cost, and seizure probability resulted in iv levetiracetam being dominant. Increasing the number of days that an ADR results in an increase in ICU stay and ICU bed stay cost resulted in iv levetiracetam being dominant. Decreasing the number of days in the ICU as a consequence of a seizure event resulted in iv levetiracetam being dominant.
Figure 2 Tornado diagram for one-way sensitivity analyses performed on the decision analysis model. The x-axis represents the incremental cost between intravenous (iv) levetiracetam and iv phenytoin. The parameters that were tested in the one-way sensitivity analyses are listed on the y-axis. Model parameters with the most influence on the base-case are listed at the top in descending order. The base-case incremental cost was $41.85. Any model parameter that crosses the threshold (incremental cost <$0) creates a scenario where levetiracetam is dominant. White bars represent scenarios where decreasing the value of the parameter leads to iv levetiracetam being dominant compared to iv phenytoin. Black bars represent scenarios where increasing the value of the parameter leads to iv levetiracetam being dominant compared to iv phenytoin.
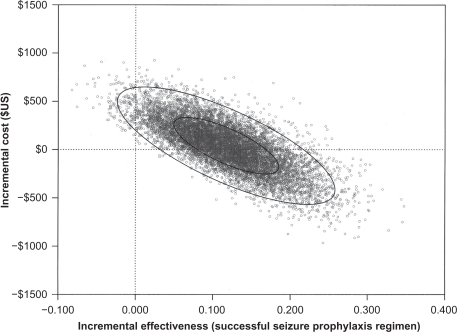
In the probabilistic sensitivity analysis, a majority of the trial simulations were displayed in the northeast (trade-off) quadrant (56.11%) with a small sample of the trial simulations in the northwest (dominated) quadrant (1.66%); but 42.33% of the scatter plots were displayed in the dominant quadrant (southeast quadrant) (). Simulations falling in the northeast quadrant were considered a “trade-off ” because the treatment (iv levetiracetam) was more expensive but more effective than the comparator (iv phenytoin).Citation31 Simulations falling in the northwest quadrant were considered dominated because the treatment (iv levetiracetam) was less effective and but more costly than the comparator (iv phenytoin).Citation31 Simulations falling into the southwest quadrant were considered a “trade-off ” because the treatment (iv levetiracetam) was less expensive but less effective than the comparator (iv phenytoin). Simulations falling in the southeast quadrant were considered dominant because the treatment (iv levetiracetam) was less costly and more effective than the comparator (iv phenytoin).Citation31
Figure 3 Probabilistic sensitivity analysis comparing intravenous (iv) levetiracetam and iv phenytoin in early seizure prophylaxis in post neurosurgical or neurological damage patients. The x-axis represents the incremental effectiveness, successful seizure prophylaxis regimen. The y-axis represents the incremental costs between iv levetiracetam and iv phenytoin. Each circle represents a single simulation for a total of 10,000 trial simulations. The inner ellipse represents the 50% distribution of the individual trials. The outer ellipse represents the 95% distribution of the individual trials.
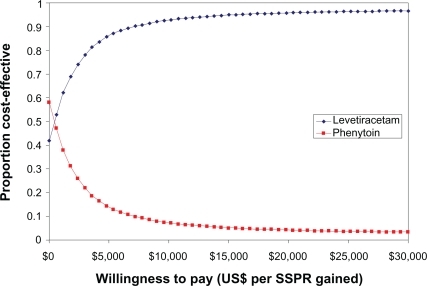
A CEAC is a common and useful way to graphically represent uncertainty in economic analyses of healthcare technologies.Citation29,Citation30 It represents the probability that a given treatment will be cost-effective when compared to an alternative at different thresholds of what decision makers are willing to pay for a given outcome.Citation29,Citation30 For example, if the WTP was defined as $15,000 for each additional SSPR gained, then iv levetiracetam is 95% cost-effective compared to iv phenytoin (). The CEAC illustrates that iv levetiracetam was cost-effective at a WTP greater than $360.82 per additional SSPR gained compared to iv phenytoin. Conversely, iv phenytoin would be cost-effective at a WTP less than $360.82 per additional SSPR gained compared to iv levetiracetam.
Figure 4 Cost-effectiveness acceptability curve between intravenous (iv) levetiracetam and iv phenytoin. The x-axis represents the willingness-to-pay for each additional successful seizure prophylaxis regimen (SSPR) gained. The y-axis represents the probability of the study medication being cost-effectiveness relative to the comparator. At a willingness-to-pay of $15,000 for each additional SSPR gained, the probability that iv levetiracetam is 95% compared to iv phenytoin.
Discussion
The results of our analysis suggest that levetiracetam may be a cost-effective early onset seizure prophylaxis strategy after neurosurgery or TBI when the WTP is greater than $360.82 per additional SSPR gained. There is no commonly accepted treatment algorithm for early seizure prophylaxis after neurosurgery or TBI; however, phenytoin has the most evidence and is commonly used in most institutions including our own local facility.Citation12 However, iv phenytoin has several limitations, including drug level monitoring, liver enzyme elevation, rare but serious skin reactions, and significant drug interactions.Citation14,Citation15 This has led to an interest in using newer AEDs for this indication, such as iv levetiracetam, which do not have the aforementioned problems.
The average cost-effectiveness ratio of levetiracetam was approximately 7% lower than that of phenytoin, with an ICER of $360.82 per SSPR gained. The additional resources spent on iv levetiracetam are partially offset by decreases in other costly healthcare resources such as length of ICU bed stay and laboratory monitoring. In our study, length of stay (which was influenced by seizure prophylaxis drug choice) was the primary cost driver. Decision maker’s consideration for this early seizure prophylaxis in neurosurgery patients will need to be determined by the institution and based on their WTP. Clearly, the reduction in LOS and improvement in seizure prevention are advantages to using iv levetiracetam; however, it was not a dominant strategy in our analysis and would require additional budget or reallocation of funds in order to realize these benefits.
The model was not robust to the sensitivity analyses as evident by the tornado diagram. Eight of the 12 parameters that underwent one-way sensitivity analyses were sensitive across the range used. These examples demonstrate that iv levetiracetam dominance is dependent on slight changes to the model parameters. Each institution may have a different patient population that is not reflected by the base-case; as a result, any deviations from the DA model parameters can result in iv levetiracetam being a dominant strategy. Careful interpretation of the study is necessary when applying the results to different institutions.
Our sensitivity analysis showed that laboratory costs associated with phenytoin was non-contributory. However, this analysis did not capture indirect costs such as staffing resource allocation needed for phenytoin monitoring. The drawing and monitoring of these labs can consume various healthcare workers’ time, including clinical pharmacists, nurses, physicians, and laboratory workers. Freeing hospital employees from this burden could free them for other patient care activities and potentially improve work flow. In our analysis, the decision to not include these additional costs most likely underestimated the overall costs of iv phenytoin. Future studies should evaluate the influence of indirect costs to overall decision making.
There are several limitations to our analysis. First, the clinical data used for model probabilities was taken from a heterogenic collection of studies that were prospective,Citation23 retrospective,Citation21 and pseudo-prospectiveCitation22 studies, and evaluated patients who underwent neurosurgeryCitation21 or had neurologic damage.Citation22,Citation23 The results may not necessarily be generalizable to indications such as different types of neurosurgery and traumatic brain injuries. Larger studies dedicated to each indication will be necessary to clarify if there are differences in seizure control. Second, the outcome parameter used in the DA model (eg, SSPR) is unique and does not have an explicit cost-effectiveness threshold associated with it. This makes it difficult to determine the cost-effectiveness across different disease states. Standardized outcomes such as quality-adjusted life years (QALYs) are able to transcend different disease states and provide decision makers with a common parameter to compare treatment interventions.Citation27 However, the short duration of the current study makes the usefulness of QALY as an outcome uncertain. Third, this study only applies to early onset seizure prophylaxis within one week of neurosurgery or neurologic damage, and cannot be extrapolated beyond this short term time frame. Seizure events outside the time frame (ie, late seizures) may be associated with the efficacy of the treatment intervention; however, there may be other confounding variables that can affect this outcome.Citation13 Decision makers should use caution when generalizing these results to their institutions.
The role that early seizures play in development of late seizures is not clear. After TBI, patients with early seizures have a 17% to 33% chance of developing late epilepsy, compared to just 2% of all TBI patients.Citation32 Multivariate analyses on this topic have had mixed results. One pooled analysis of 783 high risk trauma patients followed for 2 years via a clinical trial found early seizures to be an independent risk factor for epilepsy.Citation6 However, another population based cohort that followed 4541 adults and children for >20 years after head injury did not find early seizures to be correlated with development of epilepsy.Citation33 More evidence is needed to determine if a relationship between early and late seizure exists. In addition, it is unclear if utilization of newer AEDs for early prophylaxis results in an increase use for late prophylaxis which may affect long term costs. Such an impact is unclear and was not assessed in this study. However, switching patients to more cost-effective agents in a stable outpatient setting seems feasible. Clinical practice on this matter will likely vary widely between institutions.
In our analysis, iv levetiracetam was more cost-effective compared to iv phenytoin for early onset seizure prophylaxis after neurosurgery or neurologic damage. There is an interest in using the newer AEDs (eg, iv levetiracetam) for seizure prophylaxis in neurosurgery patients due to concerns with constant laboratory monitoring, unpredictable drug concentration with older agents (eg, phenytoin), and ADRs. Some institutions have switched to newer agents like levetiracetam for early onset seizure prophylaxis, but practice still varies from institution to institution. Moreover, there is no clear consensus on which prophylactic regimen should be used at this time and some institutions may use agents which were not assessed in this analysis.
Future prospective clinical studies are needed to provide more complete evidence to support use of the newer AEDs in this indication. Future pharmacoeconomic analyses should take advantage of new clinical data when it becomes available and reevaluate the cost-effectiveness analysis of iv levetiracetam versus iv phenytoin in early seizure prophylaxis in postoperative neurosurgical or TBI patients.
Disclosures
The authors have no conflict of interest to report. This study received no funding.
References
- DeutschmanCSHainesSJAnticonvulsant prophylaxis in neurosurgeryNeurosurgery19851735105172864654
- ManankaSIshijimaBMayanagiYPostoperative seizures: epidemiology, pathology, and prophylaxisNeurol Med Chir (Tokyo)2003431258960014723265
- NorthJBPenhallRKHaniehAFrewinDBTaylorWBPhenytoin and postoperative epilepsy: a double-blind studyJ Neurosurg19835856726776339686
- ShawMDFoyPMEpilepsy after craniotomy and the place of prophylactic anticonvulsant drugs: discussion paperJ R Soc Med19918442212232027149
- FoyPMCopelandGPShawMDThe natural history of post operative seizuresActa Neurochir (Wien)1981571–215227270268
- TemkinNRRisk factors for posttraumatic seizures in adultsEpilepsia200344Suppl 10182014511390
- FreyLCEpidemiology of posttraumatic epilepsy: a critical reviewEpilepsia200344Suppl 10111714511389
- LeeSTLuiTNEarly seizures after mild closed head injuryJ Neurosurg19927634351738023
- ChangBSLowensteinDHPractice parameter: antiepileptic drug prophylaxis in severe traumatic brain injury: report of the Quality Standards Subcommittee of the American Academy of NeurologyNeurology2003601101612525711
- SchierhoutGRobertsIAnti-epileptic drugs for preventing seizures following acute traumatic brain injuryCochrane Database Syst Rev.20014CD00017311687070
- AgrawalATimothyJPanditLManjuMPost-traumatic epilepsy: an overviewClin Neurol Neurosurg2006108543343916225987
- CoplinWRhoneyDJohnsonSJohnsonRZafonteRLevineSNational survey of the use of anticonvulsant prophylaxis after aneurysmal subarachnoid interim findings. [abstract]Proceedings of the 70th American Association of Neurological Surgeons Annual Meeting2001 April 21–26Toronto, Ontario, Canada
- TemkinNRProphylactic anticonvulsants after neurosurgeryEpilepsy Curr20022410510715309132
- Phenytoin drug information www.uptodateonline.com. Accessed July 23, 2009.
- AllenJLuddenTBurrowSClementiWStavchanskySPhenytoin accumulation kineticsClin Pharmacol Ther1979264445448487692
- ScheinfeldNImpact of phenytoin therapy on the skin and skin diseaseExpert Opin Drug Saf20043665566515500423
- TemkinNRAntiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trialsEpilepsia200142451552411440347
- GlötznerFLHaubitzIMiltnerFKappGPflughauptKWAnfallsprophylaxe mit Carbamazepin nach schweren SchädelhirnverletzungenNeurochirurgia198326366796410292
- HollandJPStapletonSRMooreAJMarshHTUttleyDBellBAA randomized double blind study of sodium valproate for the prevention of seizures in neurosurgical patientsProceedings of the 125th Meeting of the Society of British Neurological Surgeons1994 September 7–9University of Dundee, Scotland, UK
- FranceschettiSBinelliSCasazzaMInfluence of surgery and antiepileptic drugs on seizures symptomatic of cerebral tumoursActa Neurochir19901031–24751
- MilliganTHurwitzSBromfieldEEfficacy and tolerability of levetiracetam versus phenytoin after supratentorial neurosurgeryNeurology200871966566918725591
- JonesKPuccioAHarshmanKLevetiracetam versus phenytoin for seizure prophylaxis in traumatic brain injuryNeurosurg Focus2008254E318828701
- SzaflarskiJPSanghaKSLindsellCJShutterLAProspective, randomized, single-blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxisNeurocrit Care2009117 [Epub ahead of print]
- SwadronSPRudisMIAzimianKBeringerPFortDOrlinskyMA comparison of phenytoin-loading techniques in the emergency departmentAcad Emerg Med200411324425215001403
- FlemingTRedbook: Pharmacy’s Fundamental ReferenceMontvale, NJThomson PDR2008
- BriggsASculpherMClaxtonKDecision Modeling for Health Economic EvaluationOxfordOxford University Press2006
- PetittiDBMeta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine2nd editionUSAOxford University Press2000
- ShawJWZachryWMApplication of probabilistic sensitivity analysis in DA modelingFormulary200237132340
- FenwickEO’BrienBJBriggsACost-effectiveness acceptability curves – facts, fallacies, and frequently asked questionsHealth Econ200413540541515127421
- FenwickEByfordSA guide to cost-effectiveness acceptability curvesBr J Psychiatry200518710610816055820
- BriggsAFennPConfidence intervals or surfaces? Uncertainty on the cost-effectiveness planeHealth Econ1998787237409890333
- PagniCAZengaFPosttraumatic epilepsy with special emphasis on prophylaxis and preventionActa Neurochir Suppl200593273415986723
- AnnegersJFHauserWACoanSPRoccaAA population-based study of seizures after traumatic brain injuriesN Engl J Med1998338120249414327