Abstract
Osteoporosis in the aging male remains an important yet under-recognized and undertreated disease. Current US estimates indicate that over 14 million men have osteoporosis or low bone mass, and men suffer approximately 500,000 osteoporotic fractures each year. Men experience fewer osteoporotic fractures than women but have higher mortality after fracture. Bisphosphonates are potent antiresorptive agents that inhibit osteoclast activity, suppress in vivo markers of bone turnover, increase bone mineral density, decrease fractures, and improve survival in men with osteoporosis. Intravenous zoledronic acid may be a preferable alternative to oral bisphosphonate therapy in patients with cognitive dysfunction, the inability to sit upright, or significant gastrointestinal pathology. Zoledronic acid (Reclast) is approved in the US as an annual 5 mg intravenous infusion to treat osteoporosis in men. The zoledronic acid (Zometa) 4 mg intravenous dose has been studied in the prevention of bone loss associated with androgen deprivation therapy.
Osteoporosis in the aging male
Osteoporosis is a systemic skeletal disorder characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture.Citation1 The National Osteoporosis Foundation (NOF) estimates that over 14 million American men had osteoporosis or low bone mass in 2002.Citation2 One in four men older than 60 years of age will experience a fracture,Citation3 yet age-related bone loss in men remains under-recognized and undertreated.
Sixteen percent of men older than 50 and 35% men older than 85 fall each year.Citation4 Hip fractures in men increase mortality by fourfold in the first three months after fracture,Citation5 and one-year mortality reaches 20%.Citation6 Epidemiologic surveys consistently show higher mortality in men than women of similar ages after fracture.Citation7 Among survivors of hip fracture, more than 1 in 4 becomes disabled in the following year,Citation8 and nearly 1 in 5 will require long-term nursing home care.Citation9 Care for patients after hip fracture is expensive to society. In the US, direct costs for patients with osteoporotic fractures is estimated at up to $18 billion/year in 2002 dollars. White men account for 18% of these costs, or $3.2 billion annually.Citation10 Vertebral fractures also increase long-term mortality,Citation3,Citation7,Citation11 cause persistent back pain, deformity, functional decline, and diminished quality of life.
Importantly, the prevalence of osteoporosis and osteoporotic fractures are expected to rise as men live longer. The NOF predicts that there will be 20.5 million American men with osteoporosis or low bone mass by 2020.Citation2 By 2025, annual costs for osteoporosis-related fractures is expected to be $25.3 billion.Citation2
Identifying men at high risk for osteoporotic fracture
Previous fracture is the strongest predictor of any future fracture. The Osteoporotic Fractures in Men (MrOS) StudyCitation12 followed 5995 men aged 65 and older and showed that men with a history of fracture after 50 years had a twofold increased risk of nonvertebral fracture compared to men without prior fracture. A second important predictor of fracture is advancing age. The Dubbo Study showed that, compared to men aged 70–74 years, men older than 80 years had 28 times as many vertebral fractures, nine times as many hip fractures, and nearly five times as many overall fractures.Citation13 Third, bone mineral density (BMD) predicts fracture risk. The use of BMD by dual energy X-ray absorptiometry (DEXA) to predict fracture risk is comparable to the use of blood pressure to predict stroke and is substantially better than using serum cholesterol to predict the risk of myocardial infarction. The MrOS investigatorsCitation12 also found that tricyclic antidepressant use (hazard ratio [HR] 2.36), the inability to complete a narrow walk trial (HR 1.70), falls in previous year (HR 1.59), and depressed mood (HR 1.72) all increased fracture risk independent of BMD.
Bone quality indicators such as cortical thickness, microarchitecture, turnover, porosity, damage accumulation, and the rate and quality of mineralization are other important predictors of fracture, yet they are difficult to incorporate into clinical practice.Citation14 Compared to women, men have bones with larger cross-sectional areas, and as men age they tend to preserve trabecular number. Apart from bone density, both of these factors are thought to ameliorate fracture risk.
In addition to age-related bone loss, osteoporosis in men is frequently secondary to other comorbidities such as glucocorticoid use, hypogonadism, vitamin D deficiency, cigarette smoking, heavy alcohol intake, immobility, or inadequate dietary calcium intake. Studies among ambulatory men with osteoporosis indicate that 30%–64% have at least one identifiable risk factor.Citation15,Citation16 The World Health Organization (WHO) has developed the FRAXTM fracture risk assessment tool, a group of country- and ethnicity-specific risk calculators which allow an estimate of fracture risk based on historical and exam findings.Citation17
Treatment strategies
Traditional nonpharmacologic therapies include treatments intended to increase bone mineral density, prevent falls, and prevent fractures with falls. Several recent papers have reviewed the literature on fall prevention, hip protectors, and calcium and vitamin D supplementation in men.Citation14,Citation18,Citation19 Weight bearing exercise can prevent fallsCitation20 and increase BMD.Citation21 Inadequate dietary calcium and vitamin D intake are risk factors for osteoporosis. The NOF recommends that individuals 50 years old and older consume 1200 mg calcium and 800–1000 IU vitamin D daily. Several studies of calcium and vitamin D supplementation have shown increased BMD, decreased fracture risk,Citation22–Citation24 and even decreased mortalityCitation23 irrespective of baseline 25-hydroxyvitamin D levels. However, another large randomized controlled trial of calcium and vitamin D in secondary prevention of osteoporotic fractures showed no benefit.Citation25
The NOF recommends drug treatment for men aged 50 and older with prior hip or vertebral fracture, with osteoporosis (T-score ≤ −2.5 at the femoral neck or spine), or with osteopenia (T-score between −1.0 and −2.5 at the femoral neck or spine) and an absolute 10-year risk of hip fracture ≥ 3% or 10-year risk of a major osteoporosis-related fracture ≥ 20% based on the FRAX calculation.Citation26 Drugs for the treatment of osteoporosis can be classified into antiresorptive or anabolic agents. Antiresorptive agents, or drugs that inhibit osteoclast action, include most of the commonly used therapies in men such as calcitonin, testosterone, and bisphosphonates.
Calcitonin
Trovas and colleaguesCitation27 randomized 28 osteoporotic men to receive daily intranasal calcitonin 200 IU or placebo. At 12 months, the treated group had significantly suppressed markers of bone turnover and greater BMD at the lumbar spine but not hip. Toth and colleaguesCitation28 studied 71 men with idiopathic osteoporosis but no prior fracture. In these men, intranasal calcitonin daily during alternate months for 18 months significantly increased BMD at the spine and hip and decreased vertebral fractures.
Calcitonin is not approved in the US for the treatment of male osteoporosis.
Testosterone
Studies have consistently shown that testosterone replacement improves BMD in hypogonadal men.Citation29–Citation32 Snyder and colleaguesCitation31 randomized 108 men aged over 65 years without osteoporosis to daily testosterone patch or placebo. They found no treatment effect on BMD in the group as a whole or in men with baseline testosterone level >400 ng/dL. Anderson and colleaguesCitation33 studied intramuscular testosterone injections every two weeks in men with osteoporosis and prior vertebral fracture. In this population, the authors found that testosterone therapy suppressed markers of bone turnover and significantly increased lumbar spine BMD.
No studies have assessed the effect of testosterone replacement on fracture risk, and testosterone is not approved in the US for the treatment of osteoporosis.
Teriparatide
Teriparatide (1–34 PTH), stimulates bone formation. Teriparatide has been shown to raise BMD in men at the hip and spine more than alendronate alone,Citation34,Citation35 and it reduces vertebral fractures by 90%.Citation35
Teriparatide is approved by the US FDA for treatment of osteoporosis in men considered at high risk for fracture.
Bisphosphonates
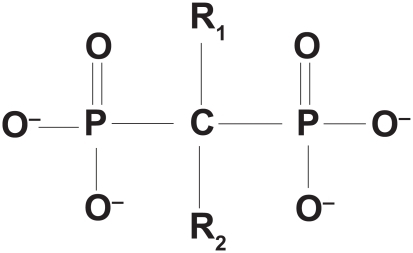
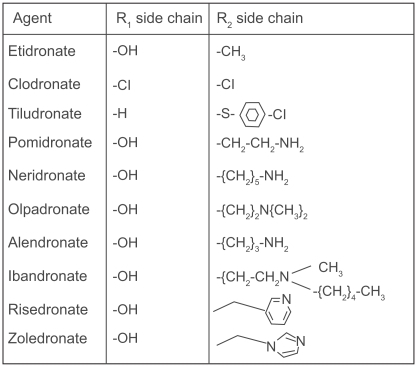
Bisphosphonates are synthetic pyrophosphate analogues with a P-C-P backbone that is resistant to hydrolysis and two phosphate groups that bind hydroxyapatite with high affinity. The central carbon binds the R1 side chain, often a hydroxyl group, which binds calcium and allows for more stable interaction with the bone matrix.Citation36 The longer R2 side chain gives each bisphosphonate its characteristic properties and potency. Bisphosphonates can be classified based on the presence or absence of a nitrogen atom in the R2 side chain. The nitrogen-containing bisphosphonates (alendronate, risedronate, ibandronate, and zoledronic acid) are more potent inhibitors of osteoclast action.Citation37
Mechanism of action
Bisphosphonates are poorly absorbed through the gut, with less than 1% bioavailability after oral administration.Citation38 The kidneys excrete approximately 50% of the drug unchanged.Citation39 The remaining bisphosphonates bind hydroxyapatite crystals on the bone surface with high affinity, where they are internalized by and accumulate in osteoclasts.
Within the osteoclast, the nonnitrogen containing bisphosphonates (etidronate, tiludronate) are incorporated into adenosine triphosphate (ATP). These nascent nonhydrolyzable ATP analogs are toxic to osteoclasts, leading to mitochondrial inhibition and osteoclast apoptosis.Citation40 The nitrogen-containing bisphosphonates inhibit the enzyme farnesyl pyrophosphate synthase (FPPS) in the HMG-CoA reductase pathway leading to cholesterol synthesis. In addition to cholesterol synthesis, this pathway produces the intermediate metabolites farnesol and gerinylgeranol that are necessary for protein prenylation. Prenylation is the posttranslational attachment of hydrophobic prenyl groups to cytoplasmic proteins to allow binding to the cell membrane.Citation40 Such modification allows the Ras superfamily of GTPases to exert changes in cytoskeletal function that produce the characteristic osteoclast ruffled border and allow osteoclast attachment to bone.Citation41 Biopsies of alendronate-treated bone show greater numbers of osteoclasts and abnormal giant, hypernucleated osteoclasts detached from bone. The number of osteoclasts increased with cumulative dose of alendronate, suggesting that the drug may prolong osteoclast survival.Citation42 Bisphosphonate potency, the ability to inhibit bone resorption in vivo, is directly related to drug ability to inhibit FPPS in vitro.Citation37 Nitrogen-containing bisphosphonates also inhibit osteoclast precursor differentiation and inhibit osteoblast-mediated osteoclast activation.Citation38
Evidence of benefit
Pamidronate
Several randomized controlled trials of pamidronate for the treatment of osteoporosis include men. Ryan and colleaguesCitation43 randomized 122 subjects with prior vertebral fracture to oral pamidronate 300 mg/day (group A) for four weeks every 16 weeks, 150 mg/day (group B) for four weeks every eight weeks or placebo (group C). At 2 years, the authors reported significant reductions in serum osteocalcin (29% group A and 33% group B) and urinary deoxypyridinoline (16% group A, 21% group B, p < 0.01 for all comparisons to placebo). They found significant increases in BMD at the spine, hip, and total body (See ).
Table 1 Relative potency of bisphosphonatesCitation37
Table 2 Studies of bisphosphonates for the treatment of osteoporosis in men
Brumsen and colleaguesCitation44 randomized 101 subjects with prior vertebral fracture to oral pamidronate 150 mg/day or placebo. At three years, the investigators found significant reductions in urinary hydroxyproline and serum alkaline phosphatase and significant increases in BMD at the lumbar spine (See ). Men and women had similar increase in BMD in response to pamidronate. There were significantly fewer morphometric vertebral fractures (ARR 22%) in the pamidronate group, and the number needed to treat (NNT) with pamidronate for three years to prevent one vertebral fracture was 5. Six subjects in the placebo group and three pamidronate-treated subjects experienced symptomatic vertebral fractures. Three subjects in the placebo group and one pamidronate-treated subject experienced nonvertebral fractures (ARR 4%). At the end of three years, 53 of the study subjects agreed to participate in an open-label two year extension of the trial. There were equal numbers from the pamidronate and placebo groups, and all subjects received oral pamidronate 150 mg/day. With pamidronate treatment, the differences in urinary hydroxyproline and serum alkaline phosphatase between the initial study groups disappeared. The BMD of the prior placebo group increased to greater extent compared to the prior treatment group such that, at the end of an additional two years there was no significant difference in femoral neck BMD between the groups. The subjects who received pamidronate for five years continued to have a small yet significant greater increase in lumbar spine BMD compared to those subjects who had initially received placebo.
Boutsen and colleaguesCitation45 studied 27 subjects who required initiation of long-term glucocorticoid therapy at a dose of at least 10 mg/day prednisolone for polymyalgia rheumatica (16), inflammatory bowel disease (4), temporal arteritis (3), and rheumatoid arthritis (2). The subjects were randomized to receive a single dose IV pamidronate 90 mg at the start of glucocorticoid therapy (group A); IV pamidronate 90 mg once followed by 30 mg IV pamidronate every three months (group B); and control (group C). Both treatment groups experienced significant decreases in bone-specific alkaline phosphatase, osteocalcin, and C-telopeptide at three months. At 12 months, however, all bone markers of the once-treated group had risen again and only group B observed a sustained decrease in bone resorption. At six and 12 months, there was significantly greater BMD at the lumbar spine, femoral neck, and total hip in both treatment groups compared to control (see ). There were no fractures recorded during the 12 month study.
Pamidronate is not approved in the US for the treatment of osteoporosis in men or women.
Alendronate
Alendronate was the first bisphosphonate to be extensively studied in men. In a study by Ho and colleagues,Citation46 80 subjects with either primary or secondary osteoporosis and prior vertebral fracture received alendronate 10 mg daily. The investigators also followed 43 matched controls. Over 12 months follow-up, the investigators found significantly improved BMD at the lumbar spine and femoral trochanter but not femoral neck (See ). Results were similar for women and men.
Orwoll and colleaguesCitation47 randomized 241 men with osteoporosis to 10 mg daily alendronate or placebo. After two years of treatment, the authors observed a significant 59% decrease in urinary N-telopeptide of type I collagen and 38% decrease in bone-specific alkaline phosphatase in the alendronate group. In the alendronate-treated group, bone mineral density at 24 months increased significantly at the lumbar spine, total hip and femoral neck compared to placebo. Quantitative analysis of spine radiographs revealed significantly fewer vertebral fractures in the alendronate group (aldosterone–renin ratio [ARR] 6.3%) but no significant difference in nonvertebral fractures (See ).
These findings were confirmed by Ringe and colleaguesCitation48 in an open label study of 134 men with osteoporosis randomized to 10 mg daily alendronate or the vitamin D analog alfacalcidol 1 μg daily. With three years follow-up, they observed significantly greater increases in BMD at the lumbar spine and femoral neck in the alendronate-treated group. The alendronate group had significantly fewer vertebral fractures (ARR 13.9%, p = 0.04) and no difference in nonvertebral fractures (See ).
A meta-analysis of the alendronate trials including 375 menCitation49 concluded that alendronate treatment significantly decreases the risk of vertebral fractures (odds ratio [OR] 0.36) in men with osteoporosis. There were not sufficient data to confirm a significant decrease in nonvertebral fractures.
Alendronate is approved in the US for the treatment of osteoporosis in men.
Risedronate
Ringe and colleaguesCitation50 studied risedronate in 316 men with primary or secondary osteoporosis. Over half of those enrolled had a vertebral fracture prior to the start of the study. Subjects were randomized to risedronate 5 mg daily or control, and all received calcium and vitamin D supplementation. At 12 months, there was a significantly greater improvement in BMD at the lumbar spine, total hip, and femoral neck. At one year, the treated subjects experienced fewer vertebral fractures (ARR 7.6%, p = 0.028), a nonsignificant 42% decrease in nonvertebral fractures, and significantly less back pain than the control group (See ).
Boonen and colleaguesCitation51 randomized 284 men with osteoporosis but without prior fracture to weekly risedronate 35 mg or placebo in 2:1 ratio. The risedronate-treated group had significant reductions in the bone turnover markers c-telopeptide, n-telopeptide, and bone-specific alkaline phosphatase at three months, and these effects persisted throughout the two-year study period. The treatment group also experienced significantly greater increases in BMD at the lumbar spine by three months and at the total hip and femoral neck by six months (see ). These treatment differences increased throughout the study. The authors noted a nonsignificant trend toward overall fracture reduction, though the study was not powered to detect a difference.
Reid and colleaguesCitation52 studied 184 men enrolled in two RCT receiving daily risedronate 2.5 mg, risedronate 5 mg, or placebo for the prevention or treatment of glucocorticoid-induced osteoporosis (GIO). Prior to enrollment, the subjects had received either <3 months (prevention study) or >6 months (treatment study) chronic oral glucocorticoid therapy of at least 7.5 mg/day prednisone or equivalent, most commonly for rheumatoid arthritis, lung disease, and polymyalgia rheumatica. At one year, the subjects treated with risedronate 5 mg/day experienced 61% decrease in urinary n-telopeptide and 20% reduction in bone-specific alkaline phosphatase. The GIO treatment study group treated with risedronate 5 mg experienced significant increases in BMD at the lumbar spine and femoral neck compared to baseline. In the GIO prevention study, risedronate 5 mg/day prevented the significant loss in BMD at the hip and lumbar spine seen in the placebo group. The authors reported that risedronate 2.5 mg/day produced similar yet smaller effects on BMD. When both risedronate treatment arms from both studies were combined, the authors found a 19% ARR in morphometric vertebral fractures at one year. However, fracture data were reported for only 96 of the 184 enrolled subjects.
Prior stroke is a risk factor for fracture, through immobility decreasing bone mineral density and by gait instability increasing risk for falls. Sato and colleaguesCitation53 studied 280 men 65 years or older after hemiplegic stroke. The subjects were randomized to risedronate 2.5 mg daily or placebo and followed for 18 months. Urinary deoxypyridinoline concentration decreased significantly compared to baseline and compared to placebo within the first six months of risedronate treatment and remained stable thereafter. The authors assessed BMD at the second metacarpal bones on both hemiplegic and unaffected sides. On the hemiplegic side, metacarpal BMD increased 2.5% in the risedronate-treated and decreased 3.5% in the untreated group. On the unaffected side, metacarpal BMD increased 3.3% in the treated and decreased 2.0% in the unaffected side. Differences between groups were highly significant (p < 0.001). During the study period, 87 men fell a total of 547 times. The risedronate group experienced fewer hip fractures (ARR 5.7%) and overall fractures (ARR 8.6%) though asymptomatic vertebral fractures were not assessed, and no vertebral fractures were reported (See ). The NNT to prevent hip fracture was 16.
Hypogonadism affects approximately 50% men with leprosy due to gonadal atrophy from testicular Mycobacterium leprae infection.Citation54 As a result, osteoporosis is very common in men with leprosy. Among 197 men with leprosy residing at a national leprosarium, Ishikawa and colleaguesCitation55 found the prevalence of osteoporosis to be 33% in men 50–59 aged years and 75% in men aged at least 80 years. Kanaji and colleaguesCitation56 followed 23 elderly men with leprosy randomized to 2.5 mg daily oral risedronate or placebo and found a significant reduction in urinary N-telopeptides and a significant increase in lumbar spine BMD at six and 12 months (See ). Though there was a low incidence of vertebral fractures observed, the treatment group experienced fewer (ARR 1.06%).
Risedronate is approved in the US for the treatment of osteoporosis in men.
Limitations of oral therapy
Because of their relative ease of use and low cost of generic alendronate, oral bisphosphonates have become mainstays of osteoporosis pharamacotherapy. Yet oral dosing is poorly absorbed, may worsen gastrointestinal toxicities, and side effects can decrease patient compliance. Esophageal injury may occur in patients on oral bisphosphonates, though randomized trials have failed to show significant association. Even in patients who strictly adhere to recommended use directions, case reports have shown oral alendronate has the potential to cause erosive esophagitis, esophageal or gastric ulcers and esophageal strictures.Citation57 Recent reports of oral bisphosphonate-associated esophageal carcinoma have emerged.Citation58 A meta-analysis of 76 randomized controlled trials of osteoporosis drug trials found that esophageal ulcerations and complications such as perforation and hemorrhage were reported in trials of all bisphosphonates except zoledronic acid.Citation59 Other gastrointestinal symptoms related to oral bisphosphonates include pyrosis, dyspepsia, abdominal pain, nausea, and vomiting.
Long-term compliance with oral bisphosphonate therapy is low, and patients most commonly cite adverse drug effects as the reason for stopping therapy.Citation60,Citation61 One systematic review of seven observational studies of bisphosphonate compliance as measured by patient surveys indicates that the discontinuation rate at one year of daily dosing ranged 19%–29%. In the study that included men, the rate was 22%. However, when compliance is measured by administrative data, the discontinuation rate was 68% for daily dosing and 56% for weekly dosing. Moreover, 76% patients had at least some interruption in bisphosphonate therapy during the first year of therapy.Citation61 The costs of bisphosphonate noncompliance are high. Patients who are less than 66% compliant with osteoporosis medications have significantly lower increases in BMD.Citation62 In an analysis of insurance claims databases involving 35,537 women prescribed bisphosphonates, only 43% women filled at least 80% of their prescriptions. However, the medication-compliant women had 37% fewer hip fractures at 24 months.Citation63
Oral bisphosphonates are poorly absorbed, and dietary calcium, calcium supplements, and antacids further impede absorption. In order to optimize bioavailability, the patient should take the medication apart from food and all other medications for at least two hours before and 30 minutes after the dose. In order to minimize gastrointestinal side effects, the patient should take the medication with 6–8 ounces of water and remain upright for 30 minutes thereafter. In a review of three observational trials of adherence to dosing instructions, up to 52% patients did not comply with at least one instruction.Citation61 In one of the studies, 26% patients were not taking risedronate correctly and among these patients 28% experienced adverse effects.Citation64
Factors associated with poorer bisphosphonate compliance include older age, male gender, nonwhite race, and greater number of comorbid conditions and nonosteoporosis medications. Compliance is improved with weekly vs daily dosing, and residence in a nursing home.Citation61,Citation65 Though not reported in these trials, compliance with the complex administration instructions is very likely also limited by cognitive dysfunction and physical debility.
Ibandronate
In an uncontrolled open label pilot, Lamy and colleaguesCitation66 enrolled 14 men with primary osteoporosis and either a prior osteoporotic fracture or a BMD at least 1.5 standard deviations below the age-matched mean. Subjects received 2 mg IV ibandronate every three months. At two years, the authors noted significant reductions in osteocalcin and β-crosslaps assay for degradation products of type I collagen. During the two years of treatment, they showed significant increases in BMD at the lumbar spine, femoral neck, and total hip (See ).
Another as yet unpublished trial of 168 men randomized to monthly oral ibandronate 150 mg or placebo has the primary outcome of change in lumbar spine BMD. One year follow-up has been completed, and data will soon be presented (personal communication).
Ringe and colleaguesCitation67 studied 115 subjects with established glucocorticoid induced osteoporosis. The subjects required chronic prednisone use of at least 7.5 mg/day for other systemic illnesses, most commonly COPD, rheumatoid arthritis, or polymyalgia rheumatica. Subjects were randomized to 2 mg intravenous (IV) ibandronate every three months vs. daily oral alfacalcidol. Ibandronate-treated subjects experienced significantly greater increases in BMD at the spine and femoral neck, and improvements were progressive throughout the 36-month study period. The ibandronate group had less lower back pain and less height loss. Though not powered to detect fracture difference, the study did show significantly fewer vertebral fractures in the ibandronate group (relative risk reduction [RRR] 62%) and a nonsignificant trend toward fewer nonvertebral fractures (See ).
Ibandronate is not approved in the US for the treatment of osteoporosis in men.
Zoledronic acid
Currently available IV nitrogen-containing bisphosphonates include pamidronate, ibandronate, and zoledronic acid. Zoledronic acid is the only IV bisphosphonate FDA-approved for the treatment of osteoporosis in men. It has the highest binding affinity to hydroxyapatite and the highest skeletal uptakeCitation68 and is the most potent osteoclast inhibitor. Zoledronic acid is the only bisphosphonate with an indication for annual use.
Evidence of benefit
The HORIZON investigators studied zoledronic acid in secondary prevention of osteoporotic fractures.Citation69 They randomized 2127 subjects (24% men) within 90 days of surgical repair of a hip fracture to receive 5 mg annual IV zoledronic acid or placebo with first dose given within 90days of hip fracture repair. At 36 months, the treated group had significantly increased bone density at the total hip and femoral neck compared to the placebo group. Over 1.9 years median follow-up, they observed significant decreases in any new fracture (139 vs 92, ARR 5.3%), clinical vertebral fracture (39 vs 21, ARR 2.1%), nonvertebral fracture (107vs 79, ARR 3.1%), and recurrent hip fracture (33 vs 23, ARR 1.5%; see ). Further, in this population at high risk for deadly recurrent fractures, the zoledronic acid-treated group experienced a survival advantage (RRR 28%, ARR 3.7%).Citation69 Men enjoyed a greater survival benefit than women (overall mortality ARR 6.4%) and a marked reduction in cardiac-related deaths (RRR 62%, RRR 4.8%).Citation70
Zoledronic acid has been approved in the US for treatment of osteoporosis in men on the basis of an as yet unpublished trial.Citation71 The study randomized 302 hypogonadal men to annual 5 mg IV zoledronic acid or weekly 70 mg oral alendronate. At two years, the zoledronic acid group had 6.1% increase in lumbar spine BMD compared to 6.2% increase in alendronate group. The all-cause mortality and serious adverse events were similar between treatment groups.
Poole and colleaguesCitation72 randomized 27 hemiplegic subjects within seven weeks after stroke zoledronic acid 4mg or placebo. The authors showed that treatment prevented significant bone loss at the hip. Without treatment, at one year the mean BMD of the total hip decreased 5.5% on the affected (hemiplegic) side and by 2.7% in the unaffected hip. With treatment, the mean BMD remained stable on the hemiplegic side and increased by 1% at the unaffected hip (see ). 72% subjects fell, but there were no fractures during the study period.
Human immunodeficiency virus (HIV) infection and antiretroviral therapy are associated with low bone density and osteoporosis. Brown and colleaguesCitation73 found that HIV-infected patients were 3.7 times as likely to have osteoporosis as uninfected individuals. Triant and colleaguesCitation74 reported that HIV-infected men and women have a significantly higher prevalence of hip, vertebral, and wrist fractures. Bolland and colleaguesCitation75 studied zoledronic acid in 43 HIV+ men with decreased bone mineral density receiving antiretroviral therapy. The men were randomized to 4 mg IV annual zoledronic acid or placebo. Urinary N-telopeptide decreased by 61%, and serum total alkaline phosphatase decreased by 21% from baseline by three months in the zoledronic acid-treated group. These values were significantly less than the untreated group, and the levels did not further decrease over the remaining two years in the study. In the zoledronic acid group, BMD increased significantly at the lumbar spine and total hip over the control group (see ).
Studies confirm both clinical efficacyCitation69,Citation72,Citation75 of annual zoledronic acid dosing and noninferiority compared to more frequent dosing,Citation76 but it is not yet known how long a single dose of zoledronic acid can suppress bone turnover. Borba and colleaguesCitation77 showed persistent suppression of C-telopeptide and bone-specific alkaline phosphatase and persistent increase in bone mineral density at the lumbar spine and total hip at 18 months after a single dose of zoledronic acid. In an extension of a previous trial, Bolland and colleaguesCitation78 showed persistence of drug effect in men at two years after a second annual dose of zoledronic acid in suppressing markers of bone turnover and increasing bone mineral density. Brown and colleaguesCitation79 studied 66 subjects with osteopenia after curative cancer treatment. All subjects received a single dose 4 mg zoledronic acid and were followed for 36 months. Data were reported for men and women separately, and both showed durable decreases in urinary NTx/Cr and increases in BMD at the spine and hip after at 36 months after a single zoledronic acid dose. Less frequent dosing regimens offer potential advantages such as reduced toxicity, greater convenience, improved compliance, and reduced cost. Though markers of bone turnover remain suppressed for more than one year, it is not known whether dosing zoledronic acid less than annually is as effective in terms of reduction in fracture risk.
Comparing therapies for osteoporosis
Few studies have made direct comparison of osteoporosis therapies in men. In theory, drugs such as teriparatide that stimulate bone formation may complement bisphosphonates (which inhibit osteoclast action) and provide even greater increases in BMD. Finkelstein and colleaguesCitation34,Citation80 randomized 83 men with low bone mass to receive alendronate, teriparatide, or both daily. Subjects receiving alendronate started treatment at the beginning of the study, and subjects receiving teriparatide started at month 6. Sixty-three men completed the study. At 30 months, the authors found that teriparatide increased BMD at the lumbar spine and femoral neck significantly more than combination therapy, and combination therapy increased BMD at the lumbar spine and femoral neck significantly more than alendronate alone.Citation34 The authors went on to show that marker of bone turnover such as serum N-telopeptide (NTX), osteocalcin, and amino-terminal propeptide of type 1 procollagen increased markedly upon starting teriparatide monotherapy and then declined toward baseline. With alendronate monotherapy, the markers of bone turnover decreased and remained stable. Combination therapy led to an initial decline in bone markers on alendronate alone followed by a rebound with teriparatide so that levels returned to baseline or above. Alendronate pre-treatment blunted the treatment effect of teriparatide,Citation80 and the authors conclude that alendronate impairs the ability of teriparatide to increase bone turnover or increase the BMD at the lumbar spine and the femoral neck in men.
Welch and colleaguesCitation81 retrospectively studied 149 men treated with testosterone, alendronate, or both for at least one year. Men in the testosterone group were referred for treatment of hypogonadism, and men in the alendronate group were referred for treatment of osteoporosis. Yet at baseline, the groups were similar in terms of age, weight, body mass index, and BMD at the lumbar spine and total hip. Compared with the baseline values, lumbar spine BMD increased significantly by 2.1% in the testosterone group, 2.6% in the alendronate group, and 2.5% in the group receiving combination therapy. There were no significant differences in BMD at the lumbar spine or total hip between groups, suggesting that the combination of testosterone and alendronate does not appear to be superior to either drug used alone.
A recently conducted, unpublished trial at our institution directly compares alendronate and zoledronic acid in the treatment of male osteoporosis. The investigators retrospectively reviewed the charts of 64 men: 26 received annual IV zoledronic acid 4 mg and 38 received weekly oral alendronate 70 mg. At one year, they found no differences in BMD at the lumbar spine or femoral neck between groups (personal communication).
Bisphosphonate use in bone loss due to androgen deprivation
Prostate cancer becomes increasingly common as men age, and androgen deprivation therapy (ADT) for prostate cancer places men at uniquely high risk for osteoporosis and fracture. ADT, which may include orchiectomy or GnRH agonist therapy, markedly reduces circulating testosterone to prepubertal levels. In one prospective cohort study of 152 men with nonmetastatic prostate cancer including 30 on acute ADT <6 months and 50 on chronic ADT >6 months, Greenspan and colleaguesCitation82 found that men starting ADT experienced significant reductions in bone mineral density (2.5% total hip, 4.0% spine) by 12 months follow-up. When compared to healthy age-matched controls and to patients with prostate cancer but not on ADT, the acute ADT had five- to tenfold increased bone density loss at multiple skeletal sites. When compared to men on chronic ADT, it appeared that the greatest bone loss occurred in the first 12 months of ADT. Shananian and colleaguesCitation83 measured fracture rates on 50,613 men with the diagnosis of prostate cancer. Among the men that survived five years after diagnosis, they found that 19.4% men on ADT experienced a fracture, as compared to 12.6% those not receiving ADT (p < 0.001). Furthermore, fractures at every site, fractures requiring hospitalization, and diagnosis of osteoporosis were all significantly increased in patients receiving ADT.
Smith and colleaguesCitation84 studied 47 men with advanced or recurrent nonmetastatic prostate cancer. The authors randomized the subjects to receive pamidronate 60 mg IV every 12 weeks plus leuprolide or leuprolide alone. All measured biomarkers of bone turnover; osteocalcin, bone-specific alkaline phosphatase, urinary deoxypyridinoline, and urinary N-telopeptide were significantly reduced in the pamidronate group. Treatment with pamidronate prevented the significant reductions in BMD experienced by the leuprolide-only group. They reported an absolute difference between groups in BMD at the lumbar spine and total hip, but there was no change in either group at the femoral neck (see ).
Table 3 Trials in prostate cancer with ADT
Table 4 Incidence of side effects in zoledronic acid trials reporting safety analyses
Greenspan and colleaguesCitation85 studied 112 men with non-metastatic prostate cancer receiving ADT in a partial-crossover design. Subjects were randomly assigned to receive alendronate 70 mg weekly or placebo at enrollment. At 12 months, all subjects in the placebo group crossed over to the alendronate group, and subjects in the alendronate group were re-randomized to continue alendronate or placebo. At two years, the subjects randomized to alendronate treatment both times had the greatest decrease in N-telopeptide, C-telopeptide, type 1 procollagen, and bone-specific alkaline phosphatase. The alendronate-alendronate group also had the highest BMD at the lumbar spine, total hip, femoral neck, and distal 1/3 radius. Fractures were not studied. The investigators found that a second year of alendronate resulted in continued improvements in bone density and decreases in bone markers.
Delay in alendronate initiation (as in the placebo–alendronate group) resulted in lower bone density and decreased suppression of bone markers compared to early treatment.Citation85 Discontinuation of alendronate (in the alendronate–placebo group) resulted in increases in markers of bone turnover and significantly decreased BMD at all skeletal sites compared to the alendronate–alendronate arm. The authors also performed post-hoc analysis of changes in BMD depending on length of ADT prior to alendronate treatment. They found that men who received ADT for less than 36 months experienced significantly greater increases in BMD on alendronate than men who received ADT for 36 months or more.
Ryan and colleaguesCitation86 studied 120 men with nonmetastatic prostate cancer on ADT for 12 months or less prior to enrollment. Subjects were randomized to zoledronic acid 4 mg IV every three months or placebo and stratified by duration of ADT (less than six months or 6–12 months). Treated subjects had significant reductions in urinary N-telopeptide and serum bone-specific alkaline phosphatase compared to the placebo group and significant improvements in BMD at one year at the femoral neck, total hip, and lumbar spine (see ). The results were not differentiated based on duration of ADT. Taken together, these studiesCitation85,Citation86 support initiation of bisphosphonates within one year of ADT and continued treatment for greater than one year in men on ADT.
Smith and colleaguesCitation87 studied zoledronic acid in 106men with nonmetastatic prostate cancer beginning ADT. Subjects were randomized to zoledronic acid 4 mg IV every three months or placebo. At one year, the placebo group lost 2%–3% BMD at the lumbar spine, femoral neck, and total hip. By comparison, treated group had gains in BMD and showed significantly greater BMD at each skeletal site (see ).
Michaelson and colleaguesCitation88 randomized 40 men with nonmetastatic prostate cancer on ADT and osteoporosis (T < −2.5), to receive a single dose 4 mg IV zoledronic acid or placebo. Subjects who received zoledronic acid had significant reductions in N-telopeptide and bone-specific alkaline phosphatase at 12 months as well as significantly greater BMD at the lumbar spine, total hip and femoral neck (see ).
Ryan and colleaguesCitation89 studied 42 men with hormone-sensitive prostate cancer with and without metastasis on ADT less than one year. The subjects were randomized to zoledronic acid 4 mg IV every three months or placebo. Skeletal sites with metastasis were excluded from DEXA measurement of BMD. At 12 months, subjects treated with zoledronic acid had significantly greater decreases in N-telopeptide and bone-specific alkaline phosphatase. Men with bone metastases had significantly higher baseline N-telopeptide and bone-specific alkaline phosphatase, but the improvements in suppression of these markers of bone turnover was independent of the presence of metastases. Treated subjects also had significantly greater BMD at the lumbar spine and femoral neck (see ).
Raloxifene in ADT
Estrogen deficiency plays a significant role in bone loss in men. Doran and colleaguesCitation90 randomized 50 elderly men without osteoporosis to receive the selective estrogen receptor modulator (SERM) raloxifene 60 mg per day or placebo. At six months, the treated group overall showed no effect in decreasing urinary N-telopeptide (NTx) excretion. However, in the subset of men with baseline low estradiol levels (mean 22 pg/mL), raloxifene treatment decreased urinary NTx. Smith and colleaguesCitation91 found a similar treatment effect in 48 men with nonmetastatic prostate cancer on GnRH agonist therapy treated with raloxifene 60 mg daily. The average estradiol level was 5 pg/mL. At 12 months, the raloxifene-treated group had significantly decreased serum amino-terminal propeptide of type I collagen and significantly increased BMD in the total hip (+1.1% vs −2.6%, p <0.001) and a nonsignificant trend to increased density in the lumbar spine (+1.0% vs −1.0%, p = 0.07).
Raloxifene is not approved in the US for the treatment of osteoporosis in men.
Indications
Zoledronic acid (Reclast) is approved in the US as an annual IV infusion of 5 mg to treat osteoporosis in men or women and to treat Paget’s disease. Zoledronic acid is also marketed as a 4 mg dose (Zometa®) IV infusion every 3–4 weeks to treat hypercalcemia of malignancy, multiple myeloma, and bone metastases from all solid tumors including lung and prostate cancer.
Zoledronic acid is the only IV bisphosphonate approved in the US for the treatment of osteoporosis in men.
Adverse drug effects
Eight trials of zoledronic acid including men for nonmetastatic bone loss have been published,Citation69,Citation72,Citation75,Citation79,Citation86–Citation89 and all eight reported basic safety data such as number of subjects who died or withdrew because of adverse events. Six of these studiesCitation69,Citation72,Citation75,Citation86,Citation87,Citation89 reported more extensive safety analyses. In the largest of these trials,Citation69 there was no association between zoledronic acid and early study withdrawal due to adverse event.
Prior studiesCitation92 have identified five typical infusion-related symptoms that occur in more than 30% subjects receiving a first dose zoledronic acid within three days of drug administration. These symptoms include myalgias, influenza-like symptoms, fever, arthralgias, and headache. The reported rates of these symptoms in the eight trials described are generally lower than in prior studies, though myalgias and fever were significantly more common in the zoledronic acid-treated subjects.Citation69 These symptoms are typically mild, resolve within 3–4 days, and occur less frequently with successive infusions of the drug. Patients should be pretreated with acetominophen.
Many symptoms were variably described among the studies. The frequency of a composite of reports of bone or back pain, myalgias, and arthralgias was 9% among zoledronic acid-treated subjects vs 5% among those receiving placebo. Currently the US FDA is investigating reports of “severe and sometimes incapacitating bone, joint, and/or muscle pains” in chronic use of bisphosphonates as a class.Citation93
Infusing zoledronic acid over at least 15 minutes reduces the risk of renal toxicity. In each of the studies cited, zoledronic acid was given over 15 minutes, and there was no significant difference in rates of rise of serum creatinine >0.5mg/dL. Citation69,Citation72 Zoledronic acid 5 mg dose (Reclast®) is not recommended for patients with creatinine clearance <35 mL/min due to lack of experience in this patient population. Zoledronic acid 4 mg (Zometa®) is recommended at full dose for patients with estimated creatinine clearance >60 mL/min with dose reductions for creatinine clearances between 30–60 mL/min. Because of the lack of clinical data to date, it is not recommended for use in patients with creatinine clearance <30 mL/min. Asymptomatic and generally transient hypocalcemia and hypophosphatemia have been reported independent of baseline 25-OH vitamin D levels.Citation72
Osteonecrosis of the jaw (ONJ) is a serious complication of bisphosphonate use defined as an area of exposed bone in the maxillofacial region that does not heal within eight weeks after identification by a health care provider.Citation94 It appears that potency of the bisphosphonate and duration of exposure to the drug increase the risk of ONJ. Patient factors which increase the risk of ONJ include cancer diagnosis, chemotherapy, glucocorticoid use, tobacco use, and pre-existing dental and periodontal disease. Although the true incidence of ONJ is unknown, recent expert consensusCitation94 estimates 1/10,000 to less than 1/100,000 patient-years in patients receiving oral bisphosphonate treatment for osteoporosis.
In the above eight zoledronic acid trials including 2563 subjects studied over 6960 patient-years, not a single case of ONJ was reported. However, these numbers must be interpreted with caution in that cumulative exposure and duration of follow-up (average 1–2 years) may not be enough to detect ONJ. The incidence of ONJ in patients receiving IV zoledronic acid for treatment of malignancy is much higher, as high as 1%–10%.Citation94 Boonyapakorn and colleaguesCitation95 prospectively studied 80 patients receiving monthly IV zoledronic acid or pamidronate for cancer diagnosis, most commonly multiple myeloma and breast cancer. Using dental radiographs and intraoral examinations, they found that 22 (28%) patients developed ONJ after bisphosphonate exposure. The mean time from exposure to ONJ in the zoledronic acid-treated patients was 26 months. Initiation of bisphosphonate therapy need not be delayed for routine dental work, but patients should be counseled on the importance of dental hygiene and recommended to have regular dental visits for routine care.
Black and colleaguesCitation92 reported an increased incidence of serious atrial fibrillation in women treated with zoledronic acid infusion, and Heckbert and colleaguesCitation96 found greater likelihood of prior alendronate use among 719 women with confirmed new onset atrial fibrillation. In contrast, Lyles and colleaguesCitation69 found no association between zoledronic acid and atrial fibrillation, stroke, myocardial infarction, or death from cardiovascular causes. Sørensen and colleaguesCitation97 retrospectively studied 13,586 Danish women with atrial fibrillation and atrial flutter and found that alendronate and etidronate use was not more common among affected patients than population controls (adjusted relative risk among women with current bisphosphonate use 0.95). US FDA review of 19,687 bisphosphonate-treated patients found no association with atrial fibrillation.Citation98 Whether bisphosphonate use predisposes a patient to atrial fibrillation remains an open question. However, this small potential risk must be considered in relation to potentially large benefits in terms of fracture reduction and survival.
Summary
Osteoporosis and low bone mass are under-recognized and undertreated in men. As the population ages, the rates of osteoporosis and osteoporotic fractures are expected to rise. Men with prior fragility fractures, older men, and men with lower BMD are at highest risk of fracture. Men with glucocorticoid use, hypogonadism, tobacco or heavy alcohol intake, vitamin D deficiency, or androgen deprivation therapy for prostate cancer are also at increased risk for osteoporosis. Laboratory evaluation for secondary causes of osteoporosis and radiographic screening for occult vertebral fractures in men with height loss are essential parts of the workup of men with osteoporosis. All men should receive adequate dietary calcium and vitamin D. Bisphosphonates are potent antiresorptive agents that inhibit osteoclast activity, suppress in vivo markers of bone turnover, and decrease fracture risk in patients at high risk of fracture. Intravenous zoledronic acid may be a preferable alternative to oral bisphosphonate therapy in patients with cognitive dysfunction, the inability to sit upright, or significant gastrointestinal pathology.
Disclosure
The authors report no conflicts of interest in this work.
References
- NIH Consensus Development Panel on Osteoporosis Prevention, Detection, and TherapyOsteoporosis prevention, diagnosis, and therapyJAMA200128578579511176917
- National Osteoporosis Foundation Advocacy News and UpdatesThe state of osteoporosis and low bone mass in the US. 2009 Cited 2009 Feb 18. Available from: http://www.nof.org/advocacy/prevalence/index.htm
- JonesGNguyenTSambrookPNKellyPJGilbertCEismanJASymptomatic fracture incidence in elderly men and women: the Dubbo Osteoporosis Epidemiology Study (DOES)Osteoporosis Int19944277282
- WinnerSJMorganCAEvansJGPerimenopausal risk of falling and incidence of distal forearm fractureBMJ19892986686148614882503081
- Office of the Surgeon GeneralThe burden of bone diseaseBone health and osteoporosis: A report of the Surgeon General2004 Cited 2009 Jan 31. Available from: http://www.surgeongeneral.gov/library/bonehealth/chapter_5.html
- LeibsonCLTostesonANGabrielSERansomJEMeltonLJMortality, disability, and nursing home use for persons with and without hip fracture: a population-based studyJ Am Geriatr Soc200250101644165012366617
- CenterJRNguyenTVSchneiderDMortality after all major types of osteoporotic fractures in men and women: an observational studyLancet199976235242
- MagazinerJFredmanLHawkesWChanges in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling agedAm J Epidemiol2003157111023103112777366
- ChrischillesEAButlerCDDavisCSWallaceRBA model of lifetime osteoporosis impactArch Intern Med199115110202620321929691
- TostesonANOrwollESEconomic impact of fracturesOsteoporosis in men: The effects of gender on skeletal healthSan DiegoAcademic Press19991527
- HasseriusRKarlssonMKJonssonBLong-term morbidity and mortality after a clinically diagnosed vertebral fracture in the elderly: a 12- and 22-year follow-up of 257 patientsCalcif Tissue Int20057623524215812579
- LewisCEEwingSKTaylorBCfor the Osteoporotic Fractures in Men (MrOS) Study Research GroupPredictors of non-spine fracture in elderly men: the MrOS studyJ Bone Miner Res200722221121917059373
- NguyenTVEismanJAKellyPARisk factors for osteoporotic fractures in elderly menAm J Epidemiol19961442552638686694
- GruntmanisUMale osteoporosis: deadly, but ignoredAm J Med Sci20073332859217301586
- KelepourisNHarperKDGannonFKaplanFSHaddadJGSevere osteoporosis in menAnn Intern Med199512364524607639446
- OrwollESKleinRFOsteoporosis in menEndocr Rev1995161871167758434
- World Health Organization Collaborating Centre for Metabolic Bone DiseasesFRAX® WHO Fracture Risk Assessment ToolSheffield, UKWorld Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK2009 Cited 2009 Feb 13. Available from: http://www.shef.ac.uk/FRAX/index.htm
- KhoslaSAminSOrwollEOsteoporosis in menEndocr Rev200829444146418451258
- EbelingPROsteoporosis in menN Engl J Med20083581474148218385499
- SherringtonCWhitneyJCLordSRHerbertRDCummingRGCloseJCEffective exercise for the prevention of falls: a systematic review and meta-analysisJ Am Geriatr Soc200856122234224319093923
- Martyn-St JamesMCarrollSHigh-intensity resistance training and postmenopausal bone loss: a meta-analysisOsteoporos Int20061781225124016823548
- LarsenERMosekildeLFoldspangAVitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3-year intervention studyJ Bone Miner Res200419337037815040824
- TrivediDPDollRKhawKTEffect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trialBMJ2003326738746912609940
- Dawson-HughesBHarrisSSKrallEADallalGEEffect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or olderN Engl J Med1997337106706769278463
- GrantAMAvenellACampbellMKfor RECORD Trial GroupOral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trialLancet200536594711621162815885294
- National Osteoporosis FoundationClinician’s guide to prevention and treatment of osteoporosis2009 Cited 2009 Feb 18. Available from: http://www.nof.org/professionals/cliniciansguide_form.asp
- TrovasGPLyritisGPGalanosARaptouPConstantelouEA randomized trial of nasal spray salmon calcitonin in men with idiopathic osteoporosis: effects on bone mineral density and bone markersJ Bone Miner Res200217352152711874243
- TothECsuporEMeszarosSThe effect of intranasal salmon calcitonin therapy on bone mineral density in idiopathic male osteoporosis without vertebral fractures – an open label studyBone2005361475115664001
- WangCCunninghamGDobsALong-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal menJ Clin Endocrinol Metab20048952085209815126525
- SnyderPJPeacheyHBerlinJAEffects of testosterone replacement in hypogonadal menJ Clin Endocrinol Metab20008582670267710946864
- SnyderPJPeacheyHBerlinJAEffect of testosterone treatment on bone mineral density in men over 65 years of ageJ Clin Endocrinol Metab19998461966197210372695
- KatznelsonLFinkelsteinJSSchoenfeldDARosenthalDIAndersonEJKlibanskiAIncrease in bone density and lean body mass during testosterone administration in men with acquired hypogonadismJ Clin Endocrinol Metab19968112435843658954042
- AndersonFHFrancisRMPeastonRTWastellHJAndrogen supplementation in eugonadal men with osteoporosis: effects of six months’ treatment on markers of bone formation and resorptionJ Bone Miner Res19971234724789076591
- FinkelsteinJSHayesAHunzelmanJLWylandJJLeeHNeerRMThe effects of parathyroid hormone, alendronate, or both in men with osteoporosisN Engl J Med20033491216122614500805
- SaagKGShaneEBoonenSTeriparatide or alendronate in glucocorticoid-induced osteoporosisN Engl J Med20073572028203918003959
- RussellRGBisphosphonates: from bench to bedsideAnn NY Acad Sci2006106836740116831938
- DunfordJEThompsonKCoxonFPStructure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonatesJ Pharmacol Exp Ther2001296223524211160603
- SmithMRBisphosphonates to prevent osteoporosis in men receiving androgen deprivation therapy for prostate cancerDrugs Aging200320317518312578398
- DrakeMTClarkeBLKhoslaSBisphosphonates: mechanism of action and role in clinical practiceMayo Clin Proc20088391032104518775204
- ReidIRBisphosphonates: new indications and methods of administrationCurr Opin Rheumatol200315445846312819475
- HallARho GTPases and the actin cytoskeletonScience199827953505095149438836
- WeinsteinRSRobersonPKManolagasSCGiant osteoclast formation and long-term oral bisphosphonate therapyN Engl J Med2009360536219118304
- RyanPJBlakeGMDavieMHaddawayMGibsonTFogelmanIIntermittent oral disodium pamidronate in established osteoporosis: a 2 year double-masked placebo-controlled study of efficacy and safetyOsteoporos Int200011217117610793877
- BrumsenCPapapoulosSELipsPDaily oral pamidronate in women and men with osteoporosis: a 3-year randomized placebo-controlled clinical trial with a 2-year open extensionJ Bone Miner Res20021761057106412054161
- BoutsenYJamartJEsselinckxWDevogelaerJPPrimary prevention of glucocorticoid-induced osteoporosis with intravenous pamidronate and calcium: a prospective controlled 1-year study comparing a single infusion, an infusion given once every 3 months, and calcium aloneJ Bone Miner Res200116110411211149473
- HoYVFraumanAGThomsonWSeemanEEffects of alendronate on bone density in men with primary and secondary osteoporosisOsteoporos Int20001129810110793867
- OrwollEEttingerMWeissSAlendronate for the treatment of osteoporosis in menN Engl J Med200034360461010979796
- RingeJDDorstAFaberHIbachKAlendronate treatment of established primary osteoporosis in men: 2-year results of a prospective, comparative, two-arm studyRheumatol Int20042411011313680141
- SawkaAMPapaioannouAAdachiJDGafniAHanleyDAThabaneLDoes alendronate reduce the risk of fracture in men? A meta-analysis incorporating prior knowledge of anti-fracture efficacy in womenBMC Musculoskelet Disord200563916008835
- RingeJDFaberHFarahmandPDorstAEfficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year studyRheumatol Int20062642743116001181
- BoonenSOrwollESWenderothDStonerKJEusebioRDelmasPDOnce-weekly risedronate in men with osteoporosis: results of a 2-year, placebo-controlled, double-blind, multicenter studyJ Bone Miner Res20092471972519049326
- ReidDMAdamiSDevogelaerJ-PChinesAARisedronate increases done density and reduces vertebral fracture risk within one year in men on corticosteroid therapyCalcif Tissue Int20016924224711730260
- SatoYIwamotoJKanokoTSatohKRisedronate sodium therapy for prevention of hip fracture in men 65 years or older after strokeArch Intern Med20051651743174816087822
- LealAMFossNTEndocrine dysfunction in leprosyEur J Clin Microbiol Infect Dis2009281718629555
- IshikawaSIshikawaAYohKTanakaHFujiwaraMOsteoporosis in male and female leprosy patientsCalcif Tissue Int1999641441479914322
- KanajiAHigashiMNamisatoMNishioMAndoKYamadaHEffects of risedronate on lumbar bone mineral density, bone resorption, and incidence of vertebral fracture in elderly male patients with leprosyLepr Rev20067714715316895071
- ParfittJRDrimanDKPathological effects of drugs on the gastrointestinal tract: a reviewHum Pathol20073852753617367604
- WysowskiDKReports of esophageal cancer with oral bisphosphonate useN Engl J Med20093601899019118315
- MacLeanCNewberrySMaglioneMSystematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosisAnn Intern Med200814819721318087050
- TostesonANGroveMRHammondCSEarly discontinuation of treatment for osteoporosisAm J Med2003115320921612947959
- PapaioannouAKennedyCCDolovichLLauEAdachiJDPatient adherence to osteoporosis medications: problems, consequences and management strategiesDrugs Aging2007241375517233546
- YoodRAEmaniSReedJILewisBECharpentierMLydickECompliance with pharmacologic therapy for osteoporosisOsteoporos Int20031496596814504697
- SirisESHarrisSTRosenCJAdherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databasesMayo Clin Proc20068181013102216901023
- HamiltonBMcCoyKTaggartHTolerability and compliance with risedronate in clinical practiceOsteoporos Int20031425926212730745
- SolomonDHAvornJKatzJNCompliance with osteoporosis medicationsArch Intern Med20051652414241916287772
- LamyOSandiniLPacheIFatioSBurnandJBurckhardtPIntravenous ibandronate in men with osteoporosis: an open pilot study over 2 yearsJ Endocrinol Invest200326872873214669826
- RingeJDDorstAFaberHIbachKSorensonFIntermittent intravenous ibandronate injections reduce vertebral fracture risk in corticosteroid-induced osteoporosis: results from a long-term comparative studyOsteoporos Int20031480180714610641
- NancollasGHTangRPhippsRJNovel insights into actions of bisphosphonates on bone: differences in interactions with hydroxy-apatiteBone20063861762716046206
- LylesKWColón-EmericCSMagazinerJSfor the HORI -ZON Recurrent Fracture TrialZoledronic acid and clinical fractures and mortality after hip fractureN Engl J Med20073571799180917878149
- Colón-EmericCMesenbrinkPLylesKPotential mediators of the reduction in mortality with zoledronic acid after hip fracture [abstract]Presented at the American Society for Bone Mineral Research meetingSept 13, 2008
- Novartis PharmaceuticalsReclast prescribing information32009 Cited 2009 May 18. Available from: http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=8476
- PooleKELoveridgeNRoseCMWarburtonEAReeveJA single infusion of zoledronate prevents bone loss after strokeStroke2007381519152517395868
- BrownTTQaqishRBAntiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic reviewAIDS200620172165217417086056
- TriantVABrownTTLeeHGrinspoonSKFracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare systemJ Clin Endocrinol Metab20089393499350418593764
- BollandMJGreyABHorneAMAnnual zoledronate increases bone density in highly active antiretroviral therapy-treated human immunodeficiency virus-infected men: a randomized controlled trialJ Clin Endocrinol Metab2007921283128817227801
- ReidIRBrownJPBurckhardtPIntravenous zoledronic acid in postmenopausal women with low bone mineral densityN Engl J Med200234665366111870242
- BorbaVZPaz-FilhoGKulakCASeibelMJBilezikianJPBone turnover 18 months after a single intravenous dose of zoledronic acidInt J Clin Pract20076161058106217504370
- BollandMJGreyABHorneAMEffects of intravenous zoledronate on bone turnover and BMD persist for at least 24 monthsJ Bone Miner Res2008231304130818627266
- BrownJEEllisSPLesterJEProlonged efficacy of a single dose of the bisphosphonate zoledronic acidClin Cancer Res200713185406541017875770
- FinkelsteinJSLederBZBurnettSMEffects of teriparatide, alendronate, or both on bone turnover in osteoporotic menJ Clin Endocrinol Metab20069182882288716684825
- WelchBJDenkeMAKermaniAAdams-HuetBGazmenNMGruntmanisUComparison of testosterone, alendronate, and a combination of both therapies in men with low bone mineral densityJ Invest Med2007554168173
- GreenspanSLCoatesPSereikaSMNelsonJBTrumpDLResnickNMBone loss after initiation of androgen deprivation therapy in patients with prostate cancerJ Clin Endocrinol Metab2005906410641716189261
- ShahinianVBKuoY-FFreemanJLGoodwinJSRisk of fracture after androgen-deprivation for prostate cancerN Engl J Med200535215416415647578
- SmithMRMcGovernFJZietmanALPamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancerN Engl J Med200134594895511575286
- GreenspanSLNelsonJBTrumpDLSkeletal health after continuation, withdrawal, or delay of alendronate in men with prostate cancer undergoing androgen-deprivation therapyJ Clin Oncol2008264426443418802155
- RyanCWHuoDDemersLMBeerTMLacernaLVfor the Zometa US05 InvestigatorsZoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancerJ Urol200617697297816890673
- SmithMREasthamJGleasonDMShashaDTchekmedyianSZinnerNRandomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancerJ Urol20031692008201212771706
- MichaelsonMDKaufmanDSLeeHRandomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist–induced bone loss in men with prostate cancerJ Clin Oncol2007251038104217369566
- RyanCWHuoDBylowKSuppression of bone density loss and bone turnover in patients with hormone-sensitive prostate cancer and receiving zoledronic acidBJU Int2007100707517552955
- DoranPMRiggsBLAtkinsonEJKhoslaSEffects of raloxifene, a selective estrogen receptor modulator, on bone turnover markers and serum sex steroid and lipid levels in elderly menJ Bone Miner Res200116112118212511697809
- SmithMRFallonMALeeHFinkelsteinJSRaloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trialJ Clin Endocrinol Metab2004893841384615292315
- BlackDMDelmasPDEastellRfor the HORIZON Pivotal Fracture TrialFracture trial once-yearly zoledronic acid for treatment of postmenopausal osteoporosisN Engl J Med20073561809182217476007
- FDA MedwatchBisphosphonates (marketed as Actonel, Actonel+Ca, Aredia, Boniva, Didronel, Fosamax, Fosamax+D, Reclast, Skelid, and Zometa)Washington DCUS Food and Drug Administration Updated 2008 Jan 7. Cited 2008 Dec 12. Available from: http://www.fda.gov/medwatch/safety/2008/safety08.htm#Bisphosphonates
- KhoslaSBurrDCauleyJBisphosphonate-associated osteone-crosis of the jaw: report of a task force of the american society for bone and mineral researchJ Bone Miner Res2007221479149117663640
- BoonyapakornTSchirmerIReichartPASturmIMassenkeilGBisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignanciesOral Oncol200844985786918282788
- HeckbertSRLiGCummingsSRSmithNLPsatyBMUse of alen-dronate and risk of incident atrial fibrillation in womenArch Intern Med2008168882683118443257
- S⊘rensenHTChristensenSMehnertFUse of bisphosphonates among women and risk of atrial fibrillation and flutter: population based case-control studyBMJ200833681381618334527
- US Food and Drug AdministrationUpdate of Safety Review Follow-up to the October 1, 2007 Early Communication about the Ongoing Safety Review of Bisphosphonates Washington DC: US Food and Drug Administration Updated 2008 Nov 12. Cited 2009 Apr 22. Available from: http://www.fda.gov/CDER/drug/early_comm/bisphosphonates_update_200811.htm