Abstract
Postmenopausal osteoporosis is common and underrecognized among elderly women. Osteoporotic fractures cause disability and disfigurement and threaten patients’ mobility, independence, and survival. Care for incident fractures in this age group must go beyond orthopedic repair, to assessment and treatment of the underlying bone fragility. Fracture risk can be reduced by vitamin D and calcium supplementation along with antiresorptive drug treatment. First-line osteoporosis pharmacotherapy employs nitrogen-containing bisphosphonates. The inconvenience of daily oral treatment has motivated development of weekly, monthly, and intermittent oral regimens, as well as quarterly and yearly intravenous (iv) regimens. Ibandronate is the first bisphosphonate to have shown direct anti-fracture efficacy with a non-daily regimen; it was approved for once-monthly oral dosing in 2005 and for quarterly iv dosing in 2006. Intermittent oral risedronate and yearly iv zoledronic acid were approved in 2007. Newly available regimens with extended dosing intervals reduce the inconvenience of bisphosphonate therapy and provide patients with a range of options from which to select a maximally sustainable course of treatment. This review discusses the development, efficacy, safety, and tolerability of extended-interval bisphosphonate regimens and examines their potential to improve patient acceptance and long-term success of osteoporosis treatment.
Background
Osteoporosis – a progressive loss of bone strength and quality that multiplies fracture risk – is a common and underrecognized hazard to postmenopausal women. Its prevalence among US women ranges from 26% at age 65 years and older to over 50% at age 85 years and older (CitationUS Department of Health and Human Services 2004), reflecting an altered balance of bone turnover that begins immediately upon menopause and increases steadily with age (CitationGarnero et al 1996b). Fortunately, osteoporosis is eminently preventable and treatable. Lifelong attention to nutrition and exercise, bone density monitoring after menopause, and appropriate treatment of diagnosed osteoporosis can reduce the burden of disability, cost, and mortality.
Bone densitometry by dual-energy X-ray absorptiometry (DXA), which measures bone mineral density (BMD), is the canonical method for diagnosing osteoporosis (CitationBriot and Roux 2005). The relationship between low BMD and fracture is stronger than the relationship between cholesterol and heart attacks (CitationUS Department of Health and Human Services 2004). Judicious BMD testing should be considered in at-risk postmenopausal women, in younger women with multiple risk factors, and in all patients who present with fragility fractures or take medications that can reduce BMD (CitationUS Department of Health and Human Services 2004). At a minimum, BMD screening of all men and women should be considered at 65 years of age.
The World Health Organization (WHO) defines osteoporosis as a BMD T-score of ≥2.5 standard deviations below the gender-specific young adult mean, as measured by DXA (CitationWorld Health Organization 1994). However, total fracture risk reflects both BMD-dependent and BMD-independent risk factors (CitationKanis et al 2007). The 2008 National Osteoporosis Foundation practice guidelines (CitationNational Osteoporosis Foundation 2008) () utilize FRAX™, the new WHO absolute fracture risk algorithm (CitationKanis 2008), which takes into account BMD, age, smoking, alcohol intake, personal or parental history of fracture, body mass index, corticosteroid use, and rheumatoid arthritis to predict individual patients’ 10-year probability of sustaining osteoporotic fractures.
Table 1 Diagnostic categories of BMD and appropriate clinical responses (CitationWorld Health Organization 1994; CitationHealy 1998; CitationHodgson et al 2001; CitationKanis 2008)
Osteoporotic fractures, with an estimated annual incidence of 2 million among US citizens aged 50 years or older (CitationBurge et al 2007), have severe physical, economic, and psychosocial consequences. Direct physical consequences include temporary or permanent disability and disfigurement. If fracture sufferers become bedfast, indirect consequences such as decubitus ulcers, pneumonia, and urinary tract infections may result (CitationUS Department of Health and Human Services 2004). Mortality risk increases 2.8- to 4-fold during the first 3 months after a hip fracture (CitationUS Department of Health and Human Services 2004). In the US, 500,000 hospitalizations, 800,000 emergency room visits, and 180,000 nursing home placements yearly are attributable to osteoporotic fractures (CitationUS Department of Health and Human Services 2004), requiring annual direct care expenditures estimated at US$16.9 billion and total costs exceeding US$19 billion (2005 dollars) (CitationBurge et al 2007). A 2007 case-control study reported incremental costs of US$4,007 per Medicaid beneficiary or US$5,370 per Medicare beneficiary during the first post-fracture year (CitationRousculp et al 2007).
A particularly distressing cost of osteoporosis to elderly patients and their families is potential loss of independence. Fracture sufferers often lose more mobility through fear than they lose to the direct effects of injury. This effect can synergize with the direct effects of fractures on mobility and morbidity to cause self-perpetuating inactivity, social isolation, and functional dependence, often culminating in institutionalization (CitationUS Department of Health and Human Services 2004).
Calcium and vitamin D supplementation are essential in the prevention and therapy of osteoporosis and have been used in all of the major trials of osteoporosis drugs (CitationSunyecz and Weisman 2005). The US Institute of Medicine’s estimated adequate intakes for adults aged ≥50 years are 1200 g calcium (with ≥700 mg preferably derived from diet) and 800–1000 units vitamin D (CitationStanding Committee on the Scientific Evaluation of Dietary Reference Intakes 1997). Calcium supplementation (1000 mg/day) prevents BMD loss and may reduce vertebral fractures in postmenopausal women, as shown in a meta-analysis by the Agency for Health Research and Quality (CitationMacLean et al 2008). Vitamin D (700–800 international units/day) with or without calcium reduced hip and non-vertebral fractures versus placebo in another meta-analysis (CitationBischoff-Ferrari et al 2005).
In addition to lifelong preventive nutrition and weight-bearing exercise, antiresorptive treatment with nitrogen-containing bisphosphonates has become standard first-line therapy for postmenopausal osteoporosis. Timely diagnosis and effective treatment of osteoporosis can reduce the incidence of fractures and their multifaceted individual and societal costs.
Objective
Bisphosphonates have become the standard first-line treatment for postmenopausal osteoporosis. This paper will trace the evolution of bisphosphonate therapies from daily to monthly or less frequent dosing regimens, outline evidence on efficacy, safety, and tolerability, and examine their potential to improve patient acceptance and clinical outcomes of osteoporosis treatment.
Bisphosphonate pharmacology and dosing frequencies
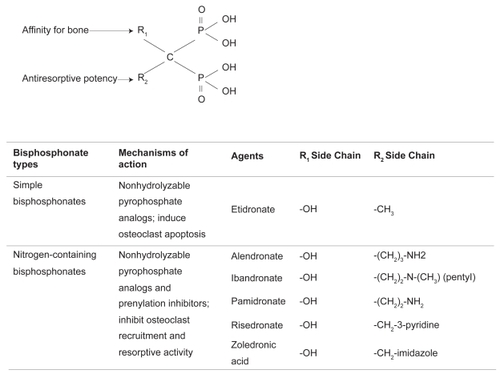
Two features of bisphosphonate structure determine biological activity (). The arrangement of 2 phosphate groups bound to a geminal carbon (rather than an oxygen as in pyrophosphate) creates a non-hydrolyzable pyrophosphate analog (CitationRogers et al 2000), and the R1 and R2 groups occupying the carbon’s other bonds mediate high-affinity calcium chelation. Thus, bisphosphonates are readily and tenaciously incorporated into bone mineral surfaces, where they are consumed by osteoclasts at remodeling sites.
Within osteoclasts, non-nitrogen-containing bisphosphonates are metabolized to apoptosis-inducing compounds. Nitrogen-containing bisphosphonates (the focus of this review) inhibit protein prenylation by farnesyl pyrophosphate synthase and thus disrupt the cellular physiology of resorption (CitationDunford et al 2001). Inhibitory potency, determined by the R2 group, is increased by tertiary nitrogen (as in ibandronate) or a heterocyclic ring (as in risedronate and zoledronic acid) (CitationDunford et al 2001). An upstream metabolite that accumulates during prenylation inhibition, isopentenyl pyrophosphate, is further metabolized to an apoptosis-inducing product (CitationMonkkonen et al 2007). Although osteoclast apoptosis is not essential for antiresorptive activity of nitrogen-containing bisphosphonates, it plays a contributory role (CitationRussell et al 2008).
Suppression of bone turnover by nitrogen-containing bisphosphonates manifests in decreased serum and urine levels of bone turnover markers (). The bone collagen breakdown products cross-linked C-telopeptide (CTX) or N-telopeptide (NTX) are often used in clinical follow-up. The rate and magnitude of their decreases in response to treatment depend on bisphosphonate potency, dose, route, and interval.
Table 2 Biomarkers of bone turnover (After CitationLeeming et al 2006)
Evolution of dosing regimens
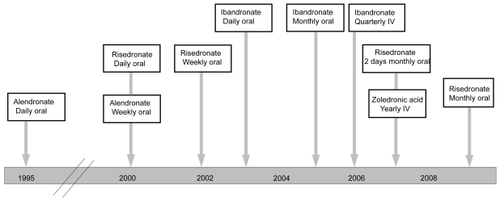
Nitrogen-containing bisphosphonates have become the standard of care for osteoporosis. US approval dates for currently available bisphosphonate regimens are shown in . Oral bisphosphonate dosing (daily, weekly, or monthly) is popular for first-line treatment in the outpatient setting. Intravenous (iv) regimens (quarterly or yearly), are newer options available to patients with postmenopausal osteoporosis. Intravenous bisphosphonates are particularly helpful for those who are bedfast or have esophageal disorders, cognitive problems, or other dosing challenges.
Alendronate, risedronate, and ibandronate all were initially approved by the Food and Drug Administration (FDA) for daily oral dosing, supported by trials with direct anti-fracture efficacy endpoints (CitationBlack et al 1996; CitationHarris et al 1999; CitationMcClung et al 2001; CitationChesnut et al 2004). The burdensome dosing requirements needed for gastrointestinal (GI) protection with daily oral bisphosphonates have motivated development of less frequent oral regimens. Weekly dosing is made possible by the long half-life of bone-bound bisphosphonates, which remain at resorption sites longer than the 2-week lifespan of individual osteoclasts (CitationBone et al 2000). Weekly oral alendronate and risedronate achieved approval based on comparisons with the respective daily regimens (CitationSchnitzer et al 2000; CitationBrown et al 2002). Weekly oral ibandronate has also shown non-inferior efficacy to the daily regimen (CitationCooper et al 2003) but has not been marketed. These weekly regimens, with 7 times the daily oral dose, maintain continuous reduction of bone turnover markers (CitationPapapoulos and Schimmer 2007).
Bisphosphonate pharmacology also makes possible monthly, intermittent, quarterly, or yearly dosing. In the quest for improved adherence and persistence, these extended-interval regimens provide important lifestyle-friendly options. Monthly oral ibandronate, the first approved monthly bisphosphonate regimen, was supported by comparison trials with the daily regimen and has been in use since 2005 (CitationMiller et al 2005; CitationReginster et al 2006). Pharmacodynamically, monthly oral ibandronate utilizes a non-linear dose increase versus the daily oral regimen (150 mg/month vs 2.5 mg/day), which more than doubles annual cumulative skeletal exposure (ACE; 10.8 mg with monthly vs 5.5 mg with daily oral treatment) (CitationZaidi et al 2007). Increased ACE maintains efficacy during the interdose periods of extended-interval regimens (CitationZaidi et al 2007). Bone turnover markers show marked suppression within 3 days after a 150 mg/month oral ibandronate dose and reach a nadir by 7 days postdose (CitationSilverman et al 2007a); although marker levels gradually increase during the interdose period, they remain throughout the month within the premenopausal range required for efficacy (CitationPapapoulos and Schimmer 2007).
An intermittent oral risedronate regimen (2 consecutive days monthly) was approved in April 2007 (CitationDelmas et al 2008a), and a once-monthly risedronate dosing regimen was approved in April 2008 (CitationDelmas et al 2008b). These risedronate regimens provide 30 times the daily oral dose. Comparison to daily oral risedronate shows that they achieve similar bone turnover marker suppression and BMD increases (CitationDelmas et al 2007a; CitationDelmas et al 2007b).
Intravenous bisphosphonate regimens decouple bisphosphonate therapy from stringent oral dosing requirements, providing alternative options for any postmenopausal osteoporosis patients and extending the benefits of bisphosphonates to patients unable to take them orally. Quarterly iv ibandronate (3 mg/3 month), approved in 2006, has shown efficacy in postmenopausal osteoporosis with a similar safety profile to the monthly oral regimen (CitationDelmas et al 2006). The lower-dose iv ibandronate regimens investigated during drug development did not achieve clinical efficacy because bone turnover returned toward untreated levels during the interdose periods (CitationAdami et al 2004; CitationRecker et al 2004). The approved 3 mg/3 month iv dose provides the highest annual cumulative skeletal exposure of approved ibandronate regimens (12 mg) (CitationZaidi et al 2007), resulting in superior efficacy to daily oral ibandronate (CitationEisman et al 2008).
Zoledronic acid was originally developed as a high-potency agent for single-dose iv infusion to treat hypercalcemia (CitationMajor 2002) and skeletal complications (CitationPerry and Figgitt 2004) of malignancy. Suppression of bone turnover in osteopenic or osteoporotic postmenopausal women infused with 5 mg zoledronic acid was more rapid and pronounced than in those receiving 70 mg/week oral alendronate (CitationSaag et al 2007); further investigations in postmenopausal osteoporosis led to the 2007 approval of a yearly iv zoledronic acid regimen (5 mg/year) for this indication (CitationBlack et al 2007).
Bisphosphonate efficacy, safety, and tolerability
Key randomized trials evaluating nitrogen-containing bisphosphonates individually and comparatively are summarized in ; their findings on efficacy, safety, and tolerability are described below.
Table 3 Overview of bisphosphonate study results by dosing frequency, route, and agent
Efficacy studies
Pivotal trials supporting approval of daily oral nitrogen-containing bisphosphonate regimens utilized fracture-based primary endpoints. After these daily regimens attained approval, “bridging trials” supporting less frequent dosing regimens were designed to show non-inferiority or equivalence to daily regimens by means of surrogate BMD endpoints.
Daily oral regimens
The Fracture Intervention Trial (FIT) evaluated oral alendronate (5 mg/day for the first 2 years; 10 mg/day thereafter) vs placebo in women with (CitationBlack et al 1996) or without (CitationCummings et al 1998) baseline vertebral fractures. Alendronate reduced the relative risk of new vertebral fractures by roughly 50% in the group with prevalent vertebral fractures (CitationBlack et al 1996); hip and wrist fracture risks also decreased by roughly 50%, and that of non-vertebral fractures by 20% (). In the group without baseline vertebral fractures (CitationCummings et al 1998), new vertebral fracture risk decreased by approximately 45%; non-vertebral fracture effects depended on baseline BMD ().
In the FIT Long-term EXtension study (FLEX) (CitationBone et al 2004), FIT participants who received alendronate for 5 years were re-randomized to continue alendronate (5 or 10 mg/day) or to switch to placebo for 5 additional years. In the group who received alendronate for a total of 10 years, BMD increased at multiple sites compared with baseline; lumbar spine BMD continued increasing throughout treatment, whereas from 5 years onward other sites showed stable or decreasing BMD (CitationBone et al 2004). Clinically evident vertebral fractures were less common than in the group who switched to placebo, who experienced hip and spine BMD declines after discontinuing alendronate (CitationBlack et al 2006).
Placebo-controlled trials supporting the approval of daily oral risedronate (5 mg/day) evaluated hip fractures among women with osteoporosis or non-skeletal risk factors (the Hip Intervention Program [HIP]) (CitationMcClung et al 2001) or new vertebral fractures among women with prevalent vertebral fractures (Vertebral Efficacy with Risedronate Trial [VERT]) (CitationReginster et al 2000). In HIP (CitationMcClung et al 2001), relative hip fracture risk was significantly reduced with risedronate vs placebo in the overall patient set and among osteoporotic patients, but did not differ significantly among non-osteoporotic patients with risk factors for falling (). Non-vertebral fracture risk, similarly, significantly decreased with risedronate overall and in osteoporotic patients. In the multinational VERT study (CitationReginster et al 2000), new vertebral fracture risk in risedronate recipients was reduced by 61% during the first year and by 49% over 3 years, whereas non-vertebral fracture risk was reduced by 33% over 3 years (). Risedronate recipients in the North American VERT study (CitationHarris et al 1999) showed 1-year vertebral fracture risk reduction of 65%, 3-year vertebral fracture risk reduction of 41%, and non-vertebral fracture risk reduction of 40% ().
In the oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe (BONE) (CitationChesnut et al 2004), oral ibandronate given daily or intermittently to women with prevalent vertebral fractures significantly reduced the relative risk of new vertebral fractures and clinical vertebral fractures compared with placebo (). Lumbar BMD significantly increased and rates of height loss decreased in both ibandronate groups as compared with placebo (CitationChesnut et al 2004). Non-vertebral fracture risk also decreased by 69% in daily oral ibandronate recipients with low baseline femoral neck T-scores < −3.0 (CitationChesnut et al 2004). BONE supported the initial approval of daily oral ibandronate in 2005. Its vertebral fracture data provided the first evidence for anti-fracture efficacy of a non-daily oral bisphosphonate regimen.
Weekly oral regimens
In the Alendronate Once-Weekly Study (CitationSchnitzer et al 2000), a 70 mg oral once-weekly dose of alendronate resulted in statistically equivalent lumbar spine BMD gains to those seen with the 10 mg daily treatment or 35 mg twice-weekly treatment (); the 70 mg/week oral alendronate regimen was approved in 2000. A bridging trial of risedronate at 35 or 50 mg/week showed non-inferior increases of lumbar BMD with both regimens as compared with the 5 mg/day approved dosing (CitationBrown et al 2002), resulting in 2002 approval for 35 mg weekly oral risedronate.
Monthly oral regimens
The currently available monthly oral ibandronate regimen was approved in 2005 on the basis of the 2-year Monthly Oral IBandronate in LadiEs (MOBILE) bridging trial (CitationMiller et al 2005; CitationReginster et al 2006). In the MOBILE trial, monthly oral ibandronate regimens (100 or 150 mg/month) proved as effective as the approved daily regimen at improving lumbar BMD (). The 150 mg/month regimen subsequently showed superiority to 100 mg/month and 2.5 mg/day at lumbar spine and total hip BMD improvement (CitationMiller et al 2005; CitationReginster et al 2006). Significantly greater percentages of patients achieved measurable 2-year BMD gains at lumbar spine, total hip, or both with the 150 mg/month regimen vs 2.5 mg/day (p ≤ 0.004) or 100 mg/month (p < 0.01) (CitationReginster et al 2006). Additionally, 150 mg/month ibandronate resulted in significantly higher percentages of patients achieving target BMD gains (≥3% or ≥6%) at these sites (CitationReginster et al 2006).
An oral risedronate regimen consisting of 75 mg/day for 2 consecutive days monthly received US approval in April 2007, based on a bridging study showing its non-inferiority to the approved daily risedronate regimen () (CitationDelmas et al 2008a). The Monthly Evaluation of Risedronate Trial in OsteoPorosis (MERIT-OP) (CitationDelmas et al 2008b) subsequently reported similar non-inferiority results for a 150 mg/month risedronate regimen approved in April 2008 ().
Quarterly iv regimens
Quarterly iv ibandronate received FDA approval in 2007 on the basis of the Intermittent Regimen iv Ibandronate Study (IRIS) (CitationAdami et al 2004) and Dosing IVAdministration (DIVA) (CitationDelmas et al 2006; CitationEisman et al 2008) trials (), whose lumbar BMD endpoints demonstrated non-inferiority to the approved daily oral ibandronate regimen. Both iv regimens in the DIVA trial achieved lumbar BMD increases statistically superior to oral dosing () (CitationDelmas et al 2006; CitationEisman et al 2008). Efficacy against non-vertebral fractures was subsequently demonstrated for the quarterly iv and monthly oral ibandronate regimens in pooled analyses (Cranney et al 2007; CitationHarris et al 2008).
Yearly iv regimen
The Health Outcomes and Reduced Incidence with Zoledronic Acid ONce Yearly (HORIZON) trial showed significant reduction of vertebral, non-vertebral, and hip fractures with yearly iv zoledronic acid vs placebo in post-menopausal osteoporosis patients with or without prevalent vertebral fractures () (CitationBlack et al 2007). A subsequent study, the HORIZON Clinical Fracture Trial (CitationLyles et al 2007), included women and men who underwent surgical repair of low-trauma hip fractures within 90 days before enrollment. Yearly zoledronic acid recipients in this high-risk population showed relative risk reduction vs placebo of 35% in new clinical fractures and 28% in 2-year all-cause mortality (CitationLyles et al 2007).
Comparative trials
The Fosamax Actonel Comparison Trial (FACT) (CitationBonnick et al 2006) compared weekly oral alendronate (70 mg/week) and risedronate (35 mg/week) regimens in a 1-year head-to-head study with a 1-year extension (). At all time points alendronate produced significantly greater BMD increases than risedronate at the hip trochanter, lumbar spine, total hip, and femoral neck. Significantly more alendronate than risedronate recipients attained measurable BMD gains (CitationSebba et al 2004; CitationBonnick et al 2006). Fractures were reported only as adverse events (AEs) in the FACT studies.
The 1-year randomized, double-blind, double-dummy Monthly Oral Therapy with Ibandronate for Osteoporosis INtervention (MOTION) trial recently demonstrated that monthly oral ibandronate provides similar efficacy to weekly oral alendronate, as assessed by BMD improvements at the lumbar spine, total hip, trochanter, and femoral neck () (CitationMiller et al 2008). Fractures were reported only as AEs in the MOTION study.
Meta-analyses of non-vertebral fracture efficacy
The FDA requires direct anti-fracture endpoints to approve new bisphosphonate agents; initial trials are often designed and powered for primary endpoints of vertebral fracture. All approval trials for currently used nitrogen-containing bisphosphonates except HIP (risedronate) and HORIZON (zoledronic acid) were designed with primary vertebral fracture endpoints. In contrast, regimen extensions of existing agents can be approved on the basis of bridging trials. Thus, no direct anti-fracture efficacy trials exist for weekly, monthly, or quarterly bisphosphonates.
Non-vertebral fractures are infrequent events; their use as a trial endpoint requires large or high-risk populations and is affected by methodological variations (CitationMiller 2008). Meta-analyses are thus often used to explore treatment effects on non-vertebral fracture risk. Daily alendronate has been associated in such meta-analyses with non-vertebral fracture risk reductions of 14%–49% (CitationKarpf et al 1997; CitationCranney et al 2002b; CitationBoonen et al 2005), and daily risedronate with non-vertebral fracture risk reductions of 19%–59% (CitationKarpf et al 1997; CitationCranney et al 2002a; CitationHarrington et al 2004; CitationLiberman et al 2006). No currently published meta-analyses have specifically examined weekly oral bisphosphonate regimens.
Meta-analyses of extended-interval ibandronate regimens based on their achieved ACE have shown significant non-vertebral fracture risk reduction. Patients receiving ACE ≥10.8 mg (ie, approved monthly oral or quarterly iv ibandronate regimens or an investigational bimonthly iv regimen) achieved relative risk reductions versus placebo of 29.9% for all non-vertebral fractures, and 34.4% for a set of 6 important non-vertebral fractures (clavicle, humerus, wrist, pelvis, hip, leg) (CitationHarris et al 2008). Ibandronate regimens with ACE ≥10.8 mg showed 38% relative risk reduction for all non-vertebral fractures versus daily oral ibandronate (ACE 5.5 mg) (CitationCranney et al 2008). These meta-analyses provide the first evidence of non-vertebral fracture efficacy for any weekly, monthly, or quarterly bisphosphonate regimen.
Safety and tolerability
The most common AEs reported with bisphosphonates affect the upper GI (UGI) system. In part such events may reflect the increased baseline incidence of UGI disorders among elderly patients and the synergistic effects of non-steroidal anti-inflammatory drugs (NSAIDs) used for comorbid arthritis. Adherence to oral dosing requirements (taking bisphosphonates with ≥8 fluid oz (237 mL) water after an overnight fast and not reclining, eating, or drinking for 30–60 minutes after dosing) reduces UGI AEs and enhances absorption. AEs affecting other body systems are somewhat less frequent.
Oral ibandronate (daily or intermittent) shows UGI AE frequencies similar to placebo, even among patients with histories of UGI disorders or concomitant anti-ulcer or NSAID treatment (CitationEpstein et al 2006). Head-to-head studies indicate that safety and tolerability profiles, and in particular UGI tolerability, appear not to vary greatly among different oral bisphosphonates. In the FACT (CitationBonnick et al 2006), overall AEs, serious AEs, and discontinuations due to AEs were similar between 70 mg/week alendronate and 35 mg/week risedronate. UGI AEs (predominantly dyspepsia, nausea, and reflux) affected 24.8% of alendronate recipients and 22.9% of risedronate recipients; and 1.7% and 1.2%, respectively, discontinued because of UGI AEs (CitationBonnick et al 2006). In the MOTION trial (CitationDelmas et al 2007b), the incidence of UGI AEs was similar between weekly oral alendronate (17.2%) and monthly oral ibandronate (17.5%); however, more alendronate users withdrew because of treatment-related UGI AEs (1.7% vs 1.0% of ibandronate users). Because UGI AEs generally result from topical irritation, agents designed for less frequent oral dosing may reduce cumulative risk (CitationEpstein et al 2006).
Osteonecrosis of the jaw (ONJ) is an infrequent but troublesome bisphosphonate-related AE thought to result from antiangiogenesis (CitationMigliorati 2003; CitationMigliorati et al 2006), impaired circulation, or excessive osteoclast suppression (CitationCarter et al 2005). Most ONJ reports are associated with iv pamidronate or zoledronic acid prescribed for hypercalcemia of malignancy or skeletal metastases (indications requiring higher and more frequent doses than are used in osteoporosis). Cumulative worldwide ONJ reports with oral bisphosphonates (as of 2006) have included 170 with alendronate, 12 with risedronate and a single patient who had received both ibandronate and iv zoledronic acid (CitationAmerican Dental Association Council on Scientific Affairs 2006). A 2007 systematic review (CitationPazianas et al 2007) collected 26 ONJ cases in adult osteoporosis patients receiving bisphosphonates (alendronate, 23; alendronate and zoledronic acid, 1; pamidronate, 1; risedronate, 1; ibandronate, 0). Advancing age, dental extractions, and corticosteroid use showed significant associations with ONJ risk.
Reports of atrial fibrillation in trials of yearly iv zoledronic acid (CitationBlack et al 2007) and daily oral alendronate (CitationCummings et al 2007) recently prompted an FDA safety review of this AE among bisphosphonate users (CitationFood and Drug Administration 2007). Although serious atrial fibrillation events in these studies (CitationBlack et al 2007; CitationCummings et al 2007) were significantly more frequent in drug recipients, no significant difference was seen between bisphosphonates and placebo when all atrial fibrillation events were considered. Neither serious nor overall atrial fibrillation events significantly differed between zoledronic acid and placebo in the HORIZON Recurrent Fracture trial, which enrolled an older patient population than the pivotal HORIZON study (CitationLyles et al 2007). Postdose electrolyte imbalances (hypocalcemia, hypophosphatemia, or hypomagnesemia) have been suggested to be responsible for atrial fibrillation during bisphosphonate use (Citationde Nijs and Westgeest 2007; CitationPoole et al 2007); however, the causality of this AE is not known with certainty. An October 2007 early communication about the ongoing safety review stated that bisphosphonate prescribing patterns need not change at this time (CitationFood and Drug Administration 2007).
Reduced kidney function has been reported in 9% to 15% of patients receiving iv zoledronic acid or pamidronate (CitationChang et al 2003). In the HORIZON trial (CitationBlack et al 2007), increases of serum creatinine >0.5 g/dL occurred at 9–11 days postdose in significantly more zoledronic acid recipients (iv 5 mg/year) than placebo recipients (1.2% vs 0.4%, p = 0.001). In 85% of cases, levels reverted in 30 days to within 0.5 g/dL of baseline, and by the third year of the study, there was no significant difference between study groups. Although iv ibandronate labeling includes a precaution regarding hypocalcemia and renal impairment, iv ibandronate has shown no evidence of affecting renal function in patients with cancer-related bone disease (CitationStrampel et al 2007) or in DIVA trial participants (CitationRizzoli and Reid 2007). In a pooled analysis of 4 trials comprising 6815 patients with osteoporosis and estimated glomerular filtration rates >30 mL/min/1.73 m2, changes in estimated creatinine clearance throughout study duration were modest and similar among iv ibandronate, daily oral ibandronate, and placebo groups (CitationMiller et al 2007). Patients receiving iv bisphosphonate medications should have their serum creatinine measured regularly.
Ocular inflammatory AEs (conjunctivitis, episcleritis, uveitis, or scleritis) are occasionally associated with nitrogen-containing bisphosphonates (particularly with regimens used in cancer) and may be mediated by tumor necrosis factor-α and interleukins 1 and 6 (CitationFraunfelder 2003; CitationFraunfelder 2007). Patients experiencing eye pain during bisphosphonate use require immediate referral to an ophthalmologist. Conjunctivitis and episcleritis may be treated topically; uveitis and scleritis require bisphosphonate discontinuation (CitationFraunfelder 2003; CitationFraunfelder 2007).
A transient flu-like illness (fever, myalgia, arthralgia, and malaise) may occur upon initial bisphosphonate use (especially with iv use or the oral dose levels used in monthly or intermittent treatment) and is thought to represent an acute phase reaction to accumulated by-products of prenylation inhibition (CitationBukowski et al 2005). Generally, symptoms are mild, last only 1–3 days, and diminish in incidence and severity with subsequent doses.
Patient perspectives: improving treatment uptake, adherence, and persistence
Osteoporosis prevention is a lifelong endeavor, and osteoporosis treatment ideally starts at or before the initial BMD decline after menopause. Care of elderly patients presenting with incident fractures must go beyond emergent repair to assessment and reduction of recurrent fracture risk. In one Midwestern community care system, process improvements utilizing follow-up of orthopedic billings provided osteoporosis evaluation and treatment to 58% of incident fragility fracture patients in 2003, in contrast to only 5% in 1999 (CitationHarrington et al 2005). Patients with incident hip or spine fractures face increased future fracture risk (CitationBlack et al 1999; CitationLindsay et al 2001) as well as acute risk of disability and mortality. Treating underlying osteoporosis in such patients may conserve medical and financial resources, functional capacity, and life expectancy.
The achievement and maintenance of fracture prevention benefits requires continuity of osteoporosis treatment. Unfortunately, adherence and persistence with treatment are problematic in all chronic diseases and pose particular challenges in osteoporosis. Oral bisphosphonate dosing requirements are inconvenient for patients; thus, less frequent treatment intervals are conducive to better patient acceptance, adherence, and persistence. The US Surgeon General’s Report on Bone Health and Osteoporosis (CitationUS Department of Health and Human Services 2004) has highlighted the need for interventions that improve adherence and for research specifically exploring the impact of dosing regimens on adherence. By making therapy less intrusive to daily life, oral regimens with less frequent dosing may provide improved real-world effectiveness.
Patient preferences and adherence: obstacles and opportunities
Patient inconvenience in osteoporosis treatment is not just a nuisance but a real hazard to effective clinical outcomes, as disruption of maintenance regimens can have severe physical consequences. Premature discontinuation of bisphosphonate therapy has been associated with up to 26% increase in fracture risk (CitationChesnut 2006; CitationGold et al 2007), and frequent oral dosing strongly predicts earlier discontinuation (CitationCramer et al 2005). Adherence and persistence with bisphosphonate therapy are important not only for direct anti-fracture benefits, but also for reduced overall healthcare requirements. In a retrospective study of 32,944 women initiating bisphosphonate treatment, those remaining adherent and persistent had significantly lower total healthcare costs than those with gaps or discontinuation of therapy (CitationCurtis et al 2006). Adherence to weekly dosing is improved over daily regimens (CitationCramer et al 2005; CitationGold et al 2007), but remains suboptimal. Only 45% of weekly bisphosphonate recipients in a large longitudinal study maintained adherence and only 57% maintained persistence over 1 year (CitationChesnut 2006). Thus, though once-weekly therapy represents a move toward a more patient-friendly regimen, it still presents obstacles to sustained treatment.
Once-monthly oral ibandronate may reduce the cumulative inconvenience of an oral bisphosphonate regimen and help patients maintain continuity of treatment. Once-monthly ibandronate users in 2 recent retrospective database studies (N =17,479 and N = 1,664) were respectively 25.1% and 37.7% less likely than once-weekly oral bisphosphonate users to discontinue therapy before 1 year (CitationSilverman et al 2007b). The naturalistic PERsistence Study of Ibandronate verSus alendronaTe (PERSIST) study in the UK (CitationCooper et al 2006) compared persistence times among women in primary care randomized to 6 months of open-label treatment with monthly oral ibandronate or weekly oral alendronate. Ibandronate recipients were offered a patient support program (PSP) available to all UK patients prescribed ibandronate; no similar program existed at the time of the study for alendronate users in the community. Kaplan–Meier analysis showed a significantly higher probability of persistence in the ibandronate/PSP group (p < .0001); mean (±SD) persistence times were 122 ± 2.5 days for ibandronate/PSP and 109 ± 2.5 days for alendronate (CitationCooper et al 2006). However, conflicting results are reported in different persistence comparisons (CitationWeiss et al 2007). Once-monthly ibandronate was preferred over once-weekly alendronate by 66.1% of patients in the randomized, crossover Boniva Alendronate Trial in Osteoporosis (CitationEmkey et al 2005).
Patients who cannot tolerate or do not prefer oral dosing may opt for quarterly iv ibandronate injection (CitationRizzoli and Reid 2007) or yearly iv zoledronic acid infusion (CitationBlack et al 2007). These regimens abrogate oral dosing requirements, but require periodic visits to a physician’s office or infusion center for administration. Outpatients with adverse GI histories on daily or weekly oral bisphosphonates achieved improved adherence to quarterly iv ibandronate (CitationLewiecki et al 2008). Yearly iv zoledronic acid has been preferred by a majority of trial outpatients who switched to it from weekly oral alendronate (CitationMcClung et al 2007; CitationSaag et al 2007). Intravenous regimens may be particularly advantageous for elderly patients residing in long-term care facilities or those with impairments affecting medication self-management. The availability of multiple oral and iv options gives patients the opportunity to decide which attributes of a bisphosphonate regimen they consider most important for long-term sustainability.
Physiologic assessments of adherence and response
The clinical silence of osteoporosis until fractures occur may create challenges in sustaining patients’ motivation for treatment. BMD change is clinically measured no more often than every 2 years (CitationBonnick and Shulman 2006). Earlier demonstration of therapeutic response may help to motivate patients and to detect non-adherence. Biomarkers of bone turnover (), measured in serum or urine, change much more rapidly than BMD (CitationBonnick and Shulman 2006), and thus may provide early objective feedback on adherence and response. Decreased CTX at various time points during bisphosphonate therapy is strongly correlated with BMD increases at 6 months and 1 year (CitationLeeming et al 2006). Conversely, high CTX levels may predict fracture risk; in the Epidemiology of Osteoporosis study (CitationGarnero et al 1996a), elderly women with elevated urinary CTX had a 4.8-fold increased risk of hip fractures.
Patients’ responses to biomarker-based feedback showed unexpected patterns in a 1-year prospective study of risedronate treatment (5 mg/day orally for 1 year) with and without a reinforcement program (urinary NTX testing and patient education at weeks 13 and 25) (CitationDelmas et al 2007a). In addition to the biomarker follow-up, persistence was measured directly with electronically monitored pill dispensers; overall persistence over 1 year was 77% in the reinforcement group and 80% in the non-reinforcement group. Counterintuitively, patients who were informed of a poor NTX response (>30% increase, indicating poor adherence) were roughly twice as likely to discontinue therapy prematurely. In contrast, patients informed of a good NTX response (>30% decrease) showed improved persistence rates after the reinforcement visit (CitationDelmas et al 2007a). Patients in the reinforcement group had less than half the incidence of new vertebral fractures as in the non-reinforcement group. Interventions that increase persistence with bisphosphonates improve fracture protection.
Recent developments thus allow a multipronged approach to maintaining the continuity of bisphosphonate treatment. Monthly or less frequent regimens lessen lifestyle disruption, preference assessments identify the attributes patients value, and bone turnover markers provide physiologic accountability.
Conclusions
Nitrogen-containing bisphosphonates have become the standard of care for postmenopausal osteoporosis, in company with calcium and vitamin D supplementation and weight-bearing physical activity. Oral bisphosphonate dosing requires stringent dosing guidelines to maximize bioavailability and minimize UGI irritation. The inconvenience this poses to patients has motivated the development of weekly and monthly oral regimens to enhance adherence, as well as quarterly and yearly iv regimens for patients unable to tolerate oral dosing. Ibandronate is the first bisphosphonate to have shown direct anti-fracture efficacy with a non-daily regimen; it was approved for once-monthly oral dosing in 2005 and for quarterly iv dosing in 2006. Yearly iv zoledronic acid was approved in 2007; it has shown improvement of fracture rates and post-fracture mortality, although the recent FDA early communication about atrial fibrillation and bisphosphonate use certainly warrants ongoing safety review. The availability of once-monthly and less frequent bisphosphonate regimens promises to improve patient satisfaction and adherence, and thus to increase the real-world effectiveness of osteoporosis therapy.
Acknowledgments
The author acknowledges Kim Coleman Healy, PhD, of Envision Pharma for editorial assistance in the development of this manuscript. This editorial assistance was supported by an independent grant from Roche.
Disclosures
The author’s financial relationships over the past 12 months include research support from GlaxoSmithKline and Roche, speakers’ bureau membership for GlaxoSmithKline, Roche, and Ortho-McNeil, and consulting for Novartis.
References
- AdamiSFelsenbergDChristiansenC2004Efficacy and safety of ibandronate given by intravenous injection once every 3 monthsBone34881915121020
- American Dental Association Council on Scientific Affairs2006Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendationsJ Am Dent Assoc13711445016873332
- Bischoff-FerrariHAWillettWCWongJB2005Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trialsJAMA29322576415886381
- BlackDMArdenNKPalermoL1999Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research GroupJ Bone Miner Res14821810320531
- BlackDMCummingsSRKarpfDB1996Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research GroupLancet3481535418950879
- BlackDMDelmasPDEastellR2007Once-yearly zoledronic acid for treatment of postmenopausal osteoporosisN Engl J Med35618092217476007
- BlackDMSchwartzAVEnsrudKE2006Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trialJAMA29629273817190893
- BoneHGAdamiSRizzoliR2000Weekly administration of alendronate: rationale and plan for clinical assessmentClin Ther22152810688387
- BoneHGHoskingDDevogelaerJP2004Ten years’ experience with alendronate for osteoporosis in postmenopausal womenN Engl J Med35011899915028823
- BonnickSSaagKGKielDP2006Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two yearsJ Clin Endocrinol Metab912631716636120
- BonnickSLShulmanL2006Monitoring osteoporosis therapy: bone mineral density, bone turnover markers, or both?Am J Med1194 Suppl 1S25S3116563938
- BoonenSLaanRFBartonIP2005Effect of osteoporosis treatments on risk of non-vertebral fractures: review and meta-analysis of intention-to-treat studiesOsteoporos Int161291815986101
- BriotKRouxC2005What is the role of DXA, QUS and bone markers in fracture prediction, treatment allocation and monitoring?Best Pract Res Clin Rheumatol199516416301189
- BrownJPKendlerDLMcClungMR2002The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosisCalcif Tissue Int711031112085156
- BukowskiJFDascherCCDasH2005Alternative bisphosphonate targets and mechanisms of actionBiochem Biophys Res Commun3287465015694409
- BurgeRDawson-HughesBSolomonDH2007Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025J Bone Miner Res224657517144789
- CarterGGossANDoeckeC2005Bisphosphonates and avascular necrosis of the jaw: a possible associationMed J Aust182413515850439
- ChangJTGreenLBeitzJ2003Renal failure with the use of zoledronic acid [letter]N Engl J Med34916769 discussion 1676–914573746
- ChesnutCH2006Treating osteoporosis with bisphosphonates and addressing adherence: a review of oral ibandronateDrugs661351916903769
- ChesnutCHEttingerMPMillerPD2005Ibandronate produces significant, similar antifracture efficacy in North American and European women: new clinical findings from BONECurr Med Res Opin2139140115811208
- ChesnutCHSkagAChristiansenC2004Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosisJ Bone Miner Res191241915231010
- CooperADrakeJBrankinE2006Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST studyInt J Clin Pract6089690516800837
- CooperCEmkeyRDMcDonaldRH2003Efficacy and safety of oral weekly ibandronate in the treatment of postmenopausal osteoporosisJ Clin Endocrinol Metab8846091514557430
- CramerJAAmonkarMMHebbornA2005Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosisCurr Med Res Opin2114536016197664
- CranneyATugwellPAdachiJ2002aMeta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosisEndocr Rev235172312202466
- CranneyAWellsGYetisirE2008Ibandronate for the prevention of nonvertebral fractures: a pooled analysis of individual patient dataOsteoporos Int Epub ahead of print July 29, 2008
- CranneyAWellsGWillanA2002bMeta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal womenEndocr Rev235081612202465
- CummingsSRBlackDMThompsonDE1998Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention TrialJAMA2802077829875874
- CummingsSRSchwartzAVBlackDM2007Alendronate and atrial fibrillationN Engl J Med3561895617476024
- CurtisJRAmonkarMMMuchaL2006Osteoporotic women adherent to bisphosphonates have reduced osteoporosis-related and total healthcare costs. Poster SU 320Twenty-eighth annual meeting of the American Society for Bone and Mineral ResearchPhiladelphia, PA, USA presentedSeptember 17, 2006
- de NijsRNWestgeestAA2007Yearly zoledronic acid in postmenopausal osteoporosis [letter]N Engl J Med357711 author reply 714–517699824
- DelmasPDAdamiSStrugalaC2006Intravenous ibandronate injections in postmenopausal women with osteoporosis: One-year results from the dosing intravenous administration studyArthritis Rheum5418384616729277
- DelmasPDVrijensBEastellR2007aEffect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosisJ Clin Endocrinol Metab92129630417244788
- DelmasPDLewieckiEMRagi-EisS2007bThe MOTION study: tolerability of monthly ibandronate and weekly alendronate in women with postmenopausal osteoporosisAbstract T383, J Bone Miner Res22Suppl 1S327
- DelmasPDBenhamouCLManZ2008aMonthly dosing of 75 mg risedronate on 2 consecutive days a month: efficacy and safety resultsOsteoporos Int1910394518087660
- DelmasPDMcClungMRZanchettaJR2008bEfficacy and safety of risedronate 150 mg once a month in the treatment of postmenopausal osteoporosisBone42364217920005
- DunfordJEThompsonKCoxonFP2001Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonatesJ Pharmacol Exp Ther2962354211160603
- EismanJACivitelliRAdamiS2008Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA studyJ Rheumatol354889718260172
- EmkeyRKoltunWBeusterienK2005Patient preference for once-monthly ibandronate versus once-weekly alendronate in a randomized, open-label, cross-over trial: the Boniva Alendronate Trial in Osteoporosis (BALTO)Curr Med Res Opin21189590316368038
- EpsteinSDelmasPDEmkeyR2006Oral ibandronate in the management of postmenopausal osteoporosis: review of upper gastrointestinal safetyMaturitas5411016522358
- Food and Drug Administration2007Early communication of an ongoing safety reviewBisphosphonates: Alendronate (Fosamax, Fosamax Plus D), Etidronate (Didronel), Ibandronate (Boniva), Pamidronate (Aredia), Risedronate (Actonel, Actonel w/Calcium), Tiludronate (Skelid) and Zoledronic acid (Reclast, Zometa) [online] Accessed October 30, 2007 URL: http://www.fda.gov/cder/drug/early_comm/bisphosphonates.htm
- FraunfelderFW2003Ocular side effects associated with bisphosphonatesDrugs Today (Barc)398293514702129
- FraunfelderFW2007Drug-induced ocular inflammatory diseasesDrugs Today (Barc)431172317353948
- GarneroPHausherrEChapuyMC1996aMarkers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective StudyJ Bone Miner Res11153188889854
- GarneroPSornay-RenduEChapuyMC1996bIncreased bone turnover in late postmenopausal women is a major determinant of osteoporosisJ Bone Miner Res11337498852944
- GoldDTMartinBCFrytakJR2007A claims database analysis of persistence with alendronate therapy and fracture risk in post-menopausal women with osteoporosisCurr Med Res Opin235859417355739
- HarringtonJTBarashHLDayS2005Redesigning the care of fragility fracture patients to improve osteoporosis management: a health care improvement projectArthritis Rheum5319820415818644
- HarringtonJTSte-MarieLGBrandiML2004Risedronate rapidly reduces the risk for nonvertebral fractures in women with postmenopausal osteoporosisCalcif Tissue Int741293514648009
- HarrisSTBlumentalsWAMillerPD2008Ibandronate and the risk of non-vertebral and clinical fractures in women with postmenopausal osteoporosis: results of a meta-analysis of phase III studiesCurr Med Res Opin242374518047776
- HarrisSTWattsNBGenantHK1999Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study GroupJAMA28213445210527181
- HealyBP1998Weak and feeble bones no more: the National Osteoporosis Foundation speaks outJ Womens Health7106789861580
- HeijckmannACJuttmannJRWolffenbuttelBH2002Intravenous pamidronate compared with oral alendronate for the treatment of postmenopausal osteoporosisNeth J Med60315912481878
- HodgsonSFWattsNBBilezikianJP2001American Association of Clinical Endocrinologists 2001 Medical Guidelines for Clinical Practice for the Prevention and Management of Postmenopausal OsteoporosisEndocr Pract729331211508261
- KanisJA2008FRAX™ WHO Fracture Risk Assessment Tool [online] Accessed March 3, 2008 URL: http://www.shef.ac.uk/FRAX/index.htm
- KanisJAOdenAJohnellO2007The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and womenOsteoporos Int1810334617323110
- KarpfDBShapiroDRSeemanE1997Prevention of nonvertebral fractures by alendronate. A meta-analysis. Alendronate Osteoporosis Treatment Study GroupsJAMA2771159649087473
- LeemingDJAlexandersenPKarsdalMA2006An update on bio-markers of bone turnover and their utility in biomedical research and clinical practiceEur J Clin Pharmacol627819216912870
- LewieckiEMBabbittAMPiziakVK2008Adherence and gastrointestinal tolerability to monthly oral or quarterly intravenous ibandronate in patients with prior gastrointestinal intolerance to oral bisphosphonates: A 12-month, open-label prospective evaluationClin Ther306052118498910
- LibermanUAHochbergMCGeusensP2006Hip and non-spine fracture risk reductions differ among antiresorptive agents: Evidence from randomised controlled trialsInt J Clin Pract60139440017026515
- LindsayRSilvermanSLCooperC2001Risk of new vertebral fracture in the year following a fractureJAMA285320311176842
- LylesKWColon-EmericCSMagazinerJS2007Zoledronic acid and clinical fractures and mortality after hip fractureN Engl J Med357179980917878149
- MacLeanCNewberrySMaglioneM2008Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosisAnn Intern Med14819721318087050
- MajorP2002The use of zoledronic acid, a novel, highly potent bisphosphonate, for the treatment of hypercalcemia of malignancyOncologist74819112490736
- McClungMReckerRMillerP2007Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronateBone41122817468062
- McClungMRGeusensPMillerPD2001Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study GroupN Engl J Med3443334011172164
- MiglioratiCA2003Bisphosphonates and oral cavity avascular bone necrosisJ Clin Oncol214253414615459
- MiglioratiCASiegelMAEltingLS2006Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatmentLancet Oncol75081416750501
- MillerPD2008Non-vertebral fracture risk reduction with oral bisphosphonates: challenges with interpreting clinical trial dataCurr Med Res Opin241071918031594
- MillerPDEpsteinSSedaratiF2008Once-monthly oral ibandronate compared with weekly oral alendronate in postmenopausal osteoporosis: results from the head-to-head MOTION studyCurr Med Res Opin242071318042311
- MillerPDLewieckiEMZaidiM2007IV ibandronate injections demonstrate favorable renal tolerability [abstract 297]American Association of Clinical Endocrinologists Sixteenth Annual Meeting and Clinical CongressSeattle, WA, USAApril 11–15, 2007
- MillerPDMcClungMRMacoveiL2005Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE studyJ Bone Miner Res2013152216007327
- MonkkonenHOttewellPDKuokkanenJ2007Zoledronic acid-induced IPP/ApppI production in vivoLife Sci8110667017850825
- National Osteoporosis Foundation2008Clinician’s Guide to Prevention and Treatment of Osteoporosis [online] Accessed March 3, 2008 URL: http://www.nof.org/professionals/NOF_Clinicians_Guide.pdf
- PapapoulosSESchimmerRC2007Changes in bone remodelling and anti-fracture efficacy of intermittent bisphosphonate therapy: implications from clinical studies with ibandronateAnn Rheum Dis66853817277001
- PazianasMMillerPBlumentalsWA2007A review of the literature on osteonecrosis of the jaw in patients with osteoporosis treated with oral bisphosphonates: prevalence, risk factors, and clinical characteristicsClin Ther2915485817919538
- PerryCMFiggittDP2004Zoledronic acid: a review of its use in patients with advanced cancerDrugs64119721115161327
- PooleKEKaptogeSReeveJ2007Yearly zoledronic acid in postmenopausal osteoporosis [letter]N Engl J Med3577112 author reply 714–517703530
- ReckerRStakkestadJAChesnutCH3rd2004Insufficiently dosed intravenous ibandronate injections are associated with suboptimal anti-fracture efficacy in postmenopausal osteoporosisBone34890915121021
- ReginsterJMinneHWSorensenOH2000Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study GroupOsteoporos Int11839110663363
- ReginsterJYAdamiSLakatosP2006Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE studyAnn Rheum Dis656546116339289
- RizzoliRReidDM2007Ibandronate: An IV injection for the treatment for postmenopausal osteoporosisBone415 Suppl 1S248
- RogersMJGordonSBenfordHL2000Cellular and molecular mechanisms of action of bisphosphonatesCancer8829617810898340
- RousculpMDLongSRWangS2007Economic burden of osteoporosis-related fractures in MedicaidValue Health101445217391423
- RussellRGWattsNBEbetinoFH2008Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacyOsteoporos Int Electronically published ahead of print Jan. 24, 2008
- SaagKLindsayRKriegmanA2007A single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral densityBone4012384317347063
- SchnitzerTBoneHGCrepaldiG2000Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study GroupAging (Milano)1211210746426
- SebbaAIBonnickSLKaganR2004Response to therapy with once-weekly alendronate 70 mg compared to once-weekly risedronate 35 mg in the treatment of postmenopausal osteoporosisCurr Med Res Opin2020314115706659
- SilvermanSLBarrett-ConnorESimonelliC2007aOral monthly ibandronate is associated with rapid suppression of serum CTX within three days of treatment initiation. [abstract W367]J Bone Miner Res22Suppl 1S455
- SilvermanSLCramerJASunyeczJA2007bWomen are more persistent with monthly bisphosphonate therapy compared to weekly bisphosphonates: 12-month results from two retrospective databases [abstract W366]J Bone Miner Res22Suppl 1S454
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes FaNB, Institute of Medicine1997Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D and FluorideWashington, DCNational Academies Press
- StrampelWEmkeyRCivitelliR2007Safety considerations with bisphosphonates for the treatment of osteoporosisDrug Saf307556317722968
- SunyeczJAWeismanSM2005The role of calcium in osteoporosis drug therapyJ Womens Health (Larchmt)141809215775736
- US Department of Health and Human Services2004Bone health and osteoporosis: a report of the Surgeon GeneralRockville, MDUS Department of Health and Human Services, Office of the Surgeon General
- WeissTWHendersonSCMcHorneyCA2007Persistence across weekly and monthly bisphosphonates: analysis of US retail pharmacy prescription refillsCurr Med Res Opin23219320317686228
- World Health Organization1994Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study GroupWorld Health Organ Tech Rep Ser84311297941614
- ZaidiMEpsteinSHarrisST2007Progression of efficacy with ibandronate: a paradigm for the development of new bisphosphonatesAnn N Y Acad Sci11172738218056047