Abstract
Sarcopenia is the progressive generalized loss of skeletal muscle mass, strength, and function which occurs as a consequence of aging. With a growing older population, there has been great interest in developing approaches to counteract the effects of sarcopenia, and thereby reduce the age-related decline and disability. This paper reviews (1) the mechanisms of sarcopenia, (2) the diagnosis of sarcopenia, and (3) the potential interventions for sarcopenia. Multiple factors appear to be involved in the development of sarcopenia including the loss of muscle mass and muscle fibers, increased inflammation, altered hormonal levels, poor nutritional status, and altered renin–angiotensin system. The lack of diagnostic criteria to identify patients with sarcopenia hinders potential management options. To date, pharmacological interventions have shown limited efficacy in counteracting the effects of sarcopenia. Recent evidence has shown benefits with angiotensin-converting enzyme inhibitors; however, further randomized controlled trials are required. Resistance training remains the most effective intervention for sarcopenia; however, older people maybe unable or unwilling to embark on strenuous exercise training programs.
Keywords:
Background
Maintaining muscle function is vital to maintain functional independence. In our growing older population, muscle mass and force reach their peak between the second and fourth decades of life and thereafter show a steady decline with age.Citation1 Sarcopenia is a syndrome characterized by progressive generalized loss of skeletal muscle mass and strength. It is usually accompanied by physical inactivity, decreased mobility, slow gait, and poor physical endurance which are also common features of the frailty syndrome.Citation2 Rockwood et alCitation3 described the concept of frailty as “a multidimensional syndrome which involves loss of reserves (energy, physical activity, cognition, and health) which gives rise to increased vulnerability”. Frailty involves a cumulative decline in multiple physiological systems including a decline in the neuromuscular system which is linked to the development of sarcopenia in later life. The loss of muscle mass during the ageing process is clinically important as it leads to reduced strength and exercise capacity, both of which are required to undertake normal daily living activities. Moreover, loss of muscle mass is a strong predictor of mortality in later life.Citation4
It has been estimated that up to 15% of people older than 65 years and as many as 50% of people older than 80 years have sarcopenia.Citation5 Sarcopenia has a major impact on public health and the cost in the united States alone was estimated at $18.5 billion in 2000.Citation6 With the increasing number of older people worldwide, the cost is ever increasing. There has, therefore, been great interest in developing approaches to counteract the effects of sarcopenia and thereby help in reducing the age-related decline and disability.
What causes sarcopenia?
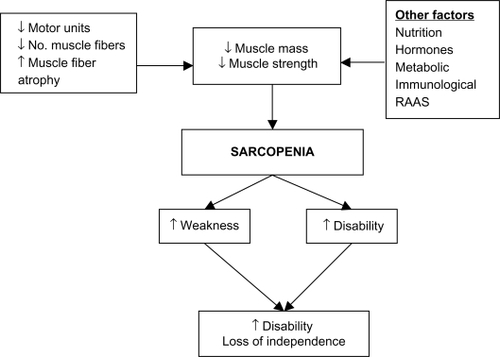
Understanding the mechanisms that have been implicated in the development of sarcopenia can help direct sarcopenia treatments. Research is still ongoing but as yet no primary cause of sarcopenia has been identified. Multiple factors appear to be involved in the development of sarcopenia (). A reduction in muscle strength is primarily linked to a reduction in overall muscle mass.Citation7 This reduction in muscle mass may occur due to a combination of the loss of muscle fibers as well as muscle fiber atrophy with a preferential atrophy of type 2 fast twitch fibers. Denervation of motor units which is then reinnervated with slow motor units can lead to increased muscle fatigability.Citation8 Although the overall biological mechanism of sarcopenia is not fully understood, observational studies have shown that satellite cells which are involved in muscle regeneration are much lower in older people and, therefore, could play a role in sarcopenia.Citation9
Other factors including hormonal changes including growth hormone (GH) and insulin-like growth factor (IGF-1) and androgens which help regulate growth and development of skeletal muscle appear to decrease in old age. It has been suggested that the renin–angiotensin system may play a role in modulating muscle function. Circulating angiotensin 2 is associated with muscle wasting, reduced IGF-1 levels, and insulin resistance and could, therefore, contribute to sarcopenia.Citation10 Sarcopenia is also associated with chronic inflammation, and observational studies have shown increased levels of proinflammatory cytokines, tumor necrosis factor-α, and interleukin-6 in aging muscle.Citation11 Studies looking at treating sarcopenia have attempted to address some of the factors implicated in the development of sarcopenia and we discuss the evidence surrounding this in our review.
Diagnosis of sarcopenia
The first step in the management of sarcopenia is to diagnose the condition. Unfortunately, at present there are no standardized diagnostic criteria for sarcopenia. Although sarcopenia is considered as a dynamic process incorporating both changes in muscle mass and function, many observational studies have concentrated on assessing changes in muscle mass. summarizes the measurement techniques, what they measure, and their limitations. Although magnetic resonance imaging (MRI) is considered to be the most accurate measure of muscle mass, the currently preferred method is dual energy X-ray absorptiometry (DXA) as it measures both fat mass and bone mass and is useful for assessing appendicular muscle mass. DXA closely correlates with measurements achieved via MRI scanning.Citation12 The main limitation with DXA is that it may underestimate the extent of sarcopenia as it can overestimate skeletal muscle mass by as much as 8% due to difficulty in distinguishing muscle from water retention and muscle fat infiltration.Citation13 Bioelectric Impedence Analysis is a quick noninvasive method for measuring body composition via tissue conductivity.Citation14 However, its reliability has been called into question as measurements can vary depending on an individual’s hydration status, ethnicity, physical fitness, and age.Citation15
Table 1 Measuring techniques for sarcopenia
BaumgartnerCitation5 in 1998 proposed a method for diagnosing sarcopenia. The degree of sarcopenia was measured by taking the muscle mass relative to a person’s height. Appendicular skeletal mass (ASM) was measured in all four limbs with DXA. Individuals with ASM/height2 (kg/m2) of two standard deviations (SDs) below the mean for gender specific healthy younger adults were more likely to have sarcopenia. Janssen et alCitation16 measured skeletal muscle mass using bioimpedence and defined sarcopenia as a skeletal muscle index (skeletal muscle mass/whole body mass × 100) less than 1 SD below the mean for young adult values. These definitions fail to incorporate measures of disability and physical performance.Citation16
Since then attempts have been made to refine the definition of sarcopenia. More recently, a joint effort has been made between the European Society on Clinical Nutrition and Metabolism and the Special Interest Needs group on geriatric nutrition on cachexia – anorexia and chronic wasting diseases.Citation17 A diagnosis of sarcopenia was based on two of the following:
A low muscle mass, ie, a percentage of muscle mass >2 SDs below the mean measured in groups of young adults of the same sex and ethnic background.
Low gait speed, ie, walking speed below 0.8 m/s in the 4-meter walking test. However, this could be replaced with one well-established functional test utilized locally as part of a comprehensive geriatric assessment.
Although this definition includes measures of functional performance and helps to eliminate variance between ethnicity and sex, it fails to set age-specific populations. It has been suggested that a T-score system similar to that for osteoporosis is needed to include reference values for different populations. However, further refinement is required.
The main effect of loss of muscle mass is loss of strength. Low muscle strength is associated with increased mortality.Citation18 Although complex measures of power and torque are available, hand-held dynamometers to measure hand grip and quadriceps strength have been commonly used with good reproducibility and validity and are simple to use and can be easily used in the clinical setting.Citation19
Physical performance measures can complement measures of muscle mass and strength in the diagnosis of sarcopenia. The Short Physical Performance Battery assesses muscle function and strength using measures which are reproducible to activities of daily living. The assessment involves balance tests, a timed 4-meter walk, and timed chair rise which can be easily performed in the clinical setting. It can predict the risk of future disability and, therefore, may be useful in identifying people in the preclinical stage of sarcopenia who may benefit from interventions.Citation20
Comorbidities and factors like pain from osteoarthritis which are unrelated to sarcopenia may limit performance and underestimate muscle strength. Fat mass may contribute to functional decline independent of muscle mass.Citation21 Individuals with sarcopenic obesity (high fat mass and low muscle mass) are more susceptible to mobility problems and disability than those who are simply obese or sarcopenic.Citation22 It is, therefore, imperative that sarcopenia is diagnosed under its paradigm as a dynamic process by assessing lean body mass and physical performance. As yet this may be more difficult to reproduce in a clinical setting.
The main difficulties in diagnosing sarcopenia are the lack of consensus in the definition of sarcopenia as well as the difficulty in measuring changes in muscle mass and function over time in older people. In clinical practice, the diagnosis is often missed as it is usually made in patients who appear to have “small muscle mass”. Simple measures of muscle strength and physical performance measures can supplement clinical diagnosis for the early recognition of people at risk of disability.
Potential interventions for sarcopenia
Exercise and physical activity
Physical activity refers to the body movement that is produced by skeletal muscle contractions and that increases energy expenditure.Citation23 Evidence has shown that older adults who are less physically active are more likely to have lower skeletal muscle mass and strength and are at increased risk of developing sarcopenia.Citation24,Citation25
In aerobic exercise, the larger muscles in the body move in a rhythmic manner for a prolonged period of time, whereas resistance exercise involves muscles working hard against an applied force or weight such as in weight-lifting. Both aerobic and resistance-type exercise training have shown to improve the rate of decline in muscle mass and strength with age ().Citation26
Table 2 Summary of treatment options
Aerobic activity (swimming, running, and walking) has long been linked to improvements in cardiovascular fitness and endurance capacity. Although aerobic exercise is less likely to contribute to muscle hypertrophy, it can increase the cross-sectional area (CSA) of muscle fibers.Citation27 Mitochondrial volume and enzyme activity increase after aerobic exercise demonstrate that muscle protein synthesis and muscle quality improve irrespective of age.Citation28 Aerobic exercise can also reduce body fat including intramuscular fat which in turn improves the functional role of muscle relative to body weight.Citation29
In contrast to aerobic exercise training, resistance exercise training appears to have a larger effect on augmenting muscle mass and strength and attenuates the development of sarcopenia.Citation30,Citation31 Improvements in muscle strength can be achieved with as little as one resistance exercise training session per week.Citation32 Frontera et alCitation33 demonstrated improvements in muscle CSA by 11% as well as improvement in muscle strength (>100%) after a 12-week period of high intensity resistance exercise training in older men. Similar improvements were seen in muscle strength even in the people aged >90 years with as little as 10–12 weeks of training.Citation34
Muscle hypertrophy occurs when muscle protein synthesis outweighs protein breakdown. Older people performing resistance exercise show a marked increase in skeletal muscle protein synthesis without an increase in whole body muscle breakdown. Resistance training in older people increases both mixed-muscle protein synthesis and specific major histocompatibility complex synthesis to the same levels as younger adults.Citation35 Evidence points to increases in size of both type 1 and type 2 muscle fibers which could explain the improvements in muscle strength and endurance.Citation33,Citation36 More recently, it has been reported that when using moderate levels of resistance exercise training, improvements in muscle strength and size in healthy older people were comparable to muscle strength seen in younger individuals. Roth et alCitation37 demonstrated that 6 months of whole body resistance training in older people (65–75 years) produced gains in muscle CSA similar to those achieved in younger individuals aged 20–30 years.
Progressive resistance training (PRT) is the most commonly used resistance therapy in older people. A Cochrane review of 121 randomized controlled trials of PRT in older people showed that doing PRT 2–3 times per week improved physical function, gait speed, timed get-up and go, climbing stairs and balance, and more importantly had a significant effect on muscle strength especially in the high intensity training groups.Citation38
The majority of studies have shown that resistance exercise training must be carried out at a high intensity in order to show substantial improvements in muscle strength.Citation33,Citation36,Citation37 In contrast, Vincent et alCitation39 performed a 26-week study in older healthy adults at both low and high intensity resistance exercise training programs and found only a modest improvement in thigh muscle strength in the high intensity resistance exercise training group.
Resistance exercise training appears to be relatively safe to perform even in participants with multiple comorbidities and can help in prevention of falls.Citation40 Resistance exercise increases muscle CSA as well as type 2 (fast twitch) muscle fibers, which leads to overall improvement in muscle power and the ability to improve physical functioning. As a result, this can lead to enhanced ability to perform activities of daily living, preventing in functional decline and disability. Even in very old nursing home residents, resistance exercise training showed substantial improvements in muscle fiber CSA (3%–9%), muscle strength (>100%) as well as improvements in physical performance such as gait speed and stair climbing.Citation36,Citation38 However, participation in regular exercise training requires motivation by the individual which may be difficult for some older individuals; therefore, nonexercise interventions may offer a useful alternative.
Nutrition
Many older adults do not consume sufficient amounts of dietary protein which leads to a reduction in lean body mass and increased functional impairment.Citation41 The current recommended dietary allowance (RDA) of protein is 0.8 g/kg/day, almost 40% of people >70 years do not meet this RDA.Citation42 Taking a low protein diet below the RDA leads to a significant decline in muscle strength and muscle mass in older women.Citation43 However, even older people who take the recommended RDA for protein continue to have a negative nitrogen balance and may require a diet containing a higher protein content than the RDA to maintain their skeletal muscle.Citation44 Protein and energy supplementation may increase muscle strength even in very old people in the short term, but a Cochrane review has found no definite functional benefit of nutritional supplementation.Citation45–Citation47
Although older people who exercise have increased protein requirements, studies investigating whether nutritional supplementation in combination with resistance training can augment muscle strength gains in older people have yielded inconsistent results. One randomized controlled trial in nursing home residents undergoing resistance training over 10 weeks found that an additional 360 calories supplement increased leg muscle strength.Citation36 Another study investigating the effect of dietary protein supplementation in combination with a 12-week resistance training period found that protein supplementation increased muscle mass but not muscle strength.Citation48 Nutritional supplementation may also result in an overall decrease in voluntary food intake and adherence to the supplements can be a problem.Citation47
Testosterone
Testosterone is secreted by the Leydig cells in men and ovarian thecal cells in women.Citation49 Testosterone appears to increase muscle mass and increase muscle protein synthesis.Citation50 It also increases the number of satellite cells in both animals and humans which are essential for muscle cell function.Citation51
A substantial number of older men are hypogonadal. Hypogonadism has been defined as a total testosterone concentration of <9.26 nmol/L (2 SD below the mean for healthy young men). As a result, approximately 20% of men >60 years and 50% men >80 years are categorized as hypogonadal.Citation52 Circulating testosterone is highly bound to sex hormone binding globulin (SHBG) and as SHBG increases with age, the total amount of bioavailable testosterone decreases. This phenomenon has been termed the “male menopause” or “andropause” in older men. Testosterone decreases gradually at a rate of 1% per year and bioavailable testosterone by 2% per year in males from the age of 30 years.Citation53 The overall reduction of testosterone is associated with loss of muscle strength, muscle mass, a reduction in bone mineral density, and increased risk of fracture risk following falls.Citation54,Citation55
Evidence to support testosterone supplementation is variable. Gruenewald and MatsumotoCitation56 analyzed 29 randomized controlled trials investigating the effects of testosterone replacement in older men. Some studies found an increase in lean body mass and hand grip strength but no effect on knee extension and flexion strength.Citation56 Other studies have shown up to 25% increase in leg strength in as little as 4 weeks of therapy.Citation57 Some studies have found no increase in muscle strength or function but an improvement in lean body mass.Citation58 Testosterone supplementation has been shown to increase the size of the prostate gland in men.Citation59 This could be detrimental to men older than 60 years in which the prevalence of early stage prostate cancer is already high.Citation60 The Baltimore Longitudinal Study on Aging involving 781 men showed a positive correlation between prostate cancer and the blood concentration of free testosterone levels. The likelihood of acquiring a high risk prostate cancer in men >65 years doubled for every 0.1 unit increase in free testosterone.Citation61 This along with other potential side effects of testosterone therapy like fluid retention, gynecomastia, polycythemia, and sleep apnea limit its usefulness as a treatment for sarcopenia.Citation59,Citation62,Citation63
Estrogens
The menopause is linked to reduced concentrations of circulating estradiol in middle aged and older women. There appears to be impaired muscle performance during the postmenopausal period when ovarian hormone production has decreased.Citation64 It is easy to hypothesize that estrogens may play a role in sarcopenia in older women.
The effect of hormone replacement therapy (HRT) in women is controversial. HRT may attenuate the loss of muscle mass which occurs in the perimenopausal period.Citation65 Estrogen replacement therapy has only modest benefits on muscle composition and this may not translate to improved physical functioning.Citation66 HRT combined with resistance training may have a role in improving lower extremity function; however, more evidence is needed.Citation67 HRT has been implicated as a risk factor for breast cancer and is, therefore, not recommended for sarcopenia.Citation68
Growth hormones
GH is required for maintenance of muscle and bone. GH exerts most of its anabolic actions through IGF-1 which is synthesized in the liver for systemic release. IGF-1 helps improve muscle function by increasing production of muscle satellite cells as well as stimulating production of muscle contractile proteins.Citation69 Not only do GH and IGF-1 levels decline with age, the amplitude and frequency of pulsatile GH release is also significantly reduced.Citation70
Despite a number of studies which have assessed the administration of GH supplementation, there is still an ongoing debate as to the use of GH supplementation on muscle mass, strength, and physical performance. The strongest evidence for the use of GH supplementation appears to be in states of reduced GH secretion. In younger GH deficient adults, GH supplementation for 3 years increased thigh muscle mass, strength, and improved exercise capacity.Citation71 However, in healthy non-GH deficient older people results are more controversial. Some studies have shown an increase in muscle mass but no improvement in muscle strength, whereas others have shown an increase in both muscle mass and strength after administration of GH supplementation.Citation72–Citation74 The failure of exogenous GH to mimic the pulsatile pattern of normal GH secretion has been blamed for the negative results. Alternative potential hormonal interventions include the use of GH releasing hormone which was found to have only a small improvement in muscle strength in older men.Citation75
It is well known that muscle strength increases as a result of resistance exercise training in older adults.Citation30,Citation33 It was hypothesized that the combination of GH replacement and exercise training may have a synergistic effect on muscle function in older people. However, results proved disappointing and the addition of GH supplementation does not augment the improvements in skeletal muscle brought about by exercise alone.Citation76,Citation77 Low GH levels alone, therefore, may not be responsible for the leveling off of muscle strength seen in older exercising people and that other pathways may be involved.
As it currently stands the evidence for the use of GH supplementation to counter the effects of sarcopenia in older people is weak. Moreover, the majority of trials involving GH replacement therapy in older people have reported a high incidence of side effects, including increased fluid retention, gynecomastia, orthostatic hypotension, and carpel tunnel syndrome.Citation72,Citation78
Vitamin D
Vitamin D levels decline with age and cutaneous vitamin D levels are up to four times lower in older compared with younger individuals.Citation79 It is known that vitamin D plays an important role in bone and muscle metabolism. Several mechanisms have been suggested for the role of vitamin D in muscle function. Vitamin D binding to the vitamin D receptor found in skeletal muscle promotes muscle protein synthesis and enhances calcium uptake across the cell membrane.Citation80 Low vitamin D levels result in atrophy predominantly of the type 2 (fast twitch) muscle fibers in common with sarcopenia.Citation81 Low levels of vitamin D have been found to be associated with an increase in sarcopenia.Citation82 A myopathy has been reported in severe vitamin D deficiency.Citation83 In older people low vitamin D levels may produce functional problems including proximal muscle weakness, difficultly rising from a chair, difficulties in ascending stairs, and axial balance problems.Citation84
The evidence for a benefit in physical performance with supplementation of vitamin D is controversial. Some studies have shown an improvement in muscle strength with intermittent dosing and others have shown small gains in lower extremity strength and less body sway with daily dosing.Citation85 This improvement has been hypothesized as the mechanism behind a fall reduction of 23%–53% in older nursing or residential home residents given vitamin D in addition to a reduction in fractures.Citation86–Citation88
Conversely, other studies have found no benefits on physical function, falls risk or quality of life with vitamin D supplementation in vitamin D deficient people.Citation89–Citation91 The difference in findings between studies may in part be attributed to differences in the dose of vitamin D used with better outcomes seen when higher doses are used.Citation92 It has also been suggested that there is a gender difference in outcomes with women standing to gain more from supplementation.Citation92 Variations between study populations may also affect outcomes with the biggest improvements in muscle function and physical performance seen in institutionalized older people.
The prevalence of vitamin D insufficiency (25(OH)D levels <40 nmol/L) in older people is high between 50% and 75% especially in the northern latitudes and low levels have been found even in summer months.Citation93–Citation95 A European epidemiological study showed the prevalence of vitamin D deficiency in older adults aged 71–76 years was 36% in older men and 47% in older women.Citation96 It is recommended that 25(OH)D levels <40 nmol/L requires supplementation and 25(OH)D levels of >75 nmol/L is the level for optimum bone and muscle health.Citation97 The recommended daily intake of vitamin D is between 400 IU and 600 IU per day which may be inadequate to raise serum vitamin D levels to a desirable level >70 nmol/L.Citation98,Citation99 Studies have shown that in order to achieve optimal levels of 75–100 nmol/L of 25(OH)D doses between 700 and 1,000 IU would be needed.Citation90 In the United States, fortification of food such as milk and orange juice is mandatory, whereas in the UK only margarine is fortified with vitamin D. The question of whether it should be mandatory for UK food products to be fortified with vitamin D remains controversial.
Although it is plausible to associate low levels of vitamin D with a reduction in muscle strength and physical function, the evidence for supplementation has been inconsistent. Safety issues surrounding vitamin D supplementation in older people include increased risk of nephrolithiasis and hypercalcemia.Citation100,Citation101 Further large randomized controlled trials are required with a longer follow-up period in order to assess the safety profile of vitamin D supplementation in older people before it is recommended as a treatment for sarcopenia in clinical practice.
Angiotensin-converting enzyme inhibitors
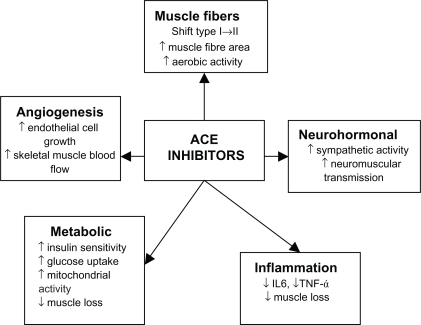
Angiotensin-converting enzyme (ACE) inhibitors have long been used as a treatment in primary and secondary prevention in cardiovascular disease as well as secondary stroke prevention. It has now been suggested that ACE inhibitors may have a beneficial effect on skeletal muscle. ACE inhibitors may exert their beneficial effects on skeletal muscles through a number of different mechanisms (). ACE inhibitors may improve muscle function through improvements in endothelial function, metabolic function anti-inflammatory effects, and angiogenesis thereby improving skeletal muscle blood flow. ACE inhibitors can increase mitochondrial numbers and IGF-I levels thereby helping to counter sarcopenia.Citation102–Citation107 People with the II genotype of the ACE gene who have low serum ACE levels show an increased response to physical endurance.Citation108,Citation109 Therefore, lowering serum ACE levels with ACE inhibitors may have a beneficial effect on physical function. Observational studies have shown that the long-term use of ACE inhibitors was associated with a lower decline in muscle strength and walking speed in older hypertensive people and a greater lower limb lean muscle mass when compared with users of other antihypertensive agents.Citation110,Citation111 Several studies have shown that ACE inhibitors improved exercise capacity in younger people with heart failure and this was also confirmed in older people with heart failure,Citation110,Citation112,Citation113 no improvement in grip strength.Citation114 Although this could be largely attributed to improvements in cardiac function, skeletal muscle atrophy is also associated with chronic heart failure so the evidence in muscle gains should not be discounted.
Figure 2 Effects of ACE inhibitors on skeletal muscle.
Few interventional studies using ACE inhibitors for physical function have been undertaken. One study looking at functionally impaired older people without heart failure has shown that ACE inhibitors increase 6-minute walking distance to a degree comparable to that achieved after 6 months of exercise training.Citation115 Another found that ACE inhibitors increased exercise time in older hypertensive men.Citation116 However, a study comparing the effects of nifedipine with ACE inhibitors in older people found no difference between treatments in muscle strength, walking distance, or functional performance.Citation117 It is possible that frailer subjects with slower walking speeds, who have a tendency to more cardiovascular problems, benefit more. This is reflected in the fact that adults with severe peripheral vascular disease significantly increase their walking time following treatment with ACE inhibitors.Citation118
Further evidence is required before recommending ACE inhibitors to counter the effects of sarcopenia. However, ACE inhibitors are associated with cardiovascular benefits and as older people frequently have underlying cardiovascular problems these agents are already commonly prescribed.
Creatine
Creatine plays an important role in protein metabolism and cellular metabolism. It has been hypothesized that creatine increases the expression of myogenic transcription factors such as myogenin and myogenic regulatory factor-4, which increases muscle mass and strength.Citation119 Creatine supplementation increases muscle phosphocreatine levels leading to a decrease in muscle relaxation time.Citation120,Citation121 This may increase the ability to perform high-intensity exercise as well as enhance muscle protein synthesis, lean skeletal muscle mass, and strength during periods of high intensity training.
To dates, several studies of creatine supplementation have shown increased muscle strength and power in younger men and women but few studies have looked at the effect of creatine supplementation in older people. Some studies have reported no effect of creatine supplementation on muscle strength or function.Citation122,Citation123 However, others have reported increments in muscle mass and increased muscle power without adverse effects.Citation122,Citation124 There is controversy over whether creatine supplementation increases the benefits of resistance training alone in older people. Some studies have found no added benefit of creatine supplementation to resistance exercise training and other studies have found a small increase in lean tissue mass with no residual benefit once resistance training was stopped.Citation125–Citation127
Creatine is a natural ingredient of food and the main source is from meat products with an average daily intake of 1 g/day. However, creatine supplementation may increase the risk of interstitial nephritis highlighting the need for particular caution about its use in older people.Citation128 Creatine is not currently recommended for sarcopenia.
Myostatin
Myostatin is a natural inhibitor of growth factor. It was initially discovered when mutations of the myostatin gene was found to correlate with exaggerated muscle hypertrophy.Citation129 The myostatin gene appears in skeletal muscle cells and functions as a negative regulator of muscle growth, antagonism of which increases satellite cell proliferation.Citation130 In animal models, it appears that over expression of myostatin induces extensive muscle loss.Citation131 Polymorphisms of the myostatin gene in humans correlated with measure of muscle mass, strength, and physical performance.Citation132
Agents which target the myostatin pathway may be useful in increasing muscle mass and, therefore, play a vital role in muscle wasting disorders as well as sarcopenia of old age. Phase II trials have been carried out in muscular dystrophy and initial results have shown that MYO-29, a recombinant antibody to myostatin, had good safety and tolerability profile.Citation133 Another potential therapeutic approach in development is a soluble activin type 2B receptor that binds to the myostatin and, therefore, reducing its availability. Initial results in mice have shown an increase in muscle weight larger than those achieved with myostatin inhibitors.Citation134
Inhibition of myostatin with follistatin (myostatin antagonist) may have potential therapeutic benefits in the treatment of sarcopenia. Although myostatin deficiency increased muscle mass in mice it impaired, the structure and function of the muscle tendons thereby making the tendons smaller, stiffer, and more brittle.Citation135,Citation136 Older people who are already at increased risk of contraction induced injury may find it more difficult to sustain regular exercise. Further studies are required.
Other potential therapies
Following suggestions of a role for both the peroxisome-prolife rator-activated receptor-δ (PPAR-δ) and adenosine monophosphate (AMP)-activated protein kinase in regulating the metabolic and contractile characteristics of myofibers, studies looking at the effect of modulating these receptors have been carried out in mice. The PPAR-δ agonist GW1516 significantly increases exercise capacity when combined with exercise but not in sedentary mice. However, the activator of AMP-activated protein kinase called AICAR (5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside) increases exercise performance by 44% even in sedentary mice and may possible be the “exercise in a pill” for older people.Citation137 It remains to be verified if these drugs are suitable for human beings especially older people.
Conclusion
Sarcopenia is an ever increasing global health concern that needs to be urgently addressed. Public awareness of the importance of physical activity need to be increased and exercise programs designed for older people should be developed. Although the primary aim would be to prevent the occurrence of the sarcopenia, like many other medical problems, we are ultimately left with having to deal with the condition and its consequences. As our review suggests, all is not lost by this stage and there is still scope for improvement even in the frail older person with a combination of measures.
The lack of diagnostic criteria to identify patients with sarcopenia hinders potential management options. Resistance exercise training remains the cornerstone of management for sarcopenia. As some older people are unable or unwilling to embark on exercise training program, alternative potential treatment options to counter the process of sarcopenia are being developed. Recent evidence has shown ACE inhibitors can improve muscle exercise capacity in functionally impaired older people; however, further randomized controlled trials are required. Other future prospects including the so called “exercise pill” have suggested potential methods to improve muscle performance in later life.
Disclosure
The authors report no conflicts of interest in this work.
References
- SayerAASyddallHMartinHPatelHBaylisDCooperCThe developmental origins of sarcopeniaJ Nutr Health Aging20081242743218615224
- CesariMLeeuwenburghCLauretaniFFrailty syndrome and skeletal muscle: results from the Invecchiare in Chianti studyAm J Clin Nutr2006831142114816685058
- RockwoodKSongXMacKnightCA global clinical measure of fitness and frailty in elderly peopleGerontologist200545386
- SzulcPMunozFMarchandFChapurlatRDelmasPDRapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: the prospective MINOS studyAm J Clin Nutr2010911227123620237137
- BaumgartnerRNEpidemiology of sarcopenia among the elderly in New MexicoAm J Epidemiol1998147755 Erratum in. Am J Epidemiol. 1999; 149:1161.9554417
- JanssenIShepardDKatzmarzykPTRoubenoffRThe health care costs of sarcopenia in the United StatesJ Am Geriatr Soc200452808514687319
- AkimaHKanoWEnomotoYMuscle function in 164 men and women aged 20–84 yrMed Sci Sports Exerc20013322022611224809
- ErimZBegMFBurkeDTde LucaCJEffects of aging on motor-unit control propertiesJ Neurophysiol1999822081209110561389
- ThornellLELindstromMRenaultVMoulyVButler-BrowneGSSatellite cells and training in the elderlyScand J Med Sci Sports200313485512535317
- BrinkMWellenJDelafontainePAngiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanismJ Clin Invest199697250925168647943
- SchaapLAPluijmSMFDeegDJHVisserMInflammatory markers and loss of muscle mass (sarcopenia) and strengthAm J Med2006119e9e1716750969
- ChenZWangZOutwaterEIs DXA a useful tool for assessing skeletal muscle mass in older women?J Bone Miner Res200520S161
- KimJWangZMHeymsfieldSBBaumgartnerRNGallagherDTotal-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry methodAm J Clin Nutr20027637838312145010
- GuoSRocheAFHoutkooperLFat-free mass in children and young-adults predicted from bioelectric impedance and anthropometric variablesAm J Clin Nutr1989504354432773822
- JanssenIHeymsfieldSBBaumgartnerRNRossREstimation of skeletal muscle mass by bioelectrical impedance analysisJ Appl Physiol20008946547110926627
- JanssenIHeymsfieldSBRossRLow relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disabilityJ Am Geriatr Soc20025088989612028177
- MuscaritoliMConsensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and nutrition in geriatricsClin Nutr20102915415920060626
- VisserMHarrisTBLangloisJBody fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart StudyJ Gerontol A Biol Sci Med Sci199853M214M2219597054
- MartinHJYuleVSyddallHEDennisonEMCooperCSayerAAIs hand-held dynamometry useful for the measurement of quadriceps strength in older people? A comparison with the gold standard biodex dynamometryGerontology20065215415916645295
- GuralnikJMFerrucciLSimonsickEMSaliveMEWallaceRBLower-extremity function in persons over the age of 70 years as a predictor of subsequent disabilityN Engl J Med19953325565617838189
- VisserMGoodpasterBHKritchevskySBMuscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older personsJ Gerontol A Biol Sci Med Sci20056032433315860469
- BaumgartnerRNBody composition in healthy agingAnn N Y Acad Sci20009044374810865787
- Chodzko-zajkoWJProctorDNFiatarone SinghMAMinsonCTSalemGJSkinnerJSAmerican College of Sports Medicine position stand. Exercise and physical activity for older adultsMed Sci Sports Exer20094115101530
- LeeJSWAuyeungTWKwokTLauEMCLeungPCWooJAssociated factors and health impact of sarcopenia in older Chinese men and women: a cross-sectional studyGerontology20075340441017700027
- RollandYCzerwinskiSvan KanGASarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectivesJ Nutr Health Aging20081243345018615225
- FrankelJEBeanJFFronteraWRExercise in the elderly: research and clinical practiceClin Geriatr Med200622256
- CogganARSpinaRJKingDSSkeletal-muscle adaptations to endurance training in 60 year-old to 70 year-old men and womenJ Appl Physiol199272178017861601786
- ShortKRVittoneJBigelowMLProctorDNNairKSAge and aerobic exercise training effects on whole body and muscle protein metabolismAm J Physiol Endocrinol Metabo2004286E92E101
- MisicMMRosengrenKSWoodsJAEvansEMMuscle quality, aerobic fitness and fat mass predict lower-extremity physical function in community-dwelling older adultsGerontology20075326026617446711
- SipilaSSuominenHEffects of strength and endurance training on thigh and leg muscle mass and composition in elderly womenJ Appl Physiol1995783343407713834
- HughesVARoubenoffRWoodMFronteraWREvansWJSinghMAFAnthropometric assessment of 10-y changes in body composition in the elderlyAm J Clin Nutr20048047548215277173
- TaaffeDRDuretCWheelerSMarcusROnce-weekly resistance exercise improves muscle strength and neuromuscular performance in older adultsJ Am Geriatr Soc1999471214
- FronteraWRMeredithCNOreillyKPKnuttgenHGEvansWJStrength conditioning in older men – skeletal-muscle hypertrophy and improved functionJ Appl Physiol198864103810443366726
- BrownABMcCartneyNSaleDGPositive adaptations to weight-lifting training in the elderlyJ Appl Physiol199069172517332272965
- YarasheskiKEAcute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and womenAm J Physiol1993265E210E2148368290
- FiataroneMAO’NeillEFRyanNDExercise training and nutritional supplementation for physical frailty in very elderly peopleN Engl J Med1994330176917758190152
- RothSMMartelGFIveyFMSkeletal muscle satellite cell characteristics in young and older men and women after heavy resistance strength trainingJ Gerontol A Biol Sci Med Sci200156B240B24711382785
- LiuCJLathamNKProgressive resistance strength training for improving physical function in older adultsCochrane Database Syst Rev2003CD00275912804434
- VincentKRBraithRWFeldmanRAResistance exercise and physical performance in adults aged 60 to 83J Am Geriatr Soc2002501100110712110072
- GillespieLDRobertsonMCGillespieWJInterventions for preventing falls in older people living in the communityCochrane Database Syst Rev20093CD00714619370674
- BartaliBFrongilloEABandinelliSLauretaniFSembaRDFriedLPLow nutrient intake is an essential component of frailty in older personsJ Gerontol A Biol Sci Med Sci20066158959316799141
- HoustonDKNicklasBJDingJZHarrisTBTylavskyFANewmanABDietary intake is associated with lean mass change in older community-dwelling adults: the health aging and body composition (The Health ABC Study) studyAm J Clin Nutr20088715015518175749
- CastanedaCCharnleyJMEvansWJCrimMCElderly women accommodate to a low-protein diet with losses of body cell mass, muscle function and immune-responseAm J Clin Nutr19956230397598064
- CampbellWWTrappeTAWolfeRREvansWJThe recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscleJ Gerontol A Biol Sci Med Sci200156M373M38011382798
- BonnefoyMCornuCNormandSThe effects of exercise and protein-energy supplements on body composition and muscle function in frail elderly individuals: a long-term controlled randomised studyBr J Nutr20038973173812720593
- PriceRDalyFPenningtonCRMcmurdoMETNutritional supplementation of very old people at hospital discharge increases muscle strength: a randomized controlled trialGerontology200551185
- MilneACPotterJVivantiAAvenellAProtein and energy supplementation in elderly people at risk from malnutritionCochrane Database Syst Rev20092CD00328819370584
- MeridithCNFronteraWRO’ReillyKPEvansWJBody-composition in elderly men – effect of dietary modification during strength trainingJ Am Geriatr Soc199240162
- BrooksRVAndrogensClin Endocrinol Metab1975450352058744
- GriggsRCKingstonWJozefowiczRFHerrBEForbesGHallidayDEffect of testosterone on muscle mass and muscle protein synthesisJ Appl Physiol198966489503
- Sinha-HikimITaylorWEGonzalez-CadavidNFZhengWBhasinSAndrogen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatmentEndocrinol Metab2004895255
- HarmanSMMetterEJTobinJDPearsonJBlackmanMRLongitudinal effects of aging on serum total and free testosterone levels in healthy menJ Clin Endocrinol Metab20018672473111158037
- MorleyJEKaiserFEPerryHMLongitudinal changes in testosterone, luteinizing hormone and follicle stimulating hormone in healthy older menMetab Clin Exp1997464104139109845
- MellstromDJohnellOLjunggrenOFree testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS SwedenJ Bone Miner Res20062152953516598372
- Srinivas-ShankarURobertsSAConnollyMJEffects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled studyJ Clin Endocrinol Metab20109563965020061435
- GruenewaldDAMatsumotoAMTestosterone supplementation therapy for older men: potential benefits and risksJ Am Geriatr Soc20035110111512534854
- UrbanRJBodenburgYHGilkisonCTestosterone administration to elderly men increases skeletal muscle strength and protein synthesisAm J Physiol1995269E820E8267491931
- Emmelot-VonkMHVerhaarHJJPourHRNEffect of testosterone supplementation on functional mobility, cognition, and other parameters in older men – a randomized controlled trialJAMA2008299395218167405
- SnyderPJPeacheyHBerlinJAEffects of testosterone replacement in hypogonadal menJ Clin Endocrinol Metab2000852670267710946864
- JemalAThomasAMurrayTThunMCancer statisticsCA Cancer J Clin200252234711814064
- PierorazioPMFerrucciLKettermannAEMetterEJCarterHBSerum testosterone is associated with aggressive prostate cancer: results from the Baltimore longitudinal study of agingJ Urol2008179150
- SchneiderBKPickettCKZwillichCWInfluence of testosterone on breathing during sleepJ Appl Physiol1986616186233745052
- RhodenELMorgentalerAMedical progress – risks of testosterone-replacement therapy and recommendations for monitoringN Engl J Med200435048249214749457
- GreevesJPCableNTReillyTKingslandCChanges in muscle strength in women following the menopause: a longitudinal assessment of the efficacy of hormone replacement therapyClin Sci199997798410369797
- DionneISarcopenia and muscle function during menopause and hormone replacement therapyJ Nutr Aging Health20004156161
- TaaffeDREstrogen replacement, muscle composition and physical function: the health ABC studyMed Sci Sports Exerc200537174177
- SipilaSEffects of hormone replacement therapy and high impact physical exercise on skeletal muscle in post-menopausal women; a double randomized placebo controlled studyClin Sci (London)200110114715111473488
- ChlebowskiRTHendrixSLLangerRDInfluence of estrogen plus progestin on breast, cancer and mammography in healthy post-menopausal women – the women’s health initiative randomized trialJAMA20032893243325312824205
- ChakravarthyMVDavisBSBoothFWIGF-I restores satellite cell proliferative potential in immobilized old skeletal muscleJ Appl Physiol2000891365137911007571
- GoyaRGBrownOABolognaniFThe thymus-pituitary axis and its changes during agingNeuroimmunomodulation199961371429876244
- JorgensenJOLThuesenLMullerJOvesenPShakkebaekNEChristiansenJS3 years of growth-hormone treatment in growth-hormone deficient adults – near normalization of body composition and physical performanceEur J Endocrinol19941302242288156094
- PapadakisMAGradyDBlackDGrowth hormone replacement in healthy older men improves body composition but not functional abilityAnn Intern Med19961247087168633830
- ThompsonJLButterfieldGEGylfadottirUKEffects of human growth hormone, insulin-like growth factor I and diet and exercise on body composition of obese postmenopausal womenJ Clin Endocrinol Metab199883147714849589642
- WelleSThorntonCStattMMcHenryBGrowth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years oldJ Clin Endocrinol Metab199681323932438784075
- VittoneJBlackmanMRBusbyWhiteheadJEffects of single nightly injections of growth hormone-releasing hormone (GHRH 1–29 in healthy elderly menMetab Clin Exp19974689969005976
- TaaffeDRPruittLReimJEffect of recombinant human growth-hormone on the muscle strength response to resistance exercise in elderly menJ Clin Endocrinol Metab199479136113667525633
- LangeKHWAndersenJLBeyerNGH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly menJ Clin Endocrinol Metab20028751352311836279
- YarasheskiKEZachwiejaJJGrowth-hormone therapy for the elderly – the fountain of youth proves toxicJAMA199327016948411498
- MacLaughlinJHAgeing decreases the capacity of human skin to produce vitamin D3J Clin Invest198576153615382997282
- BischoffHABorchersMGudatFIn situ detection of 1,25-dihydroxyvitamin D-3 receptor in human skeletal muscle tissueHistochem J200133192411352397
- ZiambarasKDagogoJackSReversible muscle weakness in patients with vitamin D deficiencyWest J Med19971674354399426489
- VisserMDeegDJHLipsPLow vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (Sarcopenia): the longitudinal aging study AmsterdamJ Clin Endocrinol Metab2003885766577214671166
- SchottGDWillsMRMuscle weakness in osteomalaciaLancet1976162662955903
- MoweMHaugEBohmerTLow serum calcidiol concentration in older adults with reduced muscular functionJ Am Geriatr Soc1999472202269988294
- Moreira-PfrimerLDFPedrosaMACTeixeiraLLazaretti-CastroMTreatment of vitamin D deficiency increases lower limb muscle strength in institutionalized older people independently of regular physical activity: a randomized double-blind controlled trialAnn Nutr Metab20095429130019729890
- BischoffHAStahelinHBDickWEffects of vitamin D and calcium supplementation on falls: a randomized controlled trialJ Bone Miner Res20031834335112568412
- FlickerLMacInnisRJSteinMSShould older people in residential care receive vitamin D to prevent falls? Results of a randomized trialJ Am Geriatr Soc2005531881188816274368
- ChapuyMCArlotMEDuboeufFVitamin D3 and calcium to prevent hip fractures in the elderly womenN Engl J Med1992327163716421331788
- WithamMDCrightonLJGillespieNDStruthersADMcMurdoMEThe effects of vitamin D supplementation on physical function and quality of life in older heart failure patients: a randomised controlled trialCirc Heart Fail2010319520120103775
- AnnweilerCBeauchetOBerrutGIs there an association between serum 25-hydroxyvitamin D concentration and muscle strength among older women? Results from baseline assessment of the EPIDOS studyJ Nutr Health Aging200913909519214335
- BrunnerRLCochraneBJacksonRDCalcium, vitamin D supplementation, and physical function in the women’s health initiativeJ Am Diet Assoc20081081472147918755319
- Bischoff-FerrariHADawson-HughesBStaehelinHBFall prevention with a supplemental and active forms of vitamin D: a meta analysis of randomised controlled trialsBMJ2009339b369219797342
- NapiorkowskaLBudlewskiTJakubas-KwiatkowskaWHamzyVGozdowskiDFranekEPrevalence of low serum vitamin D concentration in an urban population of elderly women in PolandPol Arch Intern Med2009119699703
- HiraniVTullKAliAMindellJUrgent action needed to improve vitamin D status among older people in EnglandAge Ageing201039626819934073
- Bischoff-FerrariHACanUStaehelinHBSevere vitamin D deficiency in Swiss hip fracture patientsBone20084259760218180211
- VanderwielenRPJLowikMRHVandenbergHSerum vitamin-D concentrations among elderly people in EuropeLancet19953462072107616799
- Geneva World Health Organization and Food Agricultural OrganizationWorld Health Organization: Vitamin and Mineral Requirements in Human Nutritionss2nd ed2004
- Bischoff-FerrariHADietrichTOravEJDawson-HughesBPositive association between 25-hydroxy, vitamin D levels and bone mineral density: a population-based study of younger and older adultsAm J Med200411663463915093761
- YetleyEABruleDCheneyMCDietary reference intakes for vitamin D: justification for a review of the 1997 valuesAm J Clin Nutr2009897192719176741
- Barger-LuxMJHeaneyRPDowellSChenTCHolickMFVitamin D and its major metabolites: serum levels after graded oral dosing in healthy menOsteoporos Int199882222309797906
- CurhanGCWillettWCKnightELStampferMJDietary factors and the risk of incident kidney stones in younger women (Nurses health study II)J Am Soc Nephrol200314698A699A
- HenriksenEJJacobSModulation of metabolic control by angiotensin converting enzyme (ACE) inhibitionJ Cell Physiol200319617117912767053
- ShortKRBigelowMLKahlJDecline in skeletal muscle mitochondrial function with aging in humansProc Natl Acad Sci U S A20051025618562315800038
- FabreJERivardAMagnerMSilverMIsnerJMTissue inhibition of angiotensin-converting enzyme activity stimulates angiogenesis in vivoCirculation1999993043304910368123
- FerderLRomanoLAErcoleLBStellaIInserraFBiomolecular changes in the aging myocardium – the effect of enalaprilAm J Hypertens199811129713049832172
- de CavanaghEMVPiotrkowskiBBassoNEnalapril and losartan attenuate mitochondrial dysfunction in aged ratsFASEB J2003171096109812709417
- MaggioMCedaGPLauretaniFRelation of angiotensin-converting enzyme inhibitor treatment to insulin-like growth factor-1 serum levels in subjects >65 years of age (the InCHIANTI study)Am J Cardiol2006971525152916679098
- WilliamsAGRaysonMPJubbMPhysiology – the ACE gene and muscle performanceNature200040361410688186
- MyersonSHemingwayHBudgetRMartinJHumphriesSMontgomeryHHuman angiotensin I-converting enzyme gene and endurance performanceJ Appl Physiol1999871313131610517757
- OnderGPenninxBWJHBalkrishnanRRelation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational studyLancet200235992693011918911
- Di BariMPoll-FranseLVOnderGAntihypertensive medications and differences in muscle mass in older persons: the health, aging and body composition studyJ Am Geriatr Soc20045296196615161462
- DosseggerLAldorEBairdMGInfluence of angiotensin-converting enzyme-inhibition on exercise performance and clinical symptoms in chronic heart-failure – a multicenter, double-blind, placebo-controlled trialEur Heart J19931418238365423
- HutcheonSDGillespieNDCrombieIKStruthersADMcmurdoMETPerindopril improves six minute walking distance in older patients with left ventricular systolic dysfunction: a randomised double blind placebo controlled trialHeart20028837337712231595
- SchellenbaumGDSmithNLHeckbertSRWeight loss, muscle strength, and angiotensin-converting enzyme inhibitors in older adults with congestive heart failure or hypertensionJ Am Geriatr Soc2005531996200016274385
- SumukadasDWithamMDStruthersADMcmurdoMETEffect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trialCan Med Assoc J200717786787417923654
- LeonettiGMazzolaCPasottiCTreatment of hypertension in the elderly – effects on blood-pressure, heart-rate, and physical-fitnessAm J Med199190S12S13
- BunoutDBarreraGde la MazaMPLeivaLBackhouseCHirschSEffects of enalapril or nifedipine on muscle strength or functional capacity in elderly subjects. a double blind trialJ Renin Angiotensin Aldosterone Syst200910778419502254
- AhimastosAALawlerAReidCMBlomberyPAKingwellBABrief communication: Ramipril markedly improves walking ability in patients with peripheral arterial disease – a randomized trialAnn Intern Med200614466066416670135
- WilloughbyDSRoseneJMEffects of oral creatine and resistance training on myogenic regulatory factor expressionMed Sci Sports Exerc20033592392912783039
- SmithSAMontainSJMatottRPZientaraGPJoleszFAFieldingRACreatine supplementation and age influence muscle metabolism during exerciseJ Appl Physiol199885134913569760327
- Van LeemputteMVandenbergheKHespelPShortening of muscle relaxation time after creatine loadingJ Appl Physiol19998684084410066694
- RawsonESAcute creatine supplementation in older menInt J Sports Med200021717510683103
- RawsonESWehnertMLClarksonPMEffects of 30 days of creatine ingestion in older menEur J Appl Physiol Occup Physiol19998013914410408325
- GotshalkLAKraemerWJMendoncaMAGCreatine supplementation improves musclular performance in older womenEur J Appl Physiol Occup Physiol2008102223231
- BermonSVenembrePSachetCValourSDolisiCEffects of creatine monohydrate ingestion in sedentary and weight-trained older adultsActa Physiol Scand19981641471559805101
- CruschMJCreatine supplementation combined with resistance training in older menMed Sci Sports Exerc2001332111211711740307
- CandowDGChilibeckPDChadKEChruschMJDavisonKSBurkeDGEffect of ceasing creatine supplementation while maintaining resistance training in older menJ Aging Phys Act20041221923115263100
- KoshyKMInterstial nephritis in patient taking creatineN Engl J Med199934081481510075534
- McPherronACLawlerAMLeeSJRegulation of skeletal muscle mass in mice by a new TGF-beta superfamily memberNature199738783909139826
- WagnerKRLiuXSChangXLAllenREMuscle regeneration in the prolonged absence of myostatinProc Natl Acad Sci U S A20051022519252415699335
- ZimmerTAInduction of cachexia in mice by systemically administered myostatinScience20022961486148812029139
- SeibertMJXueQLFriedLPWalstanJDPolymorphic variation in human myostatin (GDF-8) gene and associations with strength measures in the Womens Health and Aging Study II cohortJ Am Geriatr Soc20014981093109611555072
- WagnerKRPhase II trial of MYO-29 in adult subjects with muscular dystrophyAnn Neurol20086356157118335515
- LeeSJRegulation of muscle growth by multiple ligands signalling through activin type II receptorsPNAS2005102181171812216330774
- MendiasCLBakhurinKIFaulknerJATendons of myostatin-deficient mice are small, brittle, and hypocellularProc Natl Acad Sci U S A200810538839318162552
- KjaerMJespersenJGThe battle to keep or lose skeletal muscle with ageingJ Physiol (London)20095871219119178
- GoodyearLJThe exercise pill – too good to be trueN Engl J Med20083591842184418946072