Abstract
Highly active antiretroviral therapy (HAART) has had a profound impact on improving the long-term prognosis for individuals infected with human immunodeficiency virus (HIV). HAART has been available for close to two decades, and now a significant number of patients with access to HAART are over the age of 50 years. Many clinical studies have indicated that HIV infection, as well as components of HAART, can increase the risk in these individuals to a variety of noninfectious complications, including a risk to bone health. There is a significant need for detailed mechanistic analysis of the aging, HIV-infected population regarding the risk of HIV infection and therapy in order to maintain bone health. Insights from basic mechanistic studies will help to shed light on the role of HIV infection and the components of HAART that impact bone health, and will help in identifying preventative countermeasures, particularly for individuals 50 years of age and older.
Introduction
There has been little research into how human immunodeficiency virus (HIV) infection and the aging process together influence the health and well being of infected individuals, which poses many challenges for health care providers, as well as for policy makers.Citation1 Given the advent of highly active antiretroviral therapy (HAART) about two decades ago, HIV-infected individuals have a significantly improved long-term survival, with many of those affected now aged 50 years and older. HIV infection and its treatment have increasingly led to concerns over comorbidities, such as cardiovascular disease, cancer, depression, dementia, and bone mineral density loss. Such concerns parallel the general concerns regarding the health and well being in the aging population. In the US, data from the Centers for Disease Control have indicated that individuals aged 50 years and older represent 29% of those living with acquired immunodeficiency syndrome (AIDS), 24% of those living with HIV/AIDS, and 15% of new HIV/AIDS diagnoses.Citation2 The rates among African-Americans and Hispanic-Americans are 12 times and five times greater, respectively. It has been estimated that by 2015, 50% of individuals living with HIV/AIDS will be 50 years of age or older.Citation3 These epidemiologic data indicate that the HIV epidemic in the US and other countries with access to HAART will cause a change in the nature of HIV clinics, changing the focus from health concerns in younger patients to a broader age range, with an increasing focus on geriatric issues, including issues relating to bone health.
Basic bone biology and pathology
Bone is a dynamic tissue in the body that is formed and maintained by two cell types, ie, osteoblasts (which form bone) and osteoclasts (which resorb bone). There is an extensive cell-signaling network between osteoblasts and osteoclasts required for maintaining the balance of activities of these two cell types that is crucial for bone remodeling and bone health. Direct signals between bone cells have recently been implicated as being important for regulating bone remodeling.Citation4–Citation6 For instance, osteoclasts can initiate bone formation via signaling to osteoblasts, independent of their ability to resorb bone.Citation7,Citation8 It has been demonstrated in patients with autosomal dominant osteopetrosis Type II that the number of osteoclasts, but not their activity, controls bone formation.Citation9 In contrast, osteoblasts regulate osteoclast differentiation by expressing two factors that are necessary and sufficient for osteoclast formation, ie, M-CSFCitation10 and RANKL.Citation11
Measurement of bone health
Several different methods are used to measure bone density. These include dual-energy X-ray absorptiometry, quantitative computed tomography, and quantitative ultrasound. While dual-energy X-ray absorptiometry is typically the most common method used and is considered the gold standard for measuring bone density, quantitative computed tomography and quantitative ultrasound can provide other useful information. Quantitative computed tomography provides measurements of cortical and trabecular volumetric bone mineral density, and studies have found using quantitative computed tomography that individual subregions of trabecular and cortical compartments are independent predictors of hip fracture and have differential responses to osteoporosis treatments.Citation12,Citation13 Quantitative ultrasound can provide further information on bone fragility and fracture risk, particularly in postmenopausal women.Citation14–Citation16 Both the spine and femur are common sites of analysis, and both regions contain cortical bone (dense outer shell) as well as trabecular bone (interconnecting sponge-like bony sheets), although the spine has relatively more cancellous bone, which can undergo the highest rate of bone turnover. Osteopenia and osteoporosis are diagnosed by comparing bone mineral density of an individual with expected normal values. The T score represents the number of standard deviation differences between an individual’s bone mineral density versus that of the mean of the population at peak bone mass. The T score creates a foundation for comparison of individuals as having osteopenia or osteoporosis, and can be of practical utility in predicting subsequent risk of fracture in men and women 50 years of age and older.
A recent report has described the use of the World Health Organization’s FRAX equation as a first-line screening of bone metabolism alteration in the HIV-infected population.Citation17,Citation18 FRAX is a computer-based algorithm that calculates the 10-year probability of fractures in men and women on the basis of classic risk factors alone or by integration with bone mineral density, which is measured by dual-energy X-ray absorptiometry. The FRAX algorithm has been proposed as a screening tool for HIV-positive individuals to identify those who have an increased clinical risk of fractures and for where bone mineral density measurements are strongly recommended. In a study of a mostly male population, reduced bone mineral density was significantly associated with particular HAART regimens. FRAX analysis of the entire population indicated a 1.2% increased risk of hip fracture and a 5.4% increased risk for major osteoporotic fracture.Citation17 Further FRAX analysis indicated that there were 22 of 139 patients in the study (15.8%) who had a 17.5% increased risk of major osteoporotic fracture and three patients (2.2%) who had a 120% increased risk of major fracture.
Perturbation in the function of either osteoblasts or osteoclasts can result in bone density loss, presenting clinically as osteopenia or osteoporosis. Osteoporosis occurs during aging and is commonly associated with women following menopause (on average at age 51 years), where it is called postmenopausal osteoporosis, but it can also occur in men or anyone with certain hormonal disorders, other chronic diseases, or as a result of medications such as glucocorticoids.Citation19–Citation25
HIV infection as a risk factor for bone health
There is clearly a large body of literature implicating a number of general factors associated with low bone mineral density in the general population, in particular those aged 50 years and older.Citation26–Citation29 One in two women and one in five men over the age of 50 years are expected to suffer a bone fracture due to osteoporosis during their remaining lifetime.Citation30 A current question that requires much more intensive investigation is whether HIV infection is a risk factor for low mineral density and the mechanisms involved. For HIV-infected individuals, HIV infection has been implicated as a risk factor for alteration of bone mineral density.Citation31–Citation34 Clearly, there are general effects of HIV infection that can be risk factors for low bone mineral density. These include a low body mass index, physical inactivity, malabsorption, and hypogonadism (). The HIV-infected population has been reported as having high rates of vitamin D deficiency, smoking, and drug and alcohol abuse, which are general risk factors for osteoporosis ().Citation35 Immune cells may also influence bone mineral density.Citation36 The loss of both immunocompetence and bone mineral density are common during aging. Importantly, both are associated with HIV infection and AIDS pathogenesis, and in some respects can resemble accelerated aging.Citation37 It is plausible that HIV infection could therefore accelerate the aging process.Citation38
Table 1 General risk factors associated with human immunodeficiency virus infection to bone mineral density loss
Mechanistic details regarding how HIV infection may reduce bone mineral density are quite limited, with minimal follow-up and demonstration of clearly identified mechanisms. However, the limited literature would support the general model that HIV infection could impact the immune system, which would then influence the skeletal system.
During aging, the reduction in T cell renewal along with the progressive enrichment of terminally differentiated T cells results in a general decline of the immune system, leading to immunosenescence. Inflammation is a clear hallmark of age-associated comorbidities, and immune activation is a well established hallmark of HIV infection.Citation39 Constant stimulation of the immune system by HIV can activate the innate and adaptive immune system. This activation results in the release of the mediators of inflammation, eg, cytokines. HIV-mediated immune activation, along with lack of anti-inflammatory responses, is a likely driver of accelerated aging as a result of HIV infection, even during HAART-suppressed HIV replication. Age-associated defects have been observed in the activation of cells in the innate immune system.Citation40 Aging is characterized by a constitutive proinflammatory environment in which persistent low-grade innate immune activation could enhance cellular, tissue, and organ damage by HIV infection, including that of the skeletal system.
Osteoimmunology is an interdisciplinary research area that studies the interface and cross-talk between the skeletal and immune systems.Citation41,Citation42 In particular, osteoimmunology focuses on the shared components and mechanisms between the two systems, which include ligands, receptors, signaling molecules, and transcription factors. Bone marrow is important for the proper development of the immune system, and has important stem cells that maintain the immune system. Cytokines produced by immune cells (eg, RANKL, macrophage colony-stimulating factor, tumor necrosis factor alpha [TNF-α], interleukins, and interferons) can also have important effects on regulating bone homeostasis. The balance between bone modeling and remodeling can be perturbed during chronic inflammation, which can lead to bone metabolic disorders as well as bone pain.
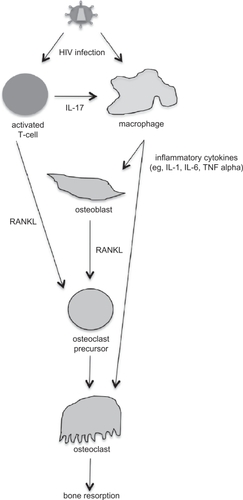
Some of the available literature shows a preliminary connection between HIV infection and osteoimmunology. For example, it has been suggested that there is an association between chronic inflammatory conditions and osteoporosis, and that RANKL is produced by activated T cells, although no studies have provided mechanistic details to support this suggestion.Citation43 During the asymptomatic phase of HIV infection, levels of inflammatory cytokines, such as interleukin-1, interleukin-6, and TNF-α are increased, and these cytokines can also stimulate bone resorption.Citation43 RANKL levels have been found to be higher in HIV-infected men and correlated with lower bone mineral density,Citation44 although another study found that bone mineral density was not associated with soluble TNF receptor 2 levels.Citation45 Well-controlled studies have not been performed to date to demonstrate a connection between altered expression of cytokines due to HIV infection and changes in bone mineral density. Such studies, particularly using well-defined animal models, would be important studies to perform in order to provide clear evidence in support of a cytokine-HIV infection-bone mineral density connection. In , a general model is proposed for how HIV infection is connected to bone mineral density loss via cell signaling. Future experiments are needed to test and refine this model.
Figure 1 Proposed model for how human immunodeficiency virus (HIV) infection is associated with bone mineral density loss. HIV can infect both activated T cells and macrophages. HIV-infected T cells express interleukin (IL)-17, which stimulates HIV-infected macrophages to produce inflammatory cytokines, including IL-1, IL-6, and tumor necrosis factor (TNF). The inflammatory cytokines can stimulate both osteoblasts and osteoclasts. HIV-infected activated T cells and osteoblasts produce RANKL, which further stimulates osteoclastogenesis. Overproduction of osteoclast activity results in an imbalance in bone remodeling and increases bone resorption.
A few other reports provide connections, the underlying mechanisms of which remain unclear and a model to associate viral infection with bone mineral density loss is not obvious. First, an older report suggested reduced bone formation and turnover in iliac crest biopsies,Citation46 but no follow-up on these studies has confirmed these observations. Further studies suggest other possible interactions between HIV infection, TNF-α, and bone mineral density. In one report, TNF-α has been reported to mediate apoptosis of human osteoblasts in response to HIV gp120.Citation47 If confirmed, this observation could be an important connection between HIV infection and osteoblasts. Another observation found that vitamin D deficiency is common among HIV-infected individuals, and a suggestion is that inhibition of 1-alpha-hydroxylase by TNF-α may have contributed to this.Citation48
In summary, imbalances in growth factors and cytokines could contribute to bone mineral density loss by increasing bone resorption, as indicated in the model proposed in the . However, it is clear that most of these data are suggestive and correlative, and there remains a strong need for very careful examination of potential mechanisms that can directly link HIV infection to bone mineral density loss.Citation49 The study of osteoimmunology in the context of HIV infection provides fertile ground for enhancing our understanding of the fundamental mechanisms that may connect HIV infection with bone metabolism.
HAART as a risk to bone health
Several of the drug classes that comprise HAART have been implicated as risk factors for low bone mineral density (). Several of the HIV protease inhibitors have been shown to alter bone mineral density.Citation50 Recent gene expression profiling has indicated that exposure of osteoblastic cells to nelfinavir and ritonavir increases gene expression of the inflammatory cytokines, MCP-1, and interleukin-8.Citation51 Protease inhibitors have also been suggested to inhibit 1-alpha-hydroxylase, as well as to reduce 1,25(OH)2D levels, which could reduce bone mineral density.Citation45 LRP5, a positive regulator of bone formation, is inhibited by protease inhibitors.Citation51,Citation52
Table 2 Drug classes in highly active antiretroviral therapy that have been implicated as risk factors for bone mineral density
Reverse transcriptase inhibitors are another class of anti-HIV drugs that have been implicated in bone metabolism. For example, azidothymidine, a nucleoside reverse transcriptase inhibitor, has been suggested to stimulate osteoclastogenesis in vitro and reduce bone mineral density in mice.Citation53 Some nucleoside reverse transcriptase inhibitors have been implicated in causing mitochondrial damage and dysfunction due to their cross-inhibition of mitochondrial DNA polymerase.Citation51,Citation53 This could be responsible for the raised lactate levels observed in some HIV-infected individuals who are on HAART, which has been associated with increased bone resorption.Citation54 Efavirenz, a non-nucleoside reverse transcriptase inhibitor, has been reported to reduce vitamin D levels by inducing hepatic enzymes.Citation55
Some of the strongest data in support of the components of HAART being associated with bone mineral density reductions have been observed with tenofovir, a nucleotide reverse transcriptase inhibitor, and been reported in human adults.Citation56 Increased bone resorption could cause a compensatory increase in osteoblast activity, which would be revealed by increased serum alkaline phosphatase levels.Citation57 Tenofovir treatment decreased bone mineral density, as well as increased urinary calcium excretion. Previous studies in macaques have demonstrated adverse effects on bone mineral density from the administration of tenofovir.Citation58–Citation60 Simian immunodeficiency virus infection of macaques was also implicated in decreasing bone mineral density. Histomorphometric analysis in one of these studies revealed an increase in tibial osteoid seam width, which can result in bone softening and can develop into osteomalacia.Citation58 The increase in osteoid seam width is likely to be associated with reduced activity in osteoblasts.
The gene expression profile of primary osteoclasts and osteoblasts has been evaluated ex vivo following tenofovir exposure.Citation61,Citation62 Specific downregulation of Gnas, Got2, and Snord32a was observed in osteoclasts. The downregulation of Gnas gene expression may result in less mitogen-activated protein kinase/extracellular signal-regulated kinase signaling and ultimately a reduction in osteoclast proliferation and actin filament formation, resulting in decreased bone resorption. Got2 is a mitochondrial enzyme involved in energy transduction, specifically amino acid metabolism as well as the urea and tricarboxylic acid cycles. Perturbation of amino acid metabolism following exposure to tenofovir in both osteoblasts and osteoclasts suggests alteration in bone homeostasis. Over 70 genes had their gene expression altered in primary osteoblasts following tenofovir exposure. The changes in gene expression profiles involved in cell signaling, cell cycle, and amino acid metabolism, would likely impact osteoblast function in bone formation.
The association of tenofovir with mitochondrial dysfunction has been investigated. In general, no mitochondrial dysfunction was observed with tenofovir.Citation63–Citation66 Other studies have reported a lowering of mitochondrial dysfunction when drug regimens were changed and nucleoside reverse transcriptase inhibitors were replaced with tenofovir.Citation67–Citation69 Potential mechanisms for tenofovir-associated bone loss include preferential uptake by osteoclasts (altering gene expression resulting in increased bone resorption), uptake by osteoblasts (altering gene expression decreasing bone formation), and uptake by both osteoclasts and osteoblasts (altering gene expression of both cells types and ultimately the balance between bone resorption and bone formation, resulting in bone loss).Citation70 The loss of bone density due to tenofovir exposure could also be associated with tenofovir-induced renal dysfunction, particularly renal proximal tubule dysfunction.Citation17,Citation57,Citation71–Citation79 The failure of renal proximal tubular cells to reabsorb filtered bicarbonate from urine would result in urinary bicarbonate wasting and subsequent acidemia and a more general dysfunction of the proximal tubular cell, a clinical condition called Fanconi syndrome. Commonly observed clinical features in Fanconi syndrome include aminoaciduria, glycosuria, tubular proteinuria, and uricosuria. Importantly, the main pathology observed in Fanconi syndrome is bone demineralization (osteomalacia or rickets) due to phosphate wasting. Therefore, tenofovir-associated bone density loss may be an outcome of renal dysfunction.
Treatment of bone mineral density loss from HIV infection and HAART
The treatment of low bone mineral density requires a complete investigation into its etiology. Secondary causes and complications, particularly relevant for individuals 50 years of age and older, including chronic liver disease, chronic malnutrition, hyperthyroidism, hypogonadism, and Type 1 diabetes, require identification and treatment where possible.Citation80–Citation82 Low vitamin D levels should be followed up by analysis of parathyroid hormone, with the goal of attempting to normalize vitamin D and parathyroid hormone levels. Diagnosis of osteoporosis, which would typically be established by a T score < 2.5 for those 50 years of age and older, would warrant treatment with bisphosphonates in conjunction with vitamin D, calcium supplementation, and strategies geared towards trying to minimize the risk of falls.
Bisphosphonates are synthetic analogs of inorganic pyrophosphate.Citation83–Citation85 They inhibit bone resorption by increasing the apoptosis of osteoclasts. To date, preliminary data suggest that the use of bisphosphonates is effective in increasing bone mineral density in the presence of HAART.Citation86–Citation88 The bisphosphonates, including alendronate (Fosamax®), ibandronate (Boniva®), risedronate (Actonel®), and zoledronate (Aclasta®, Zometa®) are all available as options for use in the treatment of bone mineral density loss.Citation83–Citation85 There are concerns regarding the use of bisphosphonates in the treatment of bone mineral density loss that occurs in HIV-infected individuals. One major concern is that the oral administration of bisphosphonates requires a very complex dosing regimen, ie, the bisphosphonate must be taken fasting, with the individual sitting or standing, with a large glass of water, and then the individual must remain upright for 30–60 minutes after taking the medication, without eating or drinking or taking other medications during that time. Another concern is the long-term administration of bisphosphonates in the context of HIV infection and HAART. To date, alendronate has been the most extensively used bisphosphonate in the treatment of bone mineral density loss in the context of HIV infection.Citation89,Citation90 However, there has not been enough statistical power in the relevant research to conclude that fracture rates are lowered. This should be a priority of future clinical studies.
Conclusion
The specific role(s) of HIV infection in bone mineral loss remains poorly characterized, and further studies are needed to identify the underlying mechanisms. The use of appropriate animal models would be highly desirable in this regard. The role of specific HAART regimens in increased risk of bone mineral density loss has been suggested but not fully analyzed, particularly in the context of many of the newly approved anti-HIV drugs available. Further studies are needed to investigate whether declines in bone mineral density are greater in individuals over 50 years of age receiving HAART compared with the non-HIV-infected population to aid further in identifying potential risk factors.Citation91 The rate of bone mineral density loss is an important parameter to determine in the identification of possible treatments using bisphosphonates. A relatively slow decline in bone mineral density loss could allow for bisphosphonates, such as zoledronate, to be used as an adjuvant therapy along with HAART. Given that the HIV-infected population is aging and many patients are now reaching the age of 50 years, there needs to be serious consideration of the impact of HIV infection and HAART on the health of those affected, particularly in the area of bone health.
Acknowledgements
The author’s research is supported by National Institutes of Health grants AR53946 and AR056642.
Disclosure
The author reports no conflict of interest in this work.
References
- HenryKInternal medicine/primary care reminder: What are the standards of care for HIV-positive patients aged 50 years and older?Curr HIV/AIDS Rep20096315316119589301
- Centers for Disease Control and PreventionHIV+ persons aged 50 and over Available at: http://www.cdc.gov/hiv/topics/over50/index.htm. Centers for Disease Control and Prevention. Accessed Aug 18, 2010.
- Graying plague: By 2015 over half of HIV in US will be in those over 50. Late diagnoses contribute to problemAIDS Alert2010253252820629255
- KarsdalMAMartinTJBollerslevJChristiansenCHenriksenKAre nonresorbing osteoclasts sources of bone anabolic activity?J Bone Miner Res200722448749417227224
- KarsdalMAHenriksenKSorensenMGAcidification of the osteoclastic resorption compartment provides insight into the coupling of bone formation to bone resorptionAm J Pathol2005166246747615681830
- MartinTJSimsNAOsteoclast-derived activity in the coupling of bone formation to resorptionTrends Mol Med2005112768115694870
- BollerslevJMarksSCJrPockwinseSUltrastructural investigations of bone resorptive cells in two types of autosomal dominant osteopetrosisBone19931468658698155410
- BollerslevJSteinicheTMelsenFMosekildeLStructural and histomorphometric studies of iliac crest trabecular and cortical bone in autosomal dominant osteopetrosis: A study of two radiological typesBone198910119242660883
- AlataloSLIvaskaKKWaguespackSGEconsMJVaananenHKHalleenJMOsteoclast-derived serum tartrate-resistant acid phosphatase 5b in Albers-Schonberg disease (type II autosomal dominant osteopetrosis)Clin Chem200450588389015016726
- CecchiniMGHofstetterWHalasyJWetterwaldAFelixRRole of CSF-1 in bone and bone marrow developmentMol Reprod Dev19974617583 discussion 83–74.8981367
- WadaTNakashimaTHiroshiNPenningerJMRANKL-RANK signaling in osteoclastogenesis and bone diseaseTrends Mol Med2006121172516356770
- BlackDMGreenspanSLEnsrudKEThe effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosisN Engl J Med2003349131207121514500804
- BlackDMBouxseinMLMarshallLMProximal femoral structure and the prediction of hip fracture in men: A large prospective study using QCTJ Bone Miner Res20082381326133318348697
- GluerCCQuantitative ultrasound techniques for the assessment of osteoporosis: Expert agreement on current status. The International Quantitative Ultrasound Consensus GroupJ Bone Miner Res1997128128012889258759
- GuglielmiGScalzoGde TerlizziFPehWCQuantitative ultrasound in osteoporosis and bone metabolism pathologiesRadiol Clin North Am201048357758820609893
- LeeHDHwangHFLinMRUse of quantitative ultrasound for identifying low bone density in older peopleJ Ultrasound Med20102971083109220587432
- CalmyAFuxCANorrisRLow bone mineral density, renal dysfunction, and fracture risk in HIV infection: A cross-sectional studyJ Infect Dis2009200111746175419874178
- GazzolaLComiLSavoldiAUse of the FRAX equation as first-line screening of bone metabolism alteration in the HIV-infected populationJ Infect Dis20102022331 author reply 331–332.
- BonnelyeEMerdadLKungVAubinJEThe orphan nuclear estrogen receptor-related receptor alpha (ERRalpha) is expressed throughout osteoblast differentiation and regulates bone formation in vitroJ Cell Biol2001153597198411381083
- LiuRHWerthVPWhat is new in the treatment of steroid-induced osteoporosis?Semin Cutan Med Surg200726420320918395668
- ShakerJLLukertBPOsteoporosis associated with excess glucocorticoidsEndocrinol Metab Clin North Am2005342341356viiiix15850846
- CaplanLSaagKGGlucocorticoids and the risk of osteoporosisExpert Opin Drug Saf200981334719236216
- EbelingPROsteoporosis in men. New insights into aetiology, pathogenesis, prevention and managementDrugs Aging19981364214349883398
- McIlwainHHGlucocorticoid-induced osteoporosis: Pathogenesis, diagnosis, and managementPrev Med200336224324912591000
- PietschmannPRaunerMSiposWKerschan-SchindlKOsteoporosis: An age-related and gender-specif ic disease – a mini-reviewGerontology200855131218948685
- CompstonJOsteoporosis: Social and economic impactRadiol Clin North Am201048347748220609886
- LookerACMeltonLJ3rdHarrisTBBorrudLGShepherdJAPrevalence and trends in low femur bone density among older US adults: NHANES 2005–2006 compared with NHANES IIIJ Bone Miner Res 210;251647119580459
- GoodfellowLREarlSCooperCHarveyNCMaternal diet, behaviour and offspring skeletal healthInt J Environ Res Public Health2010741760177220617058
- WinsloeCEarlSDennisonEMCooperCHarveyNCEarly life factors in the pathogenesis of osteoporosisCurr Osteoporos Rep20097414014419968918
- WHOAssessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study GroupWorld Health Organ Tech Rep Ser199484311297941614
- ArpadiSMHorlickMThorntonJCuffPAWangJKotlerDPBone mineral content is lower in prepubertal HIV-infected childrenJ Acquir Immune Defic Syndr200229545045411981360
- TebasPPowderlyWGClaxtonSAccelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapyAIDS2000144F63F6710770534
- ViganoAMoraSAdverse effects of antiretroviral therapy: Focus on bone densityExpert Opin Drug Saf20043319920815155148
- MadedduGSpanuASolinasPBone mass loss and vitamin D metabolism impairment in HIV patients receiving highly active anti-retroviral therapyQ J Nucl Med Mol Imaging2004481394815195003
- AmorosaVTebasPBone disease and HIV infectionClin Infect Dis200642110811416323100
- LiYToraldoGLiAB cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivoBlood200710993839384817202317
- DeeksSGImmune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapyTop HIV Med200917411812319890183
- NguyenNHolodniyMHIV infection in the elderlyClin Interv Aging20083345347218982916
- DesaiSLandayAEarly immune senescence in HIV diseaseCurr HIV/AIDS Rep20107141020425052
- ShawACJoshiSGreenwoodHPandaALordJMAging of the innate immune systemCurr Opin Immunol7262010 [Epub ahead of print].
- RaggattLJPartridgeNCCellular and molecular mechanisms of bone remodellingJ Biol Chem2010 13;28533251032510820501658
- TakayanagiHOsteoimmunology and the effects of the immune system on boneNat Rev Rheumatol200951266767619884898
- RaiszLGPathogenesis of osteoporosis: Concepts, conflicts, and prospectsJ Clin Invest2005115123318332516322775
- GibelliniDBorderiMde CrignisERANKL/OPG/TRAIL plasma levels and bone mass loss evaluation in antiretroviral naive HIV-1-positive menJ Med Virol200779101446145417705184
- ChirchLMFeinerJGoROsteopenia in patients with HIV infection is not associated with elevated sTNFR2 levelsClin Infect Dis20064381084108516983627
- SerranoSMarinosoMLSorianoJCBone remodelling in human immunodeficiency virus-1-infected patients. A histomorphometric studyBone19951621851917756046
- GibelliniDde CrignisEPontiCHIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNFalpha activationJ Med Virol20088091507151418649336
- VillamorEA potential role for vitamin D on HIV infection?Nutr Rev2006645 Pt 122623316770943
- VikulinaTFanXYamaguchiMAlterations in the immunoskeletal interface drive bone destruction in HIV-1 transgenic ratsProc Natl Acad Sci U S A201010731138481385320643942
- BrownTTQaqishRBAntiretroviral therapy and the prevalence of osteopenia and osteoporosis: A meta-analytic reviewAIDS200620172165217417086056
- MaliziaAPVioreanuMHDoranPPPowderlyWGHIV1 protease inhibitors selectively induce inflammatory chemokine expression in primary human osteoblastsAntiviral Res2007741727617240460
- TranHRobinsonSMikhailenkoIStricklandDKModulation of the LDL receptor and LRP levels by HIV protease inhibitorsJ Lipid Res200344101859186912837856
- PanGYangZBallingerSWMcDonaldJMPathogenesis of osteopenia/osteoporosis induced by highly active anti-retroviral therapy for AIDSAnn N Y Acad Sci2006106829730816831930
- CarrAMillerJEismanJACooperDAOsteopenia in HIV-infected men: Association with asymptomatic lactic acidemia and lower weight pre-antiretroviral therapyAIDS200115670370911371684
- GyllenstenKJosephsonFLidmanKSaafMSevere vitamin D deficiency diagnosed after introduction of antiretroviral therapy including efavirenz in a patient living at latitude 59 degrees NAIDS200620141906190716954738
- GallantJEStaszewskiSPozniakALEfficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviralnaive patients: A 3-year randomized trialJAMA2004292219120115249568
- FuxCARauchASimcockMTenofovir use is associated with an increase in serum alkaline phosphatase in the Swiss HIV Cohort StudyAntivir Ther20081381077108219195333
- CastilloABTarantalAFWatnikMRMartinRBTenofovir treatment at 30 mg/kg/day can inhibit cortical bone mineralization in growing rhesus monkeys (Macaca mulatta)J Orthop Res20022061185118912472227
- TarantalAFMarthasMLShawJPCundyKBischofbergerNAdministration of 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) to gravid and infant rhesus macaques (Macaca mulatta): Safety and efficacy studiesJ Acquir Immune Defic Syndr Hum Retrovirol199920432333310096575
- van RompayKKBrignoloLLMeyerDJBiological effects of short-term or prolonged administration of 9-[2-(phosphonomethox) propyl]adenine (tenofovir) to newborn and infant rhesus macaquesAntimicrob Agents Chemother20044851469148715105094
- GrigsbyIFPhamLGopalakrishnanRManskyLMManskyKCDownregulation of Gnas, Got2 and Snord32a following tenofovir exposure of primary osteoclastsBiochem Biophys Res Commun201039131324132920026012
- GrigsbyIFPhamLManskyLMGopalakrishnanRCarlsonAEManskyKCTenofovir treatment of primary osteoblasts alters gene expression profiles: Implications for bone mineral density lossBiochem Biophys Res Commun20103941485320171173
- VidalFDomingoJCGuallarJIn vitro cytotoxicity and mitochondrial toxicity of tenofovir alone and in combination with other antiretrovirals in human renal proximal tubule cellsAntimicrob Agents Chemother200650113824383216940060
- CihlarTBirkusGGreenwaltDEHitchcockMJTenofovir exhibits low cytotoxicity in various human cell types: Comparison with other nucleoside reverse transcriptase inhibitorsAntiviral Res2002541374511888656
- BirkusGHitchcockMJCihlarTAssessment of mitochondrial toxicity in human cells treated with tenofovir: Comparison with other nucleoside reverse transcriptase inhibitorsAntimicrob Agents Chemother200246371672311850253
- BieseckerGKarimiSDesjardinsJEvaluation of mitochondrial DNA content and enzyme levels in tenofovir DF-treated rats, rhesus monkeys and woodchucksAntiviral Res200358321722512767469
- GerschensonMKimCBerzinsBMitochondrial function, morphology and metabolic parameters improve after switching from stavudine to a tenofovir-containing regimenJ Antimicrob Chemother20096361244125019321503
- RiberaEParadineiroJCCurranAImprovements in subcutaneous fat, lipid profile, and parameters of mitochondrial toxicity in patients with peripheral lipoatrophy when stavudine is switched to tenofovir (LIPOTEST study)HIV Clin Trials20089640741719203906
- MiroOGarrabouGLopezSShort communication metabolic and mitochondrial effects of switching antiretroviral-experienced patients to enfuvirtide, tenofovir and saquinavir/ritonavirAntivir Ther200611562563016964831
- GrigsbyIFPhamLManskyLMGopalakrishnanRManskyKCTenofovir-associated bone density lossTher Clin Risk Manag20106414720169035
- EarleKESeneviratneTShakerJShobackDFanconi’s syndrome in HIV+ adults: Report of three cases and literature reviewJ Bone Miner Res200419571472115068493
- ParsonageMJWilkinsEGSnowdenNIssaBGSavageMWThe development of hypophosphataemic osteomalacia with myopathy in two patients with HIV infection receiving tenofovir therapyHIV Med20056534134616156882
- WilliamsJChadwickDRTenofovir-induced renal tubular dysfunction presenting with hypocalcaemiaJ Infect2006524e107e10816125779
- NelsonMRKatlamaCMontanerJSThe safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: The first 4 yearsAIDS200721101273128117545703
- LabargaPBarreiroPMartin-CarboneroLKidney tubular abnormalities in the absence of impaired glomerular function in HIV patients treated with tenofovirAIDS200923668969619262355
- KohlerJJHosseiniSHHoying-BrandtATenofovir renal toxicity targets mitochondria of renal proximal tubulesLab Invest200989551351919274046
- WoodwardCLHallAMWilliamsIGTenofovir-associated renal and bone toxicityHIV Med200910848248719459988
- PerrotSAslangulESzwebelTCaillat-VigneronNLe JeunneCBone pain due to fractures revealing osteomalacia related to tenofovir-induced proximal renal tubular dysfunction in a human immunodeficiency virus-infected patientJ Clin Rheumatol2009152727419265350
- Di BiagioARossoRMontefortePRussoRRovettaGViscoliCWhole body bone scintigraphy in tenofovir-related osteomalacia: A case reportJ Med Case Reports20093813619830218
- McComseyGAHuangJSWoolleyIJFragility fractures in HIV-infected patients: Need for better understanding of diagnosis and managementJ Int Assoc Physicians AIDS Care (Chic Ill)200433869115573712
- McComseyGAKendallMATebasPAlendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIVAIDS200721182473248218025884
- WohlDAMcComseyGTebasPCurrent concepts in the diagnosis and management of metabolic complications of HIV infection and its therapyClin Infect Dis200643564565316886161
- DrakeMTClarkeBLKhoslaSBisphosphonates: Mechanism of action and role in clinical practiceMayo Clin Proc20088391032104518775204
- DrakeMTCremersSCBisphosphonate therapeutics in bone disease: The hard and soft data on osteoclast inhibitionMol Interv201010314115220539033
- RussellRGBisphosphonates: From bench to bedsideAnn N Y Acad Sci2006106836740116831938
- LinDRiederMJInterventions for the treatment of decreased bone mineral density associated with HIV infectionCochrane Database Syst Rev20072CD00564517443607
- BollandMJGreyABHorneAMAnnual zoledronate increases bone density in highly active antiretroviral therapy-treated human immunodeficiency virus-infected men: A randomized controlled trialJ Clin Endocrinol Metab20079241283128817227801
- HuangJMeixnerLFernandezSMcCutchanJAA double-blinded, randomized controlled trial of zoledronate therapy for HIV-associated osteopenia and osteoporosisAIDS2009231515719050386
- ClayPGVossLEWilliamsCDaumeECValid treatment options for osteoporosis and osteopenia in HIV-infected personsAnn Pharmacother200842567067918413693
- PaccouJVigetNLegrout-GerotIYazdanpanahYCortetBBone loss in patients with HIV infectionJoint Bone Spine200976663764119945322
- OnenNFOvertonETSeyfriedWAging and HIV infection: A comparison between older HIV-infected persons and the general populationHIV Clin Trials201011210010920542846