Abstract
Testosterone compounds have been available for almost 70 years, but the pharmaceutical formulations have been less than ideal. Traditionally, injectable testosterone esters have been used for treatment, but they generate supranormal testosterone levels shortly after the 2- to 3-weekly injection interval and then testosterone levels decline very rapidly, becoming subnormal in the days before the next injection. The rapid fluctuations in plasma testosterone are subjectively experienced as disagreeable. Testosterone undecanoate is a new injectable testosterone preparation with a considerably better pharmacokinetic profile. After 2 initial injections with a 6-week interval, the following intervals between two injections are almost always 12-weeks, amounting eventually to a total of 4 injections per year. Plasma testosterone levels with this preparation are nearly always in the range of normal men, so are its metabolic products estradiol and dihydrotestosterone. The “roller coaster” effects of traditional parenteral testosterone injections are not apparent. It reverses the effects of hypogonadism on bone and muscle and metabolic parameters and on sexual functions. Its safety profile is excellent due to the continuous normalcy of plasma testosterone levels. No polycythemia has been observed, and no adverse effects on lipid profiles. Prostate safety parameters are well within reference limits. There was no impairment of uroflow. Testosterone undecanoate is a valuable contribution to the treatment options of androgen deficiency.
Introduction to the various testosterone preparations for treatment of hypogonadal men
Soon after its chemical identification more than 70 years ago, the male hormone testosterone (T) became pharmaceutically available. But it took considerable time before convenient and safe preparations were developed. Three approaches have been used to make T therapeutically effective: (1) routes of administration, (2) esterification in the 17β-position, and (3) chemical modification of the molecule, or a combination of approaches. In clinical practice, particularly in the perception of the patient, the route of administration is most relevant, and is used to categorize the preparations described here. Recent reviews of treatment modalities may be recommended (CitationGooren and Bunck 2004; CitationNieschlag 2006).
Free unesterified T is absorbed well from the gut but is effectively metabolized and inactivated in the liver before it reaches the target organs. Pharmacological changes of the T molecule in the 17α-position render the molecule orally effective. Alkylated derivatives of T including methyltestosterone and fluoxymesterone are administered orally or sublingually. They are metabolized by the liver, like natural T, but more slowly, and, like T, interact directly with androgen receptors. Clinical responses are variable and plasma levels cannot be determined accurately, because alkylated androgens are not recognized by most T assays. Prolonged use (especially the 17α– alkylated androgens) has been associated with hepatotoxicity including hepatocellular adenoma, cholestatic jaundice, and hemorrhagic liver cysts (CitationGooren and Bunck 2004; CitationBagatell and Bremner 1995).
Oral testosterone undecanoate
Testosterone undecanoate (TU) is T esterified in the 17β-position with a long aliphatic sidechain, undecanoic acid, dissolved in oil and encapsulated in soft gelatin. Of the 40 mg capsules, 63% (25 mg) is T. After ingestion, for a portion (around 5%) of the administered dose the route of absorption from the gastrointestinal tract is shifted from the portal vein to the thoracic duct. Due to its aliphatic chain it travels with lipids in the lymph and reaches the general circulation via the subclavian vein, thus avoiding a first pass through the liver and subsequent metabolism of T (CitationBehre et al 2004). The dosing is as a rule 80 mg twice daily.
For its adequate absorption from the gastrointestinal tract it is essential that oral TU is taken with a meal that contains dietary fat. Maximum serum levels are reached 2–6 hours after ingestion, and result in fluctuating serum T levels (for a review see CitationBehre et al 2004). To increase shelf-life the preparation was recently reformulated and the oil in the capsule is now castor oil. Recent studies show that there is dose proportionality between serum T levels and the dose range of 20–80 mg. With a dose of 120–240 mg per day, over 80% of hypogonadal men showed plasma T levels in the normal range over 24 hours (CitationBagchus et al 2003).
Transbuccal T administration
Transbuccal administration of T provides a means of oral administration of T. It is marketed as a biopellet to be pressed on the gum above the incisor tooth; then the buccal film that develops is be put between the lower gum and cheek. The resorption of T through the oral mucosa avoids intestinal absorption and subsequent hepatic inactivation of T. An informative study is the report of CitationDobs et al (2004). This study compared treatment with T buccal system (Striant®, Columbia Laboratories, Inc.) 30 mg twice daily, with a transdermal gel delivery system, 5 g containing 1% (50 mg) T, daily for 14 days in androgen-deficient men. 92.3% of T buccal system and 83.3% of T gel patients had C(ave[0–24]) within the normal range of 10.4–36.4 nmol/L (3.0–10.5 ng/mL). Mean total T values were not different in the T buccal system group (C[ave(0–24)] 16.7 ± 4.7 nmol/L; 4.8 ± 1.4 ng/mL) compared with the T gel group (C[ave(0–24)] 15.9 ± 4.8 nmol/L; 4.6 ± 1.4 ng/mL). The effects of buccal T on sexual functioning were comparable with those of parenteral T enanthate (CitationWang et al 2004b).
Sublingual T administration
Sublingual application of T has been tested with the inclusion of the hydrophobic T molecule with 2-hydroxypropyl-β-cyclodextrin (HPBCD). HPBCD enhances T solubility and absorption but HPBCD itself is not absorbed (CitationStuenkel et al 1991). The integrated dihydrostestosterone (DHT)/T ratio was normal. Serum E2 remained in the normal range. There were no accumulations of steroid hormones over the 7 day test period (CitationStuenkel et al 1991; CitationWang et al 1996). Effects on sexual behavior were comparable to those of parenteral administration of 200 mg T enanthate every 20 days.
Transdermal delivery
Testosterone can be delivered to the circulation through the intact skin, both genital and non-genital (CitationGooren and Bunck 2004; CitationNieschlag 2006). Transdermal administration delivers T at a controlled rate into the systemic circulation avoiding hepatic first pass and reproducing the diurnal rhythm of T secretion, without the peak and through levels observed in long-acting T injections.
Scrotal T patch
Scrotal patches were first designed to deliver T through the scrotal skin, where the permeability is 5 times greater than for other skin sites (CitationBehre et al 2004). Clinical results were satisfactory. Transdermal scrotal T administration is associated with high levels of DHT as a result of high concentrations of 5α-reductase in the scrotal skin (CitationBehre et al 2004). The patch may be irritating and the use is not feasible if the scrotal surface is not adequate. The scrotal patches sometimes fell off the scrotum, leaving the patient undersubstituted. To overcome these limitations, non-scrotal skin patches have been developed.
Non-scrotal T patch
These patches have a reservoir containing T with a permeation-enhancing vehicle and gelling agents (CitationMeikle et al 1996). Clinical efficacy was as good as with conventional T ester injections. Though pharmacokinetically and clinically satisfactory, there are adverse effects such as local skin reactions. Fifty percent of men participating in a clinical trial reported transient, mild to moderate erythema at sometime during therapy (CitationMeikle et al 1996).
Testosterone gel
Testosterone gel is also used for replacement therapy. Testosterone gel is hydro-alcoholic, 1% (10 mg T/g of gel), and administered at between 5 and 10 g of gel a day, amounting to 50 and 100 mg T, respectively. The pharmacokinetics of T gel have been extensively studied (CitationMeikle et al 2004). Serum T levels rose 2- to 3-fold 2 hours after application and rose further to 4- to 5-fold after 24 hours. Thereafter serum T remained steady in the upper range of normal and returned to baseline within 4 days after termination of application of T gel (CitationMeikle et al 2004). Mean DHT levels followed the same pattern as T and were at or above the normal adult male range. Serum E2 levels rose and followed the same patterns as T. Later studies showed that 9%–14% of the T administered is bioavailable. Steady state T levels are achieved 48–72 hours after the first application (CitationWang et al 2000). The formulation of the T gel allows easy dose adjustments (50–75–100 mg T gel) (CitationWang et al 2004a).
The clinical efficacy of transdermal T gel on various androgen-dependent target organ systems has been well documented. The safety profile showed that prostate-specific antigen levels rose in proportion to the increase of T levels but did not exceed normal values. Skin irritation was noted in 5.5% of patients in the study (CitationWang et al 2004a). Transfer from one person to another was found to be insignificant (CitationRolf et al 2002). No increase of serum T was found after intense rubbing of skin with persons whose endogenous T levels had been suppressed (CitationRolf et al 2002). But recently, three cases of transfer from fathers to their children have been reported (CitationBrachet et al 2005).
Intramuscular administration
Testosterone implants
Subdermal pellet implantation was among the earliest effective treatment modalities for clinical use of T and became an established form of androgen replacement by 1940 (for review (CitationKelleher et al 2004)). Several reports have outlined its desirable pharmacological properties but its use and merits and its complications (such as infection and extrusion) have been best documented by the group of Handelsman (CitationKelleher et al 2004).
Testosterone esters
The most commonly used forms of androgen replacement therapy include 17β-hydroxyl esters of T administered with slow-release, oil-based vehicles. Commonly used intramuscular-injectable T esters are T enanthate and cypionate (CitationBehre et al 2004; CitationGooren and Bunck 2004; CitationNieschlag 2006).
Testosterone enanthate is one of the most widely used intramuscular T esters. At a dose of 200–250 mg the optimal injection interval is 2–3-weeks but peak and through values are clearly above and below the normal range (CitationBehre et al 2004).
Testosterone propionate has a terminal half-life of only 19 hours. After a single injection of 50 mg the maximum concentration is reached after approximately 14 hours (CitationBehre et al 2004). On the basis of this profile, injection intervals are only 2–3 days with peak and through values above and below the normal range and therefore not suitable for monotherapy of T deficiency. Alternatively, 100 mg weekly may be given.
Other T esters are T cypionate and T cyclohexanocarboxylate. The pharmacokinetics of these T esters are very simlar to those of T enanthate (CitationBehre et al 2004). Administration of 200 mg every 2-weeks provides an acceptable form of T replacement.
Several commercially available T preparations contain a number of short- and longer-acting T esters aiming to deliver more even serum T levels. Pharmacokinetic studies of these preparations show that this goal is not achieved. The peak values are higher than in single T ester preparations and resulting plasma T levels show even larger fluctuations (CitationBehre et al 2004). So, most intramuscular presentations of T are not ideal. With the most commonly used T esters a maximum concentration follows approximately 72 hours after injection. T levels slowly diminish during the following 10–14 days showing an exponential decline of serum T levels, reaching baseline at approximately day 21 (CitationBehre et al 2004). As a result the T levels before the next injection are low (CitationBehre et al 2004; CitationGooren and Bunck 2004). The normal pattern of circadian rhythm of T is not provided, though it is questionable whether the circadian rhythm has much therapeutic relevance. The injections are painful (CitationGooren and Bunck 2004). Although levels of 5α dihydrotestosterone are normal, androgen metabolites are frequently not physiological and estradiol concentrations may become excessive in some men. The profile of T levels may be accompanied by disturbing fluctuations in sexual function, energy level, and mood (CitationGooren and Bunck 2004; CitationNieschlag 2006). High post-injection levels of T predispose the patient to acne and polycythemia, and elevated estradiol predisposes to gynecomastia. In some patients, injections may be associated with bleeding or bruising (CitationNieschlag 2006). However, these long-acting testosterone preparations have long been the mainstay of T treatment and they are the most cost-effective methods, with administration of 200 to 400 mg every 2–4-weeks. The 200 mg injection will maintain normal T for approximately 2-weeks while 300 mg doses are required for eugonadal ranges for approximately 3-weeks (CitationBehre et al 2004).
Androgen replacement with 5α reduced testosterone: 5α dihydrotestosterone.
The effects of T are mediated directly as testosterone or after conversion to either 5α DHT or estradiol locally in target tissues. The reduction of T to DHT is an amplification mechanism of the androgenizing effects of T. DHT binds to the same receptor as T but its receptor binding is stronger, resulting in a considerable higher biopotency than T itself. DHT, as opposed to T, cannot be aromatized to estradiol and acts, therefore, as a pure androgen. In certain clinical conditions a pure androgen might have advantages over aromatizable testosterone, such as cases of a microphallus, hypogonadal men with a susceptibility to gynecomastia or constitutionally delayed puberty in boys. Oestrogens are pivotal in closure of the epiphyses in puberty, and a nonaromatizable androgen might allow some extra gain in height by slowing the closure of the pubertal epiphyses. Oestrogen effects on the prostate might be deleterious (CitationCarruba 2006) and in this regard DHT might be the preferred androgen for the androgen-deficient aging male. Studies of DHT administration to hypogonadal men show that DHT maintains sex characteristics, increases muscle mass and improves sexual functions without significant increases in prostate size (CitationLy et al 2001; CitationWang and Swerdloff 2002).
Review of pharmacology, mode of action, pharmacokinetics of testosterone depot injection
In search of a better intramuscular long-acting testosterone preparation for male contraception, the world health organization (WHO) Special Programme of Research, Development and Research Training in Human Reproduction initiated search activities for identification of suitable fatty acid side chains for esterification of T (CitationCrabbe et al 1980). It appeared that T esterified with TU showed more favorable long-term kinetics (CitationHerz et al 1985; CitationBehre et al 2004). The first TU preparation was developed in China (CitationGu et al 2003; CitationZhang et al 2006). Unfortunately, the injection volume of 8 mL for 1000 mg TU caused problems at the injection site. Subsequently, by use of a special galenic formulation based on benzyl benzoate and refined castor oil, Jenapharm/Schering were able to develop a suitable intramuscular TU preparation with a volume of 4 mL containing 1000 mg TU. Intramuscular TU is currently not approved for use in the US, but is prescribed in Europe, Latin America, and Asia under the tradename Nebido® (Bayer Schering Pharma AG, Berlin, Germany), and in Australia and Spain under the tradename Reandron 1000® (Bayer Schering Pharma AG, Berlin, Germany) for the treatment of male hypogonadism.
Toxicology and pharmacology
The active pharmacological principle of TU is testosterone itself. After entering the peripheral circulation, TU (molecular weight 456.7 Da) is hydrolyzed to T which may then exert its androgenic activity (CitationHorst et al 1976). Therefore, in principle, the toxicology of TU is the same as for other cleavable T fatty acid esters such as T propionate (3 carbon atoms), T enanthate (7 carbon atoms), or T cypionate (8 carbon atoms). In contrast to these fatty acid esters the kinetics for side chain cleavage of the saturated aliphatic fatty acid undecanoic acid with 11 carbon atoms turned out to be considerably longer, permitting much longer injection intervals, at the same time as preventing supra- or sub-physiological serum T levels.
TU had been available as an oral preparation for more than 30 years. It is well-tolerated, but the bioavailability of T leaves much to be desired. It requires careful dosing at least two times a day and it must be taken with fatty meals in order to achieve acceptable plasma T levels (CitationBagchus et al 2003).
Pharmacokinetics of injectable depot TU: The relationships between intramuscular TU dose (31, 62.5, 125, 250, and 500 mg/kg body weight s.c.) and T serum levels were investigated in male rats (CitationCallies et al 2003). A single injection of 125 mg TU/kg body weight is effective in inducing physiological T levels in orchiectomized rats for a minimum of 4-weeks. High dose TU (500 mg/kg body weight) given as a single injection results in supraphysiological T serum concentrations for up to 6-weeks in non-orchiectomized animals. TU was superior to other preparations releasing T (T pellets s.c. T filled s.c. Silastic® [Dow Corning Corp.] implants, s.c. testosterone propionate) in inducing physiological T lelvels.
CitationPartsch et al (1995) compared T serum levels after single intramuscular injections of 10 mg TU or T enanthate (TE)/kg body weight in 5 long-term orchidectomized cynomolgus monkeys (Macaca fascicularis). For pharmacokinetic characteristics such AUC (4051 vs 1771 nmol x h/L), residence time (41 vs 12 days), terminal half-life (26 vs 10 days), maximal T concentration (73 vs 177 nmol/L), and time at maximal T concentration (11 vs 1 days), TU showed pharmacokinetic and pharmacodynamic properties clearly superior to those of TE. Animals treated with TU also showed a significantly longer ejaculatory response (14-weeks) than those treated with TE (7-weeks).
Pharmacokinetics in men
The first detailed pharmacokinetic investigation of the injectable TU preparation manufactured in China (CitationZhang et al 1998), administering 2 single doses in hypogonadal men. Eight patients with Klinefelter’s syndrome received either 500 mg or 1000 mg of TU by intramuscular injection; 3 months later, the other dose was given to each of the participants. Every week or every second week after the injections, the serum total T concentrations were measured. The whole observation period was only 8 or 9-weeks. The single-dose injections maintained serum T levels within the normal range for at least 7-weeks without immediately apparent side effects. Of considerable interest was the observation that the pharmacokinetic profiles of T were different when 500 mg TU was given as the first injection or as the second one. Somewhat unexpectedly, the peak T values obtained were lower when the 500 mg dose given as the second injection, compared with when the 500 mg dose was given first. The authors speculate that long-term hypogonadism of these men may have induced faster cleavage or clearance mechanisms for TU and T by the time of the second injection. Another explanation proposed was that the residual endogenous T is suppressed by the first injection (decrease of LH and FSH), and that after the second injection, only exogenous T was measured.
In a following study (CitationNieschlag et al 1999) 13 hypogonadal men received 4 intramuscular injections of TU at 6-week intervals. T serum levels were never found to lie below the lower limit of normal, and only briefly after the 3rd and 4th injection were T serum levels above the upper limit of normal () while values peak and trough levels increased over the 24-week observation period. Serum estradiol and DHT followed the same pattern, not exceeding the normal limits. In order to better establish (Citationvon Eckardstein and Nieschlag 2002) suitable injection intervals for TU, 7 hypogonadal men received injections at gradually increasing intervals between the 5th and 10th injection (starting with 6-weeks injection interval) and from then on every 12-weeks. Steady state kinetics were assessed after the 13th injection. Cmax was 32.0 ± 11.7 nmol/L and half-life was 70.2 ± 21.1 days. The mean Cmax of 32 nmol/L seen during steady-state with TU administration was lower than that achieved by Testogel® (Bayer) 100 mg/day (37.5 nmol/L); however, it was higher than with Testogel® 50 mg/day (28.8 nmol/L) and Androderm® (Watson Pharmaceuticals, Inc.,) patch 5mg/day (26.5 nmol/L). Before the next injection, the serum levels for T and its metabolites DHT and estradiol were mostly within the normal (eugonadal) range and showed a tendency to decrease with increasing injection intervals. The study concluded that after initial loading doses at 0 and 6-weeks, injection intervals of 12-weeks establish eugonadal values of serum testosterone in almost all men. A later study (CitationYassin and Saad 2005) analyzing 58 hypogonadal men receiving TU treatment every 3 months did not report elevations of DHT levels exceeding the physiological threshold.
Figure 1 Trough levels of testosterone after repeated injections of testosterone enanthate and testosterone undecanoate in 40 hypogonadal men (mean age 41, range: 18–74 years).
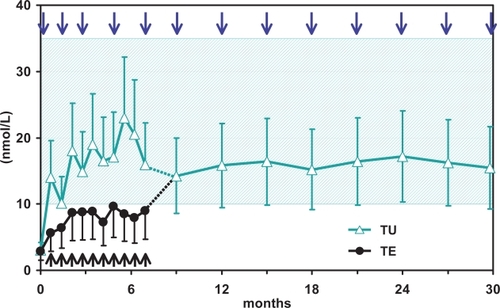
In an open label, randomized, prospective study (CitationSchubert et al 2004), TU (1000 mg TU 3 times every 6-weeks, thereafter every 9-weeks) was compared with TE (250 mg every 3-weeks) in 40 hypogonadal men. In contrast to the group treated with TE, trough T levels (measured immediately before the new injection) in patients receiving TU remained within the physiological (eugonadal) range. This study was extended as a follow-up study for approximately 2.5 years of treatment (CitationMorales et al 2006). All the patients in this study phase received 1000 mg TU every 12-weeks (the patients who had earlier received TU)) or 2 x 1000 mg TU every 8-weeks followed by 1000 mg TU every 12-weeks (the patients who had earlier received TE). This regimen resulted in stable mean serum trough levels of T (ranging from 14.9 ± 5.2 to 16.5 ± 8.0 nmol/L) and estradiol (ranging from 98.5 ± 45.2 to 80.4 ± 14.4 pmol/L). For T therapy with TU the authors recommended an initial loading dose of 3 × 1000 mg TU every 6-weeks followed by 1000 mg TU every 12-weeks. On the basis of pharmacokinetic studies the conclusion was reached that patients receiving TE could be switched to TU without interruption in therapy, but with an additional loading dose of TU 2 × 1000 mg every 8-weeks after switching from the short-acting TE to TU (CitationSchubert et al 2004; CitationNieschlag 2006).
Clinical long-term experience up to 120-weeks was published in 2004 (CitationSchubert et al 2004). Twenty-six hypogonadal patients received TU (1000 mg TU/4mL) in a first stage of the study in weeks 0, 6, 16, 26, and 36, followed by an additional stage of up to 120-weeks with injections every 12-weeks. The supranormal peak concentrations of total and free T occurred 2-weeks after the first injection, then a decrease to within the physiological range was observed. At the end of the study, during washout period, serum T levels declined to the low pre-treatment levels 14-weeks after the final injection. A parallel increase of 17β-estradiol levels was seen, but there was an earlier decrease to pre-treatment levels by 4-weeks after the last injection. Serum LH and FSH were suppressed during the treatment period, while sex hormone binding globulin (SHBG) remained stable. The latter is probably an expression of the physiological range of achieved plasma testosterone levels; administration of high dose testosterone leads to a fall in plasma SHBG levels (CitationAnderson et al 1996).
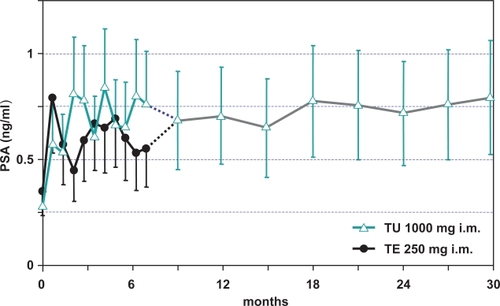
Serum prostate specific antigen (PSA) rose from 0.660 to 0.976 ng/mL (p < 0.01) after 120-weeks, but did not exceed the normal range. Prostate volume increased from 19.6 to 26 mL (p < 0.05). Osteocalcin rose from 0.734 to 1.049 nmol/L (p < 0.01). Bone mineral density (BMD) did not change. Standard laboratory tests and uroflow did not change. Sexual interest (assessed by use of the AMS questionnaire) increased (reviewed in CitationHarle et al 2005).
There is now long-term experience of up to more than 8 years with TU in 22 hypogonadal men (CitationZitzmann 2006). Individual dosing intervals ranged from 10 to 14-weeks. Serum trough levels of T were generally within the low-normal range, indicating sufficient substitution. In contrast to short-acting T esters, sensations of fluctuations in androgen serum concentrations were rarely observed. When they were, they occurred during the last 2-weeks before the next injection, indicating loss of androgenic psychotropic effects. Summarizing two key studies (Citationvon Eckardstein and Nieschlag 2002; CitationSchubert et al 2004) the following administration regimen is recommended for TU therapy in hypogonadal men: After the first injection of 1000 mg TU, the second injection of 1000 mg TU is to be administered 6-weeks after the first injection (loading dose) followed by injections every 12-weeks. While this schedule will generally be adequate, an individualization of TU therapy may be desirable (CitationZitzmann 2006). If the T serum concentration before the 4th injection lies between 10 and 15 nmol/L then the injection interval should be every 12-weeks. Should the T serum concentration at this time be lower than 10 nmol/L, then the injection interval is shortened to every 10-weeks. If the T level is greater than 15 nmol/L, then the injection interval should be extended to every 14-weeks. Additionally, clinical symptoms should be considered for individualization of injection intervals with TU therapy. The loading dose of TU achieved by the first two injections with an interval of 8-weeks is also recommended for patients who are being transferred from short-acting T injections (eg, testosterone enanthate 250 mg) to treatment with TU.
Efficacy studies, including any comparative studies
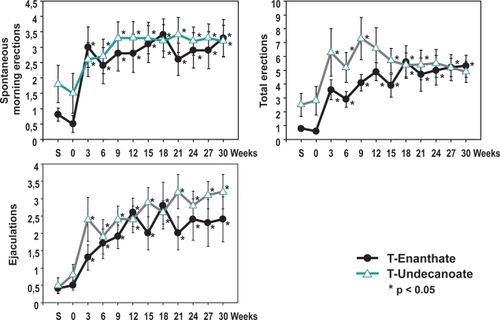
The efficacy of TU has been compared with the previous gold standard of 250 mg TE i.m. every 3-weeks in a 30-week controlled, prospective, randomized, parallel-group study (CitationSchubert et al 2004). During the first 30-weeks of the comparative phase, 40 hypogonadal men with T levels below 5 nmol/L were randomly assigned to either 250 mg TE i.m. every 3-weeks (n = 20) or TU 3 times at 6-week intervals followed by a 9-week interval. Following the first 30-weeks of the comparative part of the study, all patients received TU every 12-weeks in the one-arm follow-up study over additional 30 months. In the first 30-weeks there were no differences in sexual parameters (spontaneous morning erections, total erections, ejaculations) between the two groups. After 30-weeks, serum PSA levels in both treatment groups had risen slightly, but remained stable during long-term TU administration and stayed within the normal range over the entire observation period. Prostate volume increased during the first 30-weeks to a similar degree with both T preparations but then remained stable until the end of the follow-up study (CitationMorales et al 2006). Comparing the mean baseline levels with the mean levels after follow-up, there was an increase of serum T (from 3.9 to 16.2 nmol/L), of PSA serum levels (from 0.27 to 0.75 ng/mL) and of prostate volume (from 14.5 to 20.2 mL), whereas a decline in serum total cholesterol (from 235.3 to 202.4 mg/dL), LDL cholesterol (from 158.8 to 134.9 mg/dL), HDL cholesterol (from 46.1 to 42.8 mg/dL), and triglycerides (from 199.9 to 161.2 mg/dL) was observed. The decline in plasma HDL must be balanced against the improvement in the other classes of lipids. In the follow-up of these patients, in those receiving TU, plasma HDL returned to pre-treatment levels and then increased significantly (CitationMinnemann et al 2007).
Using a standardized self-evaluation questionnaire for assessing psychosexual effects of TU treatment, it was found that scores for sexual thoughts/fantasies and sexual interest/desire doubled. Also the score for satisfaction of sex life increased. Improvements were seen for waking erections, total number of erections, and of ejaculations (). The psychological parameters for depression, fatigue, and anxiety decreased within the first 3–6-weeks and remained stable. There were no statistically significant differences between TE and TU. No significant change was observed in the score for aggressiveness in either group, indicating that this parameter was not affected by the treatment provided. These results obtained in hypogonadal men are paralleled in some respects by the study of CitationO’Connor et al (2004) showing that a single injection of 1000 mg TU to 28 eugonadal young men, elevating mean T levels above normal, was associated with significant increases in anger-hostility from baseline to week 2 after the injection. It was accompanied by an overall reduction in fatigue-inertia, and did not increase aggressive behavior or induce any changes in non-aggressive or sexual behavior.
Figure 2 Sexual parameters in 40 hypogonadal men (mean age 41, range: 18–74 years) under treatment with testosterone enanthate or testosterone undecanoate.
It is clear now that TU is at least as effective and safe as the standard injectable formulation and requires only 4 injections per year in long-term treatment while maintaining serum T levels within the physiological range. There are data to confirm the safety and efficacy of long-term TU therapy of hypogonadal patients treated over a period of more than 8 years (CitationZitzmann 2006). The study included 22 patients who received TU up to 8.5 years at injection intervals of approximately 12-weeks. Patients reported restoration of sexual functions and positive changes in mood patterns. In contrast to short-acting TE preparations, patients rarely reported perceptions of fluctuations in androgen concentrations. Over the whole treatment period, prostate specific antigen (PSA) concentrations did not exceed the normal range and prostate size remained below 30 mL in all patients (). Hemoglobin and hematocrit increased initially during treatment but remained within the normal range over the entire treatment period. Computer tomography of the lumbar spine showed that bone density improved in all patients during the first 2 years and remained stable thereafter. Body mass index (BMI) decreased during the first 2 years of treatment. Serum total cholesterol levels did not change over the treatment period and serum LDL levels decreased slightly, concurring with the decrease of BMI, and serum HDL levels increased slightly over time. There were no relevant changes in blood pressure or heart rate. Overall, treatment with intramuscular TU appeared to have beneficial effects on body composition and lipid profile (CitationZitzmann et al 2005).
Figure 3 Effect of testosterone undecanoate (intervals of 12-weeks) on prostate-specific antigen (PSA) in 40 hypogonadal men (mean age 41, range: 18–74 years).
The above studies were confirmed recently by a study in elderly men (CitationJacobeit and Schulte 2006).
Thirty-three hypogonadal men with primary, secondary, or late-onset hypogonadism between the ages of 45 and 79 years were treated with TU. Serum T levels increased from 9.0 ± 3.8 nmol/L at baseline to 13.5 ± 4.6 nmol/L after 6-weeks and to 16.4 ± 6.4 nmol/L after 30-weeks of treatment. DHT levels increased from 0.98 ± 0.48 nmol/L to 3.1 ± 1.0 nmol/L. Serum PSA levels fluctuated minimally in the normal range. In 2 patients the length between two injections could be prolonged from 12 to 14-weeks. All patients reported improved mood, sexual function and quality of life.
Testosterone gel versus long-acting injectable testosterone undecanoate
In a study of CitationYassin et al (2007), 2 modern modes of testosterone treatment were compared: transdermal T gel and injectable long-acting TU. Thirty-four men between the ages of 49 and 70 years (median 61) were hypogonadal by clinical and laboratory criteria (T <2.3 ng/mL). They received first 5 g daily of T gel for 9 months and subsequently TU for 9 months. Large numbers of variables were monitored under both T regimens and analyzed with Student’s t-tests From the observations in androgen-deficient men, treated with T gel followed by TU, it appeared that plasma T rose slowly over 9 months in patients receiving T gel, and that there was an additional rise of plasma T when patients are treated with TU, with T values remaining in the normal range. The waist circumference declined upon elevation of plasma T. Waist circumference is a key diagnostic marker of the metabolic syndrome. Further, there was an improvement of lipid profiles (associated with the decline in waist circumference). Hemoglobin and hematocrit values rose in patients whose plasma T levels were normalized and there was an improvement of the scores of the AMS test. There was no effect on PSA values or international prostate symptom score, no effect on liver functions, and no effect on DHT:T ratio.
So far, the two types of testosterone treatment have not been compared for their benefits and potential drawbacks. While both types meet largely the specifications for requirements of present day androgen treatment, the higher levels achieved with TU compared to the T gel appeared to be efficacious.
Treatment of erectile dysfunction (ED) with TU
There is a new interest in the treatment of erectile dysfunction with testosterone. Apart from its well-known effects on libido, testosterone appears to have significant direct effects on anatomical and physiological properties of erectile tissue (CitationGooren and Saad 2006; CitationTraish and Guay 2006), and there are some interesting new observations. In support of a direct effect of testosterone on penile tissues, treatment with TU appeared to improve veno-occlusive dysfunction evidenced by cavernosographic changes in hypogonadal patients with severe ED, diabetes mellitus, obesity, and/or metabolic syndrome who had earlier not responded to PDE5 inhibitors and intracavernosal alprostadil injections (CitationYassin and Saad 2006b). One patient having venous leakage to T administration received treatment with TU at 12- to 14-week intervals following a loading dose of 6-weeks. The patient showed improved sexual function after 9-weeks of treatment and repeated cavernosography after 12-weeks revealed that the venous leakage had receded (CitationYassin and Saad 2006a). The results from this case study suggest that TU has a positive impact on the veno-occlusive properties of penile trabecular tissues in hypogonadal ED patients (). This finding has been replicated in 5 out of 12 hypogonadal patients (CitationYassin et al 2006) and could suggest the correction of venous leak, which could be at least, in part, a metabolic lesion, rather than a mechanical one, such as in venous anatomic abnormality or venous valves dysfunction, and could also perhaps clarify the higher recurrence rate in penile venous surgery (). These results confirm data obtained from animal studies showing that androgen insufficiency leads to veno-occlusive dysfunction which cannot be restored with PDE-5 inhibitor treatment alone (CitationTraish et al 2005).
Figure 4 Disappearance of venous leakage evidenced by cavernosography in a hypogonadal man with erectile dysfunction at baseline (left) and after 3 months’ administration of testosterone undecanoate (right).
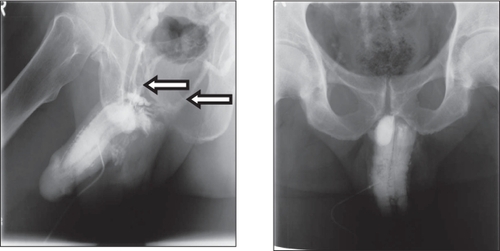
Figure 5 Another subject showing absence of venous leakage evidenced by cavernosography in a hypogonadal man with erectile dysfunction at baseline (left) and after 4.5 months’ administration of testosterone undecanoate (right) with better penile composition and opacification.
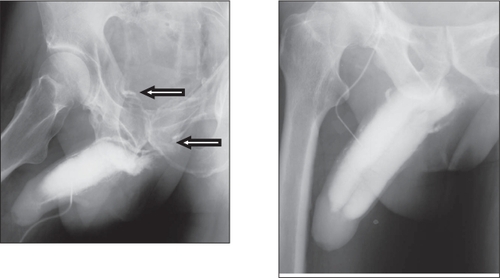
Figure 6 Improvement in scores on the international index of erectile function (IIEF) in 71 of 122 patients receiving treatment with long-acting testosterone undecanoate.
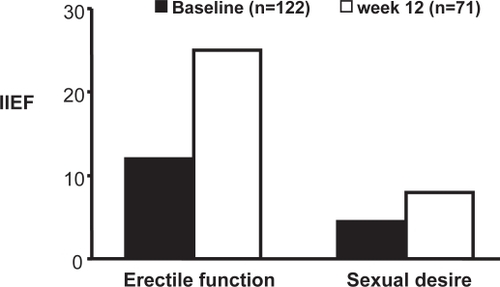
In a study assessing the impact of T therapy alone on ED, 22 hypogonadal men with ED received injections with TU, and sexual function was assessed using the International Index of Erectile Function (IIEF). While in all patients T therapy alone significantly improved the sexual desire domain of the IIEF (from 4.5 to 8.4 on a scale of 10), in 12 out of 22 patients (54%), the erectile function domain score increased from 12 at baseline (moderate ED) to 25 (indicating normal erectile function) at week 24. It is of note that the effect of T on erectile function may appear as late as after 12–24-weeks of administration of T (CitationYassin and Saad 2006b).
These observations have been extended to larger groups of patients, confirming the results of the above study (CitationYassin et al 2006) (). Data obtained from this cohort are realistic. Since the subjects are testosterone deficient, they have primarily organic reasons for ED, but of course, psychogenic components might play a role in increasing average of erectile function to this level.
Safety and tolerability
TU is generally well tolerated. Local irritation at the site of injection is moderate, does not last longer than 3 days and can be minimized by administering TU slowly over a period of at least 1 minute. Very few patients reported irritation or pain at the sight of injection despite the large volume of injection of 4 mL. No patient discontinued treatment due to problems of local discomfort. TU should be injected deeply into the gluteal muscle. The patient should be in a prone position. During the first year of TU treatment, for safety reasons, erythropoiesis parameters and prostate size and serum PSA as well as international prostate symptoms score (IPSS) should be monitored in men above the age of 45 years at quarterly intervals and then yearly thereafter (CitationNieschlag et al 2005).
The conventional injectable T esters, such as TE with injection intervals of 2–3-weeks, are associated with supraphysiological peak values shortly after the injection and to subphysiological levels in the days before the new injection. This often leads to mood swings or emotional instability. Another important consequence of the supraphysiological T levels under treatment with TE is the induction of elevations of the hematocrit. Of 70 older men with low serum T receiving 200 mg of TE every other week, 30% developed a hematocrit greater than 52% (CitationDobs et al 1999; CitationGui et al 2004). In another study, of 32 hypogonadal men receiving 200 mg TE every other week, 14 patients (43.8%) had at least one occurrence of an elevated hematocrit value (CitationJockenhovel et al 1997). The evidence that elevated hematocrit values may lead to thrombo-embolic events is not very well documented. It is clear now that TU is at least as effective and safe as the standard injectable formulation and eventually requires only 4 injections per year while maintaining serum T levels within the physiological range. There are data to confirm the safety and efficacy of long-term TU therapy in hypogonadal patients treated over a period of more than 8 years.
No major adverse effects were encountered in the clinical trials of TU. This is not surprising since the pharmaceutically active component is T itself. Common side effects of T administration, such as gynecomastia, breast tenderness, and acne were, reported in only a minority of patients. This absence of side effects is probably to be ascribed to the largely normal physiological levels of T, and its derivatives DHT and estradiol, achieved with TU. Adverse effects were observed in the initial studies when the dosing schedule was not yet well established and the higher frequency of administration of TU led to higher-than-normal levels of testosterone. Significant increase in PSA and prostate size were noted in some of these trials; however, this is due to the fact that hypogonadal men have subnormal PSA values and small prostate sizes at baseline and is observed with every treatment modality of T administration upon normalization of plasma T levels (CitationBehre et al 1994; CitationBhasin et al 2006). In a systematic review of the effect of T administration on the prostate in hypogonadal men, the average PSA increase after initiation of testosterone therapy was 0.3 ng/mL in young, hypogonadal men and 0.44 ng/mL in older men (CitationBhasin et al 2003).
In the further course of treatment with TU, PSA levels and prostate size remained stable and within the normal range. Similarly, increases of parameters of erythropoiesis to eugonadal values were observed, but there was no occurrence of polycythemia as observed in studies with the more traditional T esters (CitationJockenhovel et al 1997; CitationDobs et al 1999). Only one study showed a transient decline in serum HDL cholesterol; however, its value remained within the normal range (for a review see CitationHarle et al 2005). So, TU appears to be a safe modality of T treatment, because of the fact that with the presently established dosage regimen, plasma T levels remain in the physiological range.
Several of the recommendations of testosterone administration to elderly men with late onset hypogonadism argue in favor of short-acting testosterone preparations (CitationNieschlag et al 2005). The reasoning is that in case an concurrent disease develops, such as prostate malignancy, the impact of the extra androgens provided by the administration of testosterone will be short-lived. It is questionable whether this recommendation is rational 1) In view of the fact that there is an early diagnosis in this group of men who supposedly are monitored, following the guidelines, with an absolute minimum of digital rectal examination plus PSA determination once a year; 2) In view of the fact that delays between diagnostic biopsies and treatment of several months up to one year did not affect the recurrence rate as measured by PSA levels. The modern testosterone preparations generate physiological testosterone levels for a maximum duration of 10–14-weeks. So, it would seem that the choice of longer-acting testosterone preparation is not of great pathophysiological relevance (CitationBoorjian et al 2005; CitationGraefen et al 2005; CitationMorgentaler and Rhoden 2006); 3) With the modern testosterone preparations, plasma testosterone remain in the normal range, so there is no over-exposure of the prostate to testosterone; 4) Even with the traditional testosterone esters, with supraphysiological levels shortly after administration, no cause and effect of prostate cancer to treatment with testosterone has been found. 5) Men diagnosed with prostate cancer do not routinely undergo androgen ablation treatment. So it would seem that there is no serious objection against treating elderly men with a long-acting testosterone preparation.
Patient-focused perspectives such as quality of life, patient satisfaction/ acceptability, adherence, and uptake
Most medical conditions requiring androgen therapy are irreversible. As a consequence, androgen replacement therapy often extends over many decades. Therefore, patient compliance is of utmost importance.
After a recommended loading dose of two injections at a 6-week interval, TU is the first intramuscular agent that can be administered every 12-weeks thus maintaining physiological plasma T levels. Depending on the trough plasma T level immediately before the next injection and clinical symptoms of the patient, adjustment of the injection interval is desirable, rarely by shortening (every 10–11-weeks) or more often by prolonging (every 13–14-weeks) the interval between two injections. TU produces fewer peaks and troughs in serum T levels in comparison with the traditional T esters. Hypogonadal men treated with TU report a general sense of well-being and normal sexual function during treatment. These parameters were not different when evaluated at the half point of injection intervals versus the end of the injection interval period. This suggests that normal physiological T values were maintained throughout the 12-week period, without major fluctuations. As a result, patients did not report mood-swings or emotional instability, which is a common complaint with other T preparations.
A major advantage of TU is that it only requires 4 injections/year compared with 26 injections/year of TE (although if taken at a dose of 200 or 250 mg every 2-weeks, which is necessary if the plasma T levels are to be kept in the physiological range, with this frequency, plasma T levels will exceed to the supraphysiological range in the days following an injection). The physician sees the patient every 12-weeks for safety and efficacy monitoring. With a 2- to 3-weekly injection of TE, the frequency is so high that not every visit will be regarded as a moment of reflection on efficacy and safety of T administration. The clinical experience with TU meets the requirements spelt out in the consensus on T as well as other recommendations regarding safety and efficacy monitoring of T administration. Therefore, TU is also well suited for elderly men because these patients will be examined 4 times per year and a prostate malignancy and other reasons for the discontinuation of the therapy can be diagnosed in a timely manner. There was no impairment of urine flow.
Conclusions, place in therapy
Treatment with the unmodified T molecule is preferred by opinion leaders, with a treatment modality and in a dose which maintains serum T in the physiological range for the full 24 hours of the day. Studies so far show that TU represents an effective, safe, and well tolerated means of androgen treatment in hypogonadal men. At present, clinical experience is available with TU treatment over 9 years (CitationZitzmann et al 2007). In view of its favorable pharmacokinetic profile TU has been well received. Its advantages over the more conventional injectable T preparations are obvious. The injection frequency is as little as 4 per year. The large fluctuations of plasma testosterone with the conventional T esters are subjectively experienced as unpleasant by many patients. TU, with its more favorable pharmocokinetic profile, did not have these side effects in clinical trials. So the merits of TU are manifest. The traditional testosterone esters developed some 50–60 years ago are relatively cheap. Health economics may delay a wide introduction of TU in the short-term in spite of the obvious advantages over the traditional T esters.
TU treatment is indicated for all forms of hypogonadism. Men with ED and low T may also benefit from TU administration, and the combination of phosphodiesterease 5-inhibitors and TU may be indicated in men who do not respond sufficiently to phosphodiesterease 5-inhibitors alone.
The open questions are related to T therapy in general and apply to other T preparations as well, and relate to prostate cancer. There is no evidence that testosterone causes prostate cancer. But larger, longer-term clinical studies with more patients (comprising 6000 men followed up for 6–8 years) are required to find definitive answers on the interrelationships between T serum levels and the pathophysiology of prostate cancer. However, experts agree that it is responsible clinical practice to treat elderly hypogonadal men with T provided the existing guidelines for monitoring are followed.
References
- AndersonRA1996Comparison between testosterone enanthate-induced azoospermia and oligozoospermia in a male contraceptive study. III. Higher 5 alpha-reductase activity in oligozoospermic men administered supraphysiological doses of testosteroneJ Clin Endocrinol Metab8190288772548
- BagatellCJBremnerWJ1995Androgen and progestagen effects on plasma lipidsProg Cardiovasc Dis38255717494886
- BagchusWM2003Important effect of food on the bioavailability of oral testosterone undecanoatePharmacotherapy233192512627930
- BehreHM1994Prostate volume in testosterone-treated and untreated hypogonadal men in comparison to age-matched normal controlsClin Endocrinol (Oxf)4034197514512
- BehreHM2004Pharmacology of testosterone preparationsNieschlagEBehreHMTestosterone, action, deficiency, substitutionCambridge University Press40544
- BhasinS2003Managing the risks of prostate disease during testosterone replacement therapy in older men: recommendations for a standardized monitoring planJ Androl2429931112721204
- BhasinS2006Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guidelineJ Clin Endocrinol Metab911995201016720669
- BoorjianSA2005Does the time from biopsy to surgery affect biochemical recurrence after radical prostatectomy?BJU Int96773616153197
- BrachetC2005Children’s virilization and the use of a testosterone gel by their fathersEur J Pediatr164646716025298
- CalliesF2003Testosterone undecanoate: a useful tool for testosterone administration in ratsExp Clin Endocrinol Diabetes111203812845558
- CarrubaG2006Estrogens and mechanisms of prostate cancer progressionAnn N Y Acad Sci10892011717261768
- CrabbeP1980Injectable contraceptive synthesis: an example of international cooperationScience20999247403868
- DobsAS1999Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal menJ Clin Endocrinol Metab8434697810522982
- DobsAS2004Short-term pharmacokinetic comparison of a novel testosterone buccal system and a testosterone gel in testosterone deficient menCurr Med Res Opin207293815140340
- GoorenLJBunckMC2004Androgen replacement therapy: present and futureDrugs6418619115329035
- GoorenLJSaadF2006Recent insights into androgen action on the anatomical and physiological substrate of penile erectionAsian J Androl83916372114
- GraefenM2005Reasonable delay of surgical treatment in men with localized prostate cancer – impact on prognosis?Eur Urol477566015925069
- GuYQ2003A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy Chinese menJ Clin Endocrinol Metab88562812574181
- GuiYL2004Male hormonal contraception: suppression of spermatogenesis by injectable testosterone undecanoate alone or with levonorgestrel implants in chinese menJ Androl25720715292101
- HarleL2005Nebido: a long-acting injectable testosterone for the treatment of male hypogonadismExpert Opin Pharmacother61751916086661
- HerzJE1985Potential long-acting contraceptive agents: esters and ethers of testosterone with alpha- and/or beta-chain branchingSteroids46947533842023
- HorstHJ1976Lymphatic absorption and metabolism of orally administered testosterone undecanoate in manKlin Wochenschr548759966635
- JacobeitJWSchulteHM2006Long-acting intramuscular testosterone undecanoate (TU, Nebido®) in treatment of aging males with hypogonadism8th European Congress of EndocrinologyGlasgow1–542006 Poster No. P184
- JockenhovelF1997Effects of various modes of androgen substitution therapy on erythropoiesisEur J Med Res229389233903
- KelleherS2004Testosterone release rate and duration of action of testosterone pellet implantsClin Endocrinol (Oxf)60420815049955
- LyLP2001A double-blind, placebo-controlled, randomized clinical trial of transdermal dihydrotestosterone gel on muscular strength, mobility, and quality of life in older men with partial androgen deficiencyJ Clin Endocrinol Metab8640788811549629
- MeikleAW1996Pharmacokinetics and metabolism of a permeation-enhanced testosterone transdermal system in hypogonadal men: influence of application site – a clinical research center studyJ Clin Endocrinol Metab811832408626843
- MeikleAW2004Transdermal testosterone gel: pharmacokinetics, efficacy of dosing and application site in hypogonadal menBJU Int937899515049991
- MinnemannT2007A four-year efficacy and safety study of the long-acting parenteral testosterone undecanoateAging Male10155817701659
- MoralesA2006Clinical experience with the new long-acting injectable testosterone undecanoate. Report on the educational symposium on the occasion of the 5th World Congress on the Aging Male, 9–12 February 2006, Salzburg, AustriaAging Male9221717178558
- MorgentalerARhodenEL2006Prevalence of prostate cancer among hypogonadal men with prostate-specific antigen levels of 4.0 ng/mL or lessUrology681263717169647
- NieschlagE2006Testosterone treatment comes of age: new options for hypogonadal menClin Endocrinol (Oxf)652758116918944
- NieschlagE1999Repeated intramuscular injections of testosterone undecanoate for substitution therapy in hypogonadal menClin Endocrinol (Oxf)517576310619981
- NieschlagE2005Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, and EAU recommendationsInt J Androl28125715910536
- O’ConnorDB2004Effects of testosterone on mood, aggression, and sexual behavior in young men: a double-blind, placebo-controlled, cross-over studyJ Clin Endocrinol Metab8928374515181066
- PartschCJ1995Injectable testosterone undecanoate has more favourable pharmacokinetics and pharmacodynamics than testosterone enanthateEur J Endocrinol13251497711892
- RolfC2002Interpersonal testosterone transfer after topical application of a newly developed testosterone gel preparationClin Endocrinol (Oxf)566374112030915
- SchubertM2004Intramuscular testosterone undecanoate: pharmacokinetic aspects of a novel testosterone formulation during long-term treatment of men with hypogonadismJ Clin Endocrinol Metab8954293415531493
- StuenkelCA1991Sublingual administration of testosterone-hydroxypropyl-beta-cyclodextrin inclusion complex simulates episodic androgen release in hypogonadal menJ Clin Endocrinol Metab72105491902483
- TraishAM2005Adipocyte accumulation in penile corpus cavernosum of the orchiectomized rabbit: a potential mechanism for veno-occlusive dysfunction in androgen deficiencyJ Androl26242815713830
- TraishAMGuayAT2006Are androgens critical for penile erections in humans? Examining the clinical and preclinical evidenceJ Sex Med3382404 discussion 404–716681465
- von EckardsteinSNieschlagE2002Treatment of male hypogonadism with testosterone undecanoate injected at extended intervals of 12-weeks: a phase II studyJ Androl234192512002444
- WangCSwerdloffRS2002Should the nonaromatizable androgen dihydrotestosterone be considered as an alternative to testosterone in the treatment of the andropause?J Clin Endocrinol Metab871462611932265
- WangC2000Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal menJ Clin Endocrinol Metab8528395310946892
- WangC1996Sublingual testosterone replacement improves muscle mass and strength, decreases bone resorption, and increases bone formation markers in hypogonadal men – a clinical research center studyJ Clin Endocrinol Metab813654628855818
- WangC2004aLong-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal menJ Clin Endocrinol Metab8920859815126525
- WangC2004bNew testosterone buccal system (Striant) delivers physiological testosterone levels: pharmacokinetics study in hypogonadal menJ Clin Endocrinol Metab893821915292312
- YassinA2007Comparison of the efficacy drawbacks of two modalities of testosterone treatment: transdermal testosterone gel and injectable long-acting testosterone undecanoateJ Sex Med4Suppl 184
- YassinASaadF2005Does long-acting testosterone injection (Nebido) have an impact on DHTInt J Androl2863
- YassinAASaadF2006aDramatic improvement of penile venous leakage upon testosterone administration. A case report and review of literatureAndrologia3834716420241
- YassinAASaadF2006bTreatment of sexual dysfunction of hypogonadal patients with long-acting testosterone undecanoate (Nebido®)World J Urol246394417048032
- YassinASaadF2007Improvement of sexual functions in men with late onset hypogonadism treated with testosterone onlyJ Sex Med449750117367445
- YassinAA2006Testosterone undecanoate restores erectile function in a subset of patients with venous leakage: a series of case reportsJ Sex Med37273516839330
- ZhangGY1998A pharmacokinetic study of injectable testosterone undecanoate in hypogonadal menJ Androl1976189876028
- ZhangL2006The acceptability of an injectable, once-a-month male contraceptive in ChinaContraception735485316627044
- ZitzmannMN2006long-term experience of more than 8 years with a novel formulation of testosterone undecanoate (Nebido) in substitution therapy of hypogonadal menAging Male55
- ZitzmannM2005Long-term experience with injections of testosterone undecanoate for substitution therapy in hypogonadal men87th Annual Meeting of the Endocrine Society200564–7San Diego, CA306
- ZitzmannM2007New models to improve safety of testosterone substitution by pharmacogenetics and obesity status: experience of 118 treatment-years with a long-acting formulation of testosterone undecanoate Endocrine Society, Abstract book4401