Abstract
Resveratrol is a potent member of the class of natural, plant-derived chemicals known as polyphenols. These help explain in part why a diet high in fruit and vegetables confers health benefits and are associated with reduced risk of common complex conditions such as cardiovascular disease, cancer, diabetes, and Alzheimer’s disease. We present the latest molecular findings that account for the beneficial actions of resveratrol. The intracellular pathways activated are crucial for anti-oxidant defence, regulation of the cell cycle, mitochondrial energy production, vascular tone, oncogene suppression, and many other phenomena which if unchecked lead to morbidity and mortality from onset and progression of these various diseases. While a healthy diet and lifestyle is strongly recommended in prevention of such conditions, the future bodes well for the use of resveratrol and analogues of higher potency than the natural form for treatment of diseases that afflict humans, particularly as they age.
Introduction
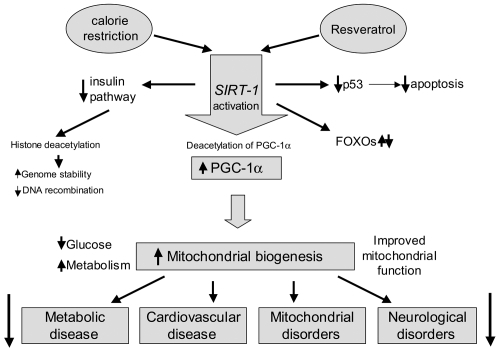
Resveratrol (trans-3,5,4’-trihydroxystilbene or 5-[(E)-2-(4-hydroxyphenyl)-ethenyl]benzene-1,3-diol; C14H12O3) (), is a polyphenolic flavonoid found in the seeds and skins of grapes, red wine, mulberries, peanuts, and rhubarb. Polyphenols exert a diversity of health benefits by activating intracellular pathways, many of which are the same as those activated by calorie restriction, an intervention long known to enhance health and prolong lifespan (CitationWood et al 2004). An early target of resveratrol is the sirtuin class of nicotinamide adenine dinucleotide (NAD)-dependent deacetylases. Seven sirtuins have been identified in mammals, of which SIRT-1 is believed to mediate the beneficial effects on health and longevity of both calorie restriction and resveratrol (CitationGuarente and Picard 2005). A number of intracellular pathways are activated by SIRT-1 (). The extent to which the sirtuin-activating actions of resveratrol are direct or indirect is still not resolved completely (CitationDenu 2005). The pathways regulated by sirtuins include gluconeogenesis and glycolysis in the liver, fat metabolism, and cell survival. Depending on cell type and circumstances, sirtuins activate or suppress members of the forkhead box O (FOXO) group of transcription factors. FOXOs then activate or suppress specific genes, leading to a decrease in apoptosis, an increase in antioxidant activities, DNA protection, anti-inflammatory effects, and modulation of various other mechanisms so as to promote the health of the cell, and thus the organism (reviewed by CitationMorris 2005, Citation2008). It may be that sirtuins benefit survival by ramping up stress resistance pathways in cells in times of adversity (CitationGuarente and Picard 2005). Several recent reports presented evidence that SIRT-1 interacts directly and deacetylates the metabolic regulator and transcriptional coactivator, peroxisome proliferator-activated receptor-γ co-activator 1α (PGC-1α). By doing so it improves mitochondrial function, induces genes for mitochondrial and fatty acid oxidation and increases mitochondrial membrane potential (CitationLagouge et al 2006; CitationGerhart-Hines et al 2007; CitationAnderson et al 2008).
Figure 2 SIRT-1 activation pathways. Resveratrol and calorie restriction activate similar SIRT-1–mediated pathways whose actions result in prevention of common age-related diseases.
A recent study defined class IA phosphoinositide 3-kinase (PI3K) as another direct target of resveratrol thus confirming earlier findings of involvement of resveratrol in the PI3K pathway (CitationFrojdo et al 2007). This could represent a sirtuin-independent pathway central to the control of lifespan implicating insulin-like signalling through the P13K/protein kinase B cascade and the downstream FOXOs.
The ability of resveratrol to mimic the beneficial effects that calorie restriction has on the lifespan of Saccharomyces cerevisiae was first noted by David Sinclair and co-workers (CitationHowitz et al 2003). Since then the effect of resveratrol on a number of other organisms such as Drosophila melanogaster, Caenorhabditis elegans, and Nothobranchius furzeri has been tested, and in each case an increase in mean lifespan of between 18% and 56% was seen (CitationWood et al 2004; CitationValenzano et al 2006). In the fruit fly and the nematode, the effect that resveratrol had on lifespan was dependent on Sir2 and SIR-2.1, the respective homologues of the mammalian protein, SIRT-1. Some reports have challenged these findings. CitationKaeberlein and co-workers (2005), for instance, were unable to reproduce the results in two different strains of yeast, instead showing that resveratrol did not significantly increase the lifespan of the cells. Likewise, another study found that resveratrol is not a general activator of Sir2 and that activation requires the presence of a covalently bound fluorophore (CitationBorra et al 2005). In the same year Guarente’s group found that resveratrol inhibits, rather than activates, SIR-2.1 in C. elegans, questioning the dogma that the wide effects of resveratrol are always a result of sirtuin activation. The involvement of the sirtuins in lifespan extension remains quite controversial. While some researchers have found that caloric restriction-mediated increases in longevity are sirtuin-dependent in yeast (CitationLin et al 2000), Drosophila (CitationRogina and Helfand 2004) and nematode (CitationWang and Tissenbaum 2006), others have found that sirtuins are not required (CitationKaeberlein et al 2004; CitationSmith et al 2007). Furthermore, overexpression of any of the human sirtuins could not extend the lifespan of normal human fibroblasts or prostate epithelial cells (CitationMichishita et al 2005). As well, resveratrol was not able to extend the replicative lifespan of human fibroblasts in culture (CitationStefani et al 2007).
On the other hand, we have found that resveratrol suppressed expression of mRNA for the senescence-inducer INK4, and by microarray analysis found that resveratol modulated expression of a number of genes such as those involved in the Ras pathway, apoptosis, cell growth, and division, G-protein signalling, cell – cell adhesids, immune system regulation, neuroendocrine signalling, muscle development, transcription, and proteolysis; ie, were mostly ones expected to affect cell lifespan (CitationStefani et al 2007).
In 2006, the effect of resveratrol on lifespan of mammals, namely a mouse model of obesity, was reported by two groups: Sinclair’s and that of Johan Auwerx (CitationBaur et al 2006; CitationLagouge et al 2006). The findings have provoked, yet again, the perennial question: ‘Can resveratrol improve human health and lifespan?’
The present review will discuss the recent resveratrol studies in mice, their relevance to longevity, and the ability to cure age-related diseases. Some new findings on resveratrol’s protection against cardiovascular disease, cancer, and neurological disorders will also be discussed.
Effects on metabolism, performance, and survival
Resveratrol is metabolized rapidly, and for this reason the question has often been posed as to whether resveratrol has any effect in mammals in vivo. The two studies in 2006, by Sinclair’s laboratory at Harvard, reported in Nature, and by Auwerx group in France, published in Cell, produced tantalizing findings in mice that support powerful actions of resveratrol in mammals. Each research team tested three groups of mice: one on a normal diet, one on a high calorie diet, and the third on a high calorie plus resveratrol regimen. The Sinclair study used a lower dose given in ‘middle age.’ The Auwerx study used a dose more than ten-fold higher and that was given from an early age. While some differences were seen, by and large the effects resveratrol elicited in the mice in each laboratory were astonishingly similar.
Sinclair’s group showed that resveratrol could prevent most of the ill effects of the high-fat diet by shifting many physiological parameters of mice on a calorie-rich diet towards those of mice on a standard diet, and thereby reducing the risk of diabetes, fatty liver, cardiovascular disease, and cancer (CitationBaur et al 2006). As a result the mice lived 30% longer, ie, had the same lifespan as the mice on a normal diet, even though the mice stayed fat and had high cholesterol (CitationBaur et al 2006). One unanswered question in this study was whether mice on a normal diet could also have their lifespan extended by resveratrol. In the Harvard study, the plasma levels of resveratrol achieved in the mice on a normal diet were lower than those in the obese mice. Therefore, to test the effect in mice on a normal diet, the experiment had to be repeated by giving the mice a diet containing higher resveratrol so as to achieve similar plasma levels as was seen in the mice on a high calorie diet. The mice lived slightly longer, but this did not reach statistical significance (D.A. Sinclair, personal communication).
Shortly after Sinclair’s 2006 study was published, Auwerx’s group reported that resveratrol, given at up to 20-fold higher dose to younger (1–2 month old) mice, could prevent diet-induced obesity and enhance the aerobic capacity of the mice (CitationLagouge et al 2006). Resistance to muscle fatigue was greatly improved in these mice, so that they could run twice as far before exhaustion (CitationLagouge et al 2006). This particular finding has attracted widespread interest in all fields relevant to human physical performance, ranging from athletic sports to active military service.
Both the Sinclair and Auwerx groups found that in resveratrol-treated mice insulin sensitivity and motor function were improved, and mitochondrial activity was increased significantly (CitationBaur et al 2006; CitationLagouge et al 2006). The latter ties in nicely with results both groups obtained from measuring the activity of PGC-1α. They found that in resveratrol-treated mice the acetylation status of PGC-1α was greatly decreased, and thus its activity was increased enormously (CitationBaur et al 2006; CitationLagouge et al 2006). This is an important result, because mitochondrial biogenesis in liver and muscle is by and large controlled by PGC-1α, deacetylation of which is positively regulated by SIRT-1 (CitationLin et al 2002; CitationGerhart-Hines et al 2007; CitationAnderson et al 2008). Thus resveratrol, via stimulation of the SIRT-1-mediated deacetylation of PGC-1, improves mitochondrial function and energy balance in mice (CitationBaur et al 2006; CitationLagouge et al 2006) (). In muscle, this leads to an increase in oxidative-type muscle fibres. As part of this action, the two studies found that genes relevant to the changes seen in liver (CitationBaur et al 2006) and skeletal muscle (CitationLagouge et al 2006) were induced, as identified by whole genome expression profiling. In the French study, skeletal muscle from resveratrol-treated mice showed the greatest increases in expression for genes involved in processes such as ribosomal mRNA processing, striated muscle contraction, the electron transport chain, oxidative phosphorylation and adenosine 5’-triphosphate (ATP) synthesis (CitationLagouge et al 2006), supporting the notion that resveratrol affected mitochondrial biogenesis and enhanced oxidative capacity of the muscle. The US study, which tested liver samples, found that the most significant alterations were in gene sets affecting the tricarboxylic acid (TCA) cycle, glycolosis, complement pathways, butanoate and propanoate metabolism, Stat3 signalling, as well as insulin signaling, insulin-like growth factor-1 and mTPR signaling, oxidative phosphorylation, and electron transport (CitationBaur et al 2006). Notably, Cidea, which regulates energy balance in brown fat and protects against obesity and diabetes, when knocked out, was shown to be the gene whose expression was most highly upregulated in the high-fat mice and second most highly downregulated in the high-fat-resveratrol group. These results highlight the similarities between resveratrol- and calorie restriction-induced pathways, and at the same time underline the observation that resveratrol-induced effects are tissue-specific.
The differences between each study might have arisen from differences in design aspects. In the Auwerx study, the mice were young and were fed resveratrol for three months, and, as a result of this, they gained nearly complete protection from high fat diet-induced obesity (CitationLagouge et al 2006). In the Sinclair study, the mice fed resveratrol were older (1 year), the dose was over ten times lower (5–22 versus 200–400 mg/kg body weight/day) and they were given resveratrol for a year in order to see whether this would improve their insulin sensitivity and lifespan when put on a high fat diet (CitationBaur et al 2006). Despite improved hepatic glucose and lipid metabolism, the mice were not protected from obesity. They did not, however, develop hepatic steosis (fatty liver), diabetes, cardiovascular disease, or cancer, each of which were seen in the high-fat fed controls. No change in liver histology was observed in the French study, but this could have been contributed by their younger age and the much shorter treatment with resveratrol that they were subjected to.
Despite the increase in deacetylated PGC-1α in liver, an effect that would normally increase gluconeogenesis, lower glucose levels were noted. This was explained by activation of adenosine monophosphate (AMP)-activated protein kinase (AMPK), which potently inhibits gluconeogenesis. AMPK would also protect from fatty liver, since it increases fatty acid oxidation by inhibiting the rate-limiting enzyme in fatty acid synthesis, acetyl coA carboxylase. This outcome comfirms earlier findings that polyphenols stimulate AMPK and lower plasma lipid concentration in diabetic low-density lipoprotein (LDL) receptor-deficient mice (CitationZang et al 2006). The findings support a parallel between the SIRT-1 and AMPK signaling pathways (CitationKoo and Montminy 2006). Both are triggered by fasting, and are energy-sensing in that they respond to changes in NAD+ and AMP. Each can increase lifespan and enhance glucose utilization as well as insulin sensitivity. Each pathway converges on PGC-1α and most certainly other regulators of glucose and lipid metabolism.
A reduction in core body temperature is associated with reduced energy expenditure and improved survival in mice, and vice versa (CitationConti et al 2006), yet the increased fat metabolism by the mitochondria-rich brown fat, leading to more adipose tissue burn off, suggests that the higher dose of resveratrol used in the Auwerx study was sufficient to trigger SIRT-1 activation (CitationKoo and Montminy 2006). The 10–20-fold lower doses of resveratrol in the study by the Sinclair group could possibly explain why core body temperature did not increase in their mice and why their mice were not protected from obesity (CitationKoo and Montminy 2006).
In humans, the possibility of a direct involvement of SIRT-1 in energy expenditure, was suggested by a case-control study carried out by Auwerx and co-workers. This showed that nondiabetic offspring of Finnish diabetic patients were more likely to show an association of particular alleles of three single nucleotide polymorphisms of SIRT-1 with energy expenditure (CitationLagouge et al 2006). They proposed that this could partially explain why different people respond differently to a high-fat diet. The finding might thus be relevant to the mechanisms responsible for obesity and/or diabetes, as well as other conditions. It should, however, be noted that genetic studies are notoriously unreproducible in different settings, so that much more research is needed to confirm the veracity of this particular genetic association before it can be generally accepted.
Cardiovascular protection
Moderate consumption of red wine is often associated with a lower incidence of cardiovascular diseases in populations with a high fat diet, a notion that has been termed the ‘French Paradox’ (CitationRenaud and de Lorgeril 1992). Since resveratrol has been found to be a component in red wine it has often been used to explain the French Paradox. The amount of red wine that a person needs to consume in order to acquire an equivalent amount of resveratrol to the amount used in the Sinclair experiments (CitationBaur et al 2006) has, however, been estimated as approx. 52 bottles per day! Therefore, resveratrol in red wine is unlikely to explain the ‘French Paradox’, despite the possibility of positive effects of red wine on the cardiovascular system; discussed later (CitationLefevre et al 2007).
The inhibitory effects of resveratrol on cardiovascular disease may be similar to those of 25% calorie restriction over 6 years in humans (CitationFontana et al 2004). These effects include a reduction in platelet aggregation, dilatation of blood vessels, antiatherosclerotic effects, lowering of lipid peroxidation, reduction in endothelin-1, protection of endothelial cells against apoptosis, lowering of blood pressure, oxidative stress and end-organ damage in hypertensive animals, as well as improvement of serum cholesterol profile and triglyceride concentrations. These effects have all been carefully reviewed (CitationBaur and Sinclair 2006; CitationPerez-Vizcaino et al 2006; CitationMorris 2007, Citation2008).
One explanation for the cardioprotective effect of resveratrol is its ability to prevent platelet aggregation through selective inactivation of the prostaglandin H2 synthase, cyclooxygenase (Cox)-1, over Cox-2 (CitationSzewczuk et al 2004), in much the same way as aspirin. Resveratrol is also a powerful inhibitor of the peroxidase reactions of Cox-1. Its weak inhibition of Cox-2, the isoform targeted by nonsteroidal anti-inflammatory drugs, is, moreover, confined to cyclooxygenase activity (CitationSzewczuk et al 2004). Cox-1 synthesizes thromboxane A2, which is a strong inducer of platelet aggregation and vasoconstriction, whereas inhibition of Cox-2 can increase thrombus formation (CitationMukherjee et al 2001). In a recent in vivo study, resveratrol (5 mg/kg) significantly prolonged platelet plug formation of mice (CitationShen et al 2007). This study suggested that the inhibitory effect of resveratrol involves inhibition of the p38 mitogen activated protein kinase (MAPK), cytosolic phospholipase A2 (cPLA2), arachidonic acid (AA), thromboxane A2 (TxA2), intracellular calcium concentration [Ca2+]i cascade, and activation of nitric oxide (NO)/cyclic guanosine monophosphate (GMP), resulting in inhibition of phospholipase C (PLC) and/or protein kinase C (PKC) activation, thereby leading to inhibition of [Ca2+]i or free radical formation, and finally inhibition of platelet aggregation (CitationShen et al 2007).
The vasodilator effect of resveratrol involves inhibition of TxA2 formation and the ability of resveratrol to stimulate Ca2+-activated K+ channels (CitationLi et al 2000), as well as NO signaling in the endothelium by inhibiting vascular NADH/NADPH oxidase activity. This then results in suppression of superoxide production and thus reduces NO inactivation (CitationOrallo et al 2002). Resveratrol, but not other flavonoids, can induce expression of endothelial NO synthase (eNOS) and inducible NO synthase (iNOS) (CitationLeikert et al 2002; CitationWallerath et al 2002; CitationDas et al 2005; CitationLeighton et al 2006). It also affects L-citrulline production by vascular endothelial cells (CitationLeikert et al 2002). The increase in eNOS in blood vessel walls in response to red wine polyphenols, particularly resveratrol, contributes to vasodilatation by enhancing NO production (CitationRäthel et al 2007). This was delineated recently in a paper that showed moderate consumption of red wine in mice improves ischemia-induced neovascularization in high-cholesterol conditions by restoring the Akt (protein kinase B) – eNOS – NO pathway and by increasing the number and function of endothelial progenitor cells (CitationLefevre et al 2007).
Another possible mechanism by which resveratrol exerts its beneficial effect on the cardiovascular system is through its antioxidant activity. An association has been found between oxidation of LDL particles and risk of heart disease and myocardial infarction. Resveratrol prevents oxidation of LDL by chelating copper and by scavenging reactive oxygen species (ROS) (CitationFrankel et al 1993). The fact that resveratrol can be detected in LDL particles after red wine consumption by humans (CitationUrpi-Sarda et al 2005) is consistent with its ability to prevent peroxidation of lipids and other macromolecules (CitationBaur and Sinclair 2006). Furthermore, resveratrol activates the cholesterol efflux genes ATP-binding cassette transporter 1 (ABCA1) and ATP-binding cassette transporter G1 (ABCG1), as well as transcription factors that influence the nuclear liver receptor-α (LXR-α) gene, resulting in a more limited cholesterol accumulation by human macrophages. Resveratrol also represses the expression of the lipid uptake genes lipoprotein lipase and scavenger receptor AII (SR-AII) (CitationSevov et al 2006).
Multiple and complex pathways are therefore involved in the beneficial effects of resveratrol and other polyphenols on cardiovascular health. Their various effects protect against hypertension, ischemic heart disease, ischemic damage during myocardial infarction, and brain damage following cerebral ischemia, so explaining the inverse association that has been seen between dietary flavonoid consumption and cardiovascular mortality (CitationBaur and Sinclair 2006; CitationPerez-Vizcaino et al 2006).
Cancer
The first-observed, and now well-established, effect of resveratrol is its potential to inhibit the initiation and growth of tumors in a range of cancer models of both mouse and rat. Amongst many other such effects, resveratrol has been shown to prevent colon cancer (CitationTessitore et al 2000) and prostate cancer (CitationHarper et al 2007), and to substantially increase survival of mice with subcutaneous neuroblastomas (CitationChen et al 2004). Its effect on breast cancer is controversial, where resveratrol either failed to have any positive effect (CitationBove et al 2002) or prevented cancer progression in vivo (CitationWhitsett et al 2006; CitationSu et al 2007). Resveratrol was, moreover, found to inhibit angiogenesis in human xenografts (CitationGarvin et al 2006).
The multiple, and perhaps cumulative, mechanisms by which resveratrol slows tumour development have been reviewed thoroughly by CitationBaur and Sinclair (2006). These include inhibition of angiogenesis, alterations in cell cycle and apoptosis, as well as antioxidant effects. Some of these mechanisms seem to be due to the suppression by resveratrol of cyclooxygenases and ornithine decarboxylase expression, both enzymes that normally promote angiogenesis (CitationBaur and Sinclair 2006). It has been reported that resveratrol also inhibits cytochrome P450s, which are enzymes involved in drug metabolism and that are considered pro-carcinogens (CitationCiolino et al 1998; CitationZhou et al 2005). This was strengthened by findings in the microarray analyses of livers from resveratrol-treated mice, in which downregulation of three cytochrome P450 enzymes was observed (CitationBaur et al 2006).
Another possible mechanism for the protective effect of resveratrol against cancer relates to its antiproliferative and proapoptotic effects (CitationAggarwal et al 2004), leading to downregulation of cell cycle proteins and an increase in apoptosis of tumor cells (reviewed in CitationBaur and Sinclair 2006). While these effects have been seen in vitro, the study of obese mice by Sinclair’s group (CitationBaur et al 2006) produced somewhat conflicting results. When they compared a parametric analysis of gene set enrichment (PAGE) from resveratrol-treated fat mice with a preexisting caloric restriction data set known as AGEMAP, they found that cell cycle checkpoint and apoptotic pathways were elevated in the caloric restriction group, but downregulated in the resveratrol-treated group. Hepatic apoptosis was, however, unchanged, and the authors suggested that downregulation of the cell cycle checkpoints may be linked to the recent finding that inhibition of checkpoint function increases stress resistance and lifespan in C. elegans (CitationOlsen et al 2006).
The antioxidant effects of resveratrol could contribute to its anticancer properties. This is because ROS, by damaging DNA and other macromolecules, can contribute to the initiation and progression of cancer (CitationBaur and Sinclair 2006). It is not clear whether these are direct effects or a consequence of the upregulation of antioxidant enzymes by resveratrol.
It will be interesting to learn the results of several phase I clinical trials that are currently being conducted and which involve oral administration of resveratrol to humans in the USA and the UK (CitationBaur and Sinclair 2006).
Neurological disorders
The underlying pathogenic processes of neurodegenerative disorders are similar and are linked to pathways that are important during the aging process. It is thus not surprising that resveratrol has been shown to confer neuronal protection in in vitro studies (reviewed by CitationAnekonda 2006). CitationChen and co-workers (2005) showed that by activating SIRT-1, resveratrol inhibited, indirectly, nuclear factor κB (NF-κB) signalling and thus protected against microglia-dependent β-amyloid toxicity in a model of Alzheimer’s disease. This progressive age-related, neurodegenerative disorder is characterized by the presence of neurofibrillary tangles and extracellular amyloid β plaques in the cortex and hippocampus, areas of the brain that are important for memory and learning. A recent study by CitationKim and associates (2007) has now provided the in vivo evidence in two mouse models of neurodegenerative diseases, namely Alzheimer’s disease and amyotrophic lateral sclerosis (ALS). They found that resveratrol reduced significantly the extent of neuronal cell death and neurotoxicity of the mutant proteins expressed in these two models (p25, a toxic coactivator of CDK5, and a mutant form of superoxide dismutase 1, respectively). Administration of resveratrol reduced neurodegeneration in the hippocampus and rescued associative learning in p25 mice (CitationKim et al 2007). Most importantly, the effect of resveratrol was mediated by SIRT-1, via increased deacetylation of p53 and/or PGC-1α, confirming earlier findings of the role of PGC-1α in neuronal metabolism (CitationSt-Pierre et al 2006) and the link between resveratrol, SIRT-1, and PGC-1α discussed above. By repressing p53, resveratrol may protect neurons from oxidative damage and prevent apoptotic neuronal death. Another possible pathway involved in promoting neuronal survival involves suppression or activation of FOXO proteins. This has been supported by work in Huntington’s disease models of both mice and C. elegans, where resveratrol rescued early neuronal dysfunction phenotypes induced by mutant polyglutamines. In the nematode this effect was dependent on daf-16, a member of the FOXO family (CitationParker et al 2005).
There is evidence that Alzheimer’s disease is caused by the accumulation of localized neuronal damage surrounding hemorrhagic mini-strokes in the vicinity (CitationCullen et al 2006). Thus, the general cardiovascular protection provided by resveratrol in the brain is another way in which it may be protecting against this disease. In addition numerous studies have shown that brain damage caused by ischemia, stroke, seizure, and epilepsy is reduced markedly after treatment with resveratrol (CitationAnekonda 2006).
Resveratrol thus offers promise for the treatment and prevention of Alzheimer’s disease, but also Parkinson’s disease, Huntington’s disease, and other neurological disorders (CitationAnekonda 2006).
Dose and metabolism
The wide range of resveratrol doses used in animal studies (0.1 to 1.500 mg/kg body weight) (CitationBaur and Sinclair 2006), as well as the fact that resveratrol is absorbed and metabolized rapidly after oral ingestion by humans (CitationWalle et al 2004), makes it difficult to estimate a dose to be recommended as a therapeutic supplement or treatment. Since it is the metabolites of resveratrol, rather than free resveratrol, that tend to be detected in the urine up to 9 hours after ingestion, it has often been suggested that it is these metabolites that retain or mediate at least some of the beneficial effects (CitationBaur and Sinclair 2006). The levels of resveratrol and its derivatives attained after ingestion of 25 mg in a 70 kg adult are approximately 20–40 nmol/L and 2000 nmol/L, respectively (CitationYu et al 2002).
The potency of polyphenol extracts from different grape vine cultivars varies considerably (CitationRäthel et al 2007). CitationBaur and Sinclair (2006) optimistically estimate that at a concentration of 5 mg/l in red wine, two glasses (375 ml) would equal a dose of 27 μg/kg in a 70 kg person. This would result in peak serum concentrations of approximately 2.4 nM of free resveratrol and 180 nM of modified resveratrol. A recent Spanish study estimated that the total resveratrol intake for men and women is on average 1629 μg/day and 235 μg/day, respectively (CitationZamora-Ros et al 2007). A pharmacologically relevant dose, however, would have to be more like 100 mg/kg body weight or about 9 μM free resveratrol and 680 μM total resveratrol (CitationBaur and Sinclair 2006). Such doses have so far shown no adverse side effects in animal models (CitationCrowell et al 2004; CitationLagouge et al 2006). Many supplement companies offer resveratrol for purchase on the internet, but, since these are not regulated, the consumer should be more than cautious about buying them. Purity, origin, and age of the product are often unknown. Many of the preparations available on the market can, moreover, exhibit substantial depletion of activity by the time they reach the consumer. Products are offered at costs of about $US1 per 300 mg of resveratrol per capsule, which, at an intake of one capsule per day, would amount to monthly costs of US$30. However, 300 mg of resveratrol would probably have less effect than the amount of resveratrol present in a daily glass of red wine, so to achieve the various substantive results discussed in the present review, the cost of 100 mg/kg body weight needs to be considered. This would amount to approximately $US600 a month for an average person of 70 kg (CitationBaur and Sinclair 2006). The development of more stable analogues and the outcomes of clinical trials may progress both efficacy and price-performance ratio of resveratrol.
What is more, since no long-term effects in mammals have been established, and given the large number of targets which have been found for resveratrol to date, it has to be emphasized that we are a long way from knowing whether this polyphenol is safe as a therapeutic or as a supplementary drug. The potential side-effects of resveratrol, especially from long-term consumption, have not been established in mammalian studies to date, and are a mystery in humans.
Lastly, it should be noted that resveratrol can be accumulated in specific tissues (CitationVitrac et al 2003) and that it may act in synergy with other dietary compounds such as quercetin, ethanol, vitamin E, and catechin (CitationWallerath et al 2005; CitationBaur and Sinclair 2006).
Effects of other phytochemicals
While a detailed discussion of other naturally-occurring polyphenols would go beyond the scope of this review, it should be stressed that many phytochemicals have been implicated in the prevention and treatment of common human diseases. Some of these include curcumin (from the roots of turmeric), epigallocatechin (from green tea), sulforaphane (found in broccoli), and quercetin. Similar to resveratrol, most of these phytochemicals have neuroprotective, cancer-preventive, and positive cardiovascular effects. The major mechanism by which these agents were traditionally thought to exert their effects was by their antioxidative properties. In recent years, however, it has become clear that there are multiple cellular stress-response pathways and mechanisms activated in response to the polyphenols listed above. These include suppression of cytochrome P450 enzymes, induction of apoptotic pathways, suppression of cell cycle progression, chromatin remodelling, inhibition of angiogenesis, and anti-inflammatory activity (for detailed reviews refer to CitationDashwood et al 2006; CitationMattson and Cheng 2006; CitationMyzak and Dashwood 2006; CitationJuge et al 2007).
Conclusion
The biologically-potent polyphenol resveratrol has wide-ranging effects on diverse biological processes in cells. Stimulation of these show enormous promise in the prevention of common ills in our society. While we are eagerly awaiting the results of clinical trials, more research is required to fully understand the mechanisms whereby resveratrol exerts its effects and to exclude any potential long-term adverse side-effects.
References
- AggarwalBBBhardwajAAggarwalRSRole of resveratrol in prevention and therapy of cancer: preclinical and clinical studies(Review)Anticancer Res200424278384015517885
- AndersonRMBargerJLEdwardsMGDynamic regulation of PGC-1alpha localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress responseAging Cell200871011118031569
- AnekondaTSResveratrol – a boon for treating Alzheimer’s disease?Brain Res Rev2006523162616766037
- BaurJAPearsonKJPriceNLResveratrol improves health and survival of mice on a high-calorie dietNature20064443374217086191
- BaurJASinclairDATherapeutic potential of resveratrol: the in vivo evidenceNat Rev Drug Discov2006549350616732220
- BorraMTSmithBCDenuJMMechanism of human SIRT1 activation by resveratrolJ Biol Chem2005280171879515749705
- BoveKLincolnDWTsanMFEffect of resveratrol on growth of 4T1 breast cancer cells in vitro and in vivoBiochem Biophys Res Commun20022911001511866465
- ChenJZhouYMueller-SteinerSSIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signalingJ Biol Chem2005280403647416183991
- ChenYTsengSHLaiHSResveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in miceSurgery2004136576615232540
- CiolinoHPDaschnerPJYehGCResveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptorCancer Res1998585707129865727
- ContiBSanchez-AlavezMWinsky-SommererRTransgenic mice with a reduced core body temperature have an increased life spanScience2006314825817082459
- CrowellJAKorytkoPJMorrisseyRLResveratrol-associated renal toxicityToxicol Sci200482614915329443
- CullenKMKócsiZStoneJMicrovascular pathology in the aging human brain: Evidence that senile plaques are sites of microhaemorrhagesNeurobiol Aging20062717869617063559
- DasSAlagappanVKBagchiDCoordinated induction of iNOS-VEGF-KDR-eNOS after resveratrol consumption: a potential mechanism for resveratrol preconditioning of the heartVascul Pharmacol200542281915905131
- DashwoodRHMyzakMCHoEDietary HDAC inhibitors: time to rethink weak ligands in cancer chemoprevention?Carcinogenesis200627344916267097
- DenuJMThe Sir 2 family of protein deacetylasesCurr Opin Chem Biol200594314016122969
- FontanaLMeyerTEKleinSLong-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humansProc Natl Acad Sci USA200410166596315096581
- FrankelENWaterhouseALKinsellaJEInhibition of human LDL oxidation by resveratrolLancet1993341110348097009
- FrojdoSCozzoneDVidalHResveratrol is a class IA phosphoinositide 3-kinase inhibitorBiochem J20074065111817550345
- GarvinSOllingerKDabrosinCResveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivoCancer Lett20062311132216356836
- Gerhart-HinesZRodgersJTBareOMetabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alphaEmbo J20072619132317347648
- GuarenteLPicardFCalorie restriction--the SIR2 connectionCell20051204738215734680
- HarperCEPatelBBWangJResveratrol suppresses prostate cancer progression in transgenic miceCarcinogenesis20072819465317675339
- HowitzKTBittermanKJCohenHYSmall molecule activators of sirtuins extend Saccharomyces cerevisiae lifespanNature2003425191612939617
- JugeNMithenRFTrakaMMolecular basis for chemoprevention by sulforaphane: a comprehensive reviewCell Mol Life Sci20076411052717396224
- KaeberleinMKirklandKTFieldsSSir2-independent life span extension by calorie restriction in yeastPLoS Biol20042E29615328540
- KaeberleinMMcDonaghTHeltwegBSubstrate-specific activation of sirtuins by resveratrolJ Biol Chem2005280170384515684413
- KimDNguyenMDDobbinMMSIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosisEmbo J20072631697917581637
- KooS-HMontminyMIn vino veritas: A tale of two Sirt1s?Cell20061271091317174885
- LagougeMArgmannCGerhart-HinesZResveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alphaCell200612711092217112576
- LefevreJMichaudSEHaddadPModerate consumption of red wine (cabernet sauvignon) improves ischemia-induced neovascularization in ApoE-deficient mice: Effect on endothelial progenitor cells and nitric oxideFaseb J20072138452217641150
- LeightonFMiranda-RottmannSUrquiagaIA central role of eNOS in the protective effect of wine against metabolic syndromeCell Biochem Funct200624291816170835
- LeikertJFRathelTRWohlfartPRed wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cellsCirculation20021061614712270851
- LiHFChenSAWuSNEvidence for the stimulatory effect of resveratrol on Ca(2+)-activated K+ current in vascular endothelial cellsCardiovasc Res20004510354510728430
- LinJWuHTarrPTTranscriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibresNature200241879780112181572
- LinSJDefossezPAGuarenteLRequirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiaeScience20002892126811000115
- MattsonMPChengANeurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responsesTrends Neurosci200629632917000014
- MichishitaEParkJYBurneskisJMEvolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteinsMol Biol Cell20051646233516079181
- MorrisBJA forkhead in the road to longevity: the molecular basis of lifespan becomes clearer. [Invited Review]J Hypertens200523128530915942449
- MorrisBJClimate not cultivars in the NO-ing of red winesJ Hypertens200725501317278961
- MorrisBJLe BourgERattanSISHow xenohormetic compounds confer health benefitsMild Stress: Applying Hormesis in Aging Research and Interventions2008NetherlandsSpringer11538
- MukherjeeDNissenSETopolEJRisk of cardiovascular events associated with selective COX-2 inhibitorsJAMA2001286954911509060
- MyzakMCDashwoodRHHistone deacetylases as targets for dietary cancer preventive agents: lessons learned with butyrate, diallyl disulfide, and sulforaphaneCurr Drug Targets200674435216611031
- OlsenAVantipalliMCLithgowGJCheckpoint proteins control survival of the postmitotic cells in Caenorhabditis elegansScience20063121381516741121
- OralloFAlvarezECaminaMThe possible implication of trans-resveratrol in the cardioprotective effects of long-term moderate wine consumptionMol Pharmacol20026129430211809853
- ParkerJAArangoMAbderrahmaneSResveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neuronsNat Genet2005373495015793589
- Perez-VizcainoFDuarteJAndriantsitohainaREndothelial function and cardiovascular disease: effects of quercetin and wine polyphenolsFree Radic Res20064010546517015250
- RäthelTRSamtlebenRVollmarAMActivation of endothelial nitric oxide synthase by red wine polyphemols: Impact of grape cultivars, growing area and the vinification processJ Hypertens200725541917278969
- RenaudSde LorgerilMWine, alcohol, platelets, and the French paradox for coronary heart diseaseLancet1992339152361351198
- RoginaBHelfandSLSir2 mediates longevity in the fly through a pathway related to calorie restrictionProc Natl Acad Sci USA200410115998600315520384
- SevovMElfinehLCavelierLBResveratrol regulates the expression of LXR-alpha in human macrophagesBiochem Biophys Res Commun200634810475416901463
- ShenMYHsiaoGLiuCLInhibitory mechanisms of resveratrol in platelet activation: pivotal roles of p38 MAPK and NO/cyclic GMPBr J Haematol20071394758517868048
- SmithDLJrMcClureJMMatecicMCalorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the SirtuinsAging Cell200766496217711561
- St-PierreJDroriSUldryMSuppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivatorsCell200612739740817055439
- StefaniMMarkusMALinRCThe effect of resveratrol on a cell model of human agingAnn N Y Acad Sci200711144071817804521
- SuJLYangCYZhaoMForkhead proteins are critical for bone morphogenetic protein-2 regulation and anti-tumor activity of resveratrolJ Biol Chem2007282193859817513867
- SzewczukLMFortiLStivalaLAResveratrol is a peroxidase-mediated inactivator of COX-1 but not COX-2: a mechanistic approach to the design of COX-1 selective agentsJ Biol Chem2004279227273715020596
- TessitoreLDavitASarottoIResveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21CIP expressionCarcinogenesis20001616192210910967
- Urpi-SardaMJaureguiOLamuela-RaventosRMUptake of diet resveratrol into the human low-density lipoprotein. Identification and quantification of resveratrol metabolites by liquid chromatography coupled with tandem mass spectrometryAnal Chem20057731495515889903
- ValenzanoDRTerzibasiEGenadeTResveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrateCurr Biol20061629630016461283
- VitracXDesmouliereABrouillaudBDistribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administrationLife Sci20037222193312628442
- WalleTHsiehFDeLeggeMHHigh absorption but very low bioavailability of oral resveratrol in humansDrug Metab Dispos20043213778215333514
- WallerathTDeckertGTernesTResveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthaseCirculation20021061652812270858
- WallerathTLiHGodtel-AmbrustUA blend of polyphenolic compounds explains the stimulatory effect of red wine on human endothelial NO synthaseNitric Oxide2005129710415740983
- WangYTissenbaumHAOverlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXOMech Ageing Dev2006127485616280150
- WhitsettTCarpenterMLamartiniereCAResveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in ratsJ Carcinog200651516700914
- WoodJGRBLavuSSirtuin activators mimic caloric restriction and delay ageing in metazoansNature2004430686915254550
- YuCShinYGChowAHuman, rat, and mouse metabolism of resveratrolPharm Res20021919071412523673
- Zamora-RosRAndres-LacuevaCLamuela-RaventosRMConcentrations of resveratrol and derivatives in foods and estimation of dietary intake in a Spanish population: European Prospective Investigation into Cancer and Nutrition (EPIC)-Spain cohortBr J Nutr2007122119 Epub ahead of print
- ZangMXuSMaitland-ToolanKAPolyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient miceDiabetes20065521809116873680
- ZhouHBChenJJWangWXAnticancer activity of resveratrol on implanted human primary gastric carcinoma cells in nude miceWorld J Gastroenterol200511280415633232