Abstract
Context
The cognitive side effects of medications with anticholinergic activity have been documented among older adults in a variety of clinical settings. However, there has been no systematic confirmation that acute or chronic prescribing of such medications lead to transient or permanent adverse cognitive outcomes.
Objective
Evaluate the existing evidence regarding the effects of anticholinergic medications on cognition in older adults.
Data sources
We searched the MEDLINE, OVID, and CINAHL databases from January, 1966 to January, 2008 for eligible studies.
Study selection
Studies were included if the anticholinergic activity was systematically measured and correlated with standard measurements of cognitive performance. Studies were excluded if they reported case studies, case series, editorials, and review articles.
Data extraction
We extracted the method used to determine anticholinergic activity of medications and its association with cognitive outcomes.
Results
Twenty-seven studies met our inclusion criteria. Serum anticholinergic assay was the main method used to determine anticholinergic activity. All but two studies found an association between the anticholinergic activity of medications and either delirium, cognitive impairment or dementia.
Conclusions
Medications with anticholinergic activity negatively affect the cognitive performance of older adults. Recognizing the anticholinergic activity of certain medications may represent a potential tool to improve cognition.
Clinical scenario
A 78-year-old Caucasian female presents to the emergency department (ED) with a chief complaint of shortness of breath, lethargy, and confusion. She was transported to the ED by her neighbor who assists with the history due to the patient’s current state of confusion. The patient lives alone in her own apartment in an independent senior living facility and has noted a decreased ability to complete her daily activities due to her shortness of breath and fatigue. Her past medical history is positive for hypertension, urinary incontinence, chronic obstructive pulmonary disease, gastroesophageal reflux disease, and atrial fibrillation. Her home medications include: ranitidine 150 mg by mouth twice daily, atenolol 50 mg by mouth daily, ipratropium inhaler 1–2 inhalations by mouth four times daily as needed, digoxin 0.125 mg by mouth daily, warfarin 3 mg by mouth daily, calcium carbonate/vitamin D 500 mg/200 IU by mouth twice daily, and Tyelonol PM® 500 mg/25 mg by mouth as needed for sleep. She is admitted for chronic obstructive pulmonary disease (COPD) exacerbation and to rule out myocardial infarction. Her cognitive testing on admission reveals a Mini-Mental Status Examination (MMSE) score of 19/30 with deficits in orientation, attention, and recall. She scores positive on a Confusion Assessment Method (CAM) evaluation due to an acute change in mental status, disorganized thinking, and fluctuating attention.
Introduction
In 2005, there were more than 36 million Americans aged 65 and older.Citation1 This population is known to suffer from multiple chronic diseases, require numerous prescribed and over-the-counter medications, and is at a higher risk of developing dementia.Citation2–Citation4 It is estimated that 20%–50% of the same cohort, including the four million with dementia, took at least one medication with some anticholinergic activities.Citation3,Citation5–Citation8
The use of drugs with anticholinergic activity has been an integral part of the routine treatment of common conditions such as asthma, urinary incontinence, and various psychiatric disorders. However, the adverse effects of these anticholinergics have been known for a long time including peripheral effects such as dry mouth and constipation, and central nervous system effects such as attention deficits and hallucinations.Citation9 The central nervous system of older patients is very sensitive to the above adverse anticholinergic effects due to the significant decrease in cholinergic neurons or receptors in the brain of older adults, the reduction in hepatic metabolism and renal excretion of medications, and the increase in blood–brain barrier permeability.Citation9
Many clinical researchers have recognized the importance of accounting for the risk of medications with central nervous system anticholinergic effects in the medical care of older patients, especially those with pre-existing cognitive disorders.Citation3,Citation9 However, there has been no systematic confirmation that acute or chronic prescribing of such medications leads to transient or permanent adverse cognitive outcomes. Thus, we conducted this systematic review of the literature to identify the various methods used to determine the central anticholinergic effects of various medications and evaluate the impact of such activities on cognitive function of older adults.
Methods
Search strategies and study selection
We searched the MEDLINE database using the search terms “cholinergic antagonists” combined with “delirium, dementia, amnestic, cognitive disorders.” We limited our search to the English language and human studies published between January 1966 and January 2008. In order to identify pertinent studies, we scanned titles and abstracts from each retrieved citation. Publications that appeared to be irrelevant on the basis of the study population and methods as indicated in the title and abstract were discarded. We were also able to retrieve additional pertinent publications using the reference lists from identified articles.
Inclusion criteria
We included all cross-sectional, case control, and retrospective or prospective observational cohort studies that evaluated the anticholinergic activity of medications and their impact on the cognitive function of older adults. We excluded case study, case series, editorial, and review articles.
Data extraction and synthesis
We extracted data from each study that met our inclusion criteria into a pre-defined table that included: citation, anticholinergic activity measurement method, association between anticholinergics and cognitive impairment, total number of patients, and baseline demographics (eg, age, gender). Each article was critically evaluated in six categories for internal validity. Articles were evaluated on the parameters of the type of data included, adjustments for confounders, attrition rates, use of standardized cognitive assessment measures, and the presence of selection and recall bias. Each parameter evaluating internal validity was awarded a score of “1” if the study sufficiently met appropriate criteria and “0” if criteria were not met. Critical appraisal scores were then tabulated and correlated with a rating of “poor” if the appraisal score was 0–2; “fair” if the appraisal score was 3–4; and “good” if the score was 5–6. The critical appraisal was carried out by three clinical researchers (NC, MB, TL).
Results
Our search strategies revealed 258 potential articles from MEDLINE. However, after scanning the titles and the abstracts, we excluded 217 studies because they did not meet our inclusion criteria. An additional 20 of the remaining 41 articles were excluded because they were reviews, case reports, or case series. From the reference lists of the identified articles we were able to find six additional pertinent publications (see Figure ).
In total, we found 27 studies that have investigated the cognitive burden of drugs with anticholinergic properties (see Table ). All but seven studies were conducted among a heterogeneous group of participants in the United States. The remaining non-US studies were conducted among French, Canadian, German, and Portuguese patients, but published in the English language. The majority of the studies included in this review took place in a hospitalized or nursing home population (n = 18), with the remaining studies (n = 9) evaluating ambulatory patients. Of the 27 studies, 13 were designed as cross-sectional, six studies were case-control, and eight were prospective or retrospective cohort studies.
Table 1 Clinical studies evaluating anticholinergic activity
The anticholinergic activity of medications was evaluated using the serum anticholinergic activities assay (SAA) or the practitioner’s knowledge of a list of drugs with known anticholinergic effects (see Table ). SAA was the main method to determine anticholinergic activity in 17 studies. SAA is usually measured using a radio receptor competitive binding assay developed by Tune and Coyle.Citation10 The SAA estimates anticholinergic activity generated not only from medications, but also from endogenous factors as a result of stress or hyperthermia.Citation11 The other remaining studies combined clinical knowledge with drug lists to determine the anticholinergic activity of certain medications.
Table 2 Association between serum anticholinergic activities and cognition
Acute anticholinergic effect on cognition
Delirium (acute and severe cognitive impairment) was diagnosed clinically using the DSM-IV criteria or using their derivatives, such as the Confusion Assessment Method (CAM), or its counterpart validated in the critically-ill population, the CAM-ICU. The CAM or CAM-ICU is a highly sensitive and specific method that evaluates subjects for the presence of four items: acute onset of cognitive changes fluctuating in course, inattention, disorganized thinking, and altered level of consciousness.Citation36 The presence of the first two items and any of the third or fourth items determines the diagnosis of delirium. Other tools evaluating concentration, wakefulness, orientation, perception, and sleep disturbances (Saskatoon Delirium Checklist [SDC]) were used to evaluate delirium. The SDCCitation11 and Delirium Symptoms Interview (DSI)Citation37 were both validated tools developed from the DSM criteria to measure cognitive deficits.
Thirteen studies evaluated the impact of anticholinergic on delirium, with eleven studies identifying a positive association between the use of such medicines and delirium. Delirium episodes experienced by study participants occurred at any point in the observation period(commonly the duration of inpatient admission). Few studies evaluated sequential blood samples to measure changes in SAA over time. Nearly 70% of the studies included in our analysis used SAA as the method for evaluating anticholinergic activity and 70% used either CAM or DSM criteria for evaluating delirium.
A recent study included in the analysis reported by Plaschke and colleagues evaluated a critically-ill population for the correlation of SAA or electroencephalography (EEG) with delirium.Citation32 The authors report that a higher SAA value was identified in the delirious cohort, though this did not correlate with a difference in the risk of developing delirium in the ICU. Additionally, an article published in 1994 by Marcantonio and colleagues failed to draw a correlation between anticholinergic and postoperative delirium risk, though the anticholinergic exposure was only documented in 9% of the study population.Citation24
Chronic anticholinergic effect on cognition
Chronic cognitive deficit was defined as mild cognitive impairment, worsening dementia or new diagnosis of dementia, or global decline in cognition not caused by delirium. Cognitive performance was evaluated using the MMSE in most studies included in this review and found an association between the use of anticholinergic and cognitive performance as determined by the MMSE (Table ). This screening tool for cognition evaluates different areas of cognition such as orientation, memory, recall, attention, and language. Any score above 24, out of a possible score of 30, is considered normal, while a score below 24 suggests a cognitive impairment.Citation38 A modified version of the MMSE, the telephone interview for cognitive status (TICS_, has also been also used. The TICS is as reliable and valid as the MMSE.Citation39
Table 3 Association between anticholinergic activity assessed by expert-based drug list and cognition
Few studies evaluated for any long-term (>12 months) impact on cognitive function in patients exposed to anticholinergic. Ancelin and colleaguesCitation12 provided one of the few studies evaluating the impact of anticholinergic over time. This study included a French population without baseline cognitive deficits and found an increased risk of mild cognitive impairment at the one-year follow-up based on criteria established by the Stockholm consensus group. However, at eight years of follow-up the authors did not find an increased risk in the diagnosis of dementia (DSM-III) between consistent users of anticholinergic and nonusers. Another study by Lu and colleaguesCitation22 revealed no impact of anticholinergic exposure on cognition among a group of patients with baseline cognitive impairment at one year, though a significant decrease in cognitive function at two years was noticed in those using anticholinergics.
Clinical interpretation of data synthesis
Our systematic evidence review of 27 studies found a consistent association between the use of anticholinergic and cognitive impairment in older adults, including delirium. Our findings were similar to a review of 80 studies that was conducted by Dyer and colleaguesCitation40 that found a positive association between the use of anticholinergic drugs and postoperative delirium. Furthermore, Tune and colleagues also correlated delirium and confusional states in demented patients as a result of anticholinergic activities.Citation10 The authors noted that this adverse effect may not arise exclusively from an individual agent with strong anticholinergic effects, but as an accumulation of multiple medications with varying degrees of anticholinergic effects. Similarly, the anticholinergic effects seem not to be related to the dosage of each individual drug, identifying the role of other factors in the development of cognitive deficits.Citation41 The presence of multiple baseline risk factors as well as the role of multiple neurotransmitter systems in the development of d elirium or cognitive impairment has been previously described.Citation42
The finding of this systematic review indicates the burden of anticholinergic has consistently been shown to negatively associate with cognitive performance. All but two studiesCitation31 included in this review support the association of anticholinergic use and worsening cognitive performance either through an acute (delirium) or chronic (mild cognitive impairment) impact. The long-term effect of anticholinergics on cognition requires further analysis, as few studies adequately quantified exposure to anticholinergics and correlated this exposure to long-term risks of developing a neurodegenerative dementing disorder such as Alzheimer disease.
The studies included in this evaluation draw a consistent correlation between SAA and worsening performance on cognitive testing. Throughout the studies evaluated in this review, investigators discovered minimal changes in global measures of cognitive function with exposure to anticholinergics, and instead identified deficits in processing speed, psychomotor performance, concentration/attention, problem solving and language skills. Delirium was frequently identified by disorientation, altered consciousness, disorganized thinking, and fluctuating alertness. Variable deficits in recall were identified, with some articles describing deficits in verbal or narrative recall, with others identifying no change in recall abilities. The significance of this comparison is that in a clinical setting many practitioners rely on global measures to evaluate cognitive performance and therefore may not accurately identify a decline in cognitive function when evaluating exposure to anticholinergics.
Most studies identified in our review have used the in vitro SAA, while few studies have used drug lists coupled with clinical judgment.Citation6 All of these different methods have limitations, such as the inability to assess anticholinergic effects of each individual drug or to determine their potential synergistic effects when combined. Although higher levels of SAA has been correlated with poor cognitive function in several previous studies, conflicting data exists that makes interpretation of the absolute SAA value difficult. Similarly, the complexity of the testing procedure, along with the intermittent availability, limits the widespread acceptance as a biomarker or predictor of delirium. Many existing medication scales compute a total score of different drugs to determine the anticholinergic burden, suggesting that special attention should be paid not only to individual drug score, but also to the accumulated anticholinergic effects of all medications taken by the patient. Carnahan and colleaguesCitation41 have established a tool, the anticholinergic drug scale (ADS), divided in an ordinal fashion from 0 to 3, with 0 signifying no known anticholinergic activity, and 3 signifying marked anticholinergic activity. They found that ADS total scores were significantly associated with SAA. However, Thomas and colleagues failed to prove a correlation between SAA and a clinical diagnosis of delirium in older patients (>age 80 years) with acute illness.Citation43 Their results suggest the SAA is limited to peripheral activity, not central anticholinergic effects that may impact cognition.
The main limitation of this review is in the selection of studies with different designs and settings that contribute to the common conclusion. This review also bears limitations inherent to each study design, whether it is a cross-sectional, a case-control or a longitudinal study. Given the heterogeneity in the included studies and populations, baseline confounders such as cognitive impairment, past medical history and reason for admissions could not be evaluated and may impact results. The heterogeneity in study populations of the included studies may have significantly affected results. It is well documented that endogenous cholinergic neurotransmitter shifts may impact cognition,Citation44,Citation45 as well as normal responses to stress that may play a role in cognitive testing. Similarly, reporting mechanisms for the medications evaluated in the included studies was inconsistent, making the generation of a comprehensive, clinically useful list of anticholinergic medications impractical from this data set. Although central anticholinergic activity is most relevant in the development of delirium, cognitive impairment, or dementia, no study stratified medications by peripheral or central activity.
Through our search strategy, it is possible to miss a small number of studies that are unpublished. All but two studies assessed either acute cognitive impairment or delirium, with few studies measuring long-term effects on cognition. Moreover, methods used to diagnose delirium were not consistent throughout the included studies. The diagnostic criteria for delirium have evolved over time; thus the reviewed studies present a variety of methods to measure the diagnosis. Despite these limitations, this study is the first comprehensive review of the measurement and cognitive impact of anticholinergic medications.
Gaps in the literature
Despite the associations that have been previously described regarding the impact of anticholinergics on cognitive function, several gaps in the existing literature can still be identified. First, our existing literature support for the association of anticholinergics and cognitive impairment lacks randomized, prospective clinical trials that describe a presumed difference in relevant outcomes. It remains to be sufficiently determined what outcomes should be expected if a reduction in anticholinergic activity is achieved in various populations with and without cognitive impairment. One might speculate that frequency and severity of acute or chronic mental status changes be reduced when the burden of anticholinergic medications is reduced, though the extent or duration of exposure minimization required to achieve a clinically significant impact remains to be delineated.
Secondly, the correlation of exposure to anticholinergics and health-related outcomes remains to be described. Many literature sources included in this review have drawn associations between anticholinergics use and cognitive impairment; however, no data source has evaluated an impact on hospitalization rates, emergency department visits, duration of hospital stay, overall quality of life, or even mortality.
Finally, long-term exposure to medications with anticholinergic activity was not evaluated in a majority of the studies. The impact of anticholinergics on cognitive impairment was often limited to short-term exposure, with limited assessment of medication regimens or SAA values. The impact of long-term exposure to medications with anticholinergic medications remains to be sufficiently examined, as in the PAQUID study of community-dwelling elders in southern France.Citation21 Similarly, a recent study by Boustani and colleagues suggests an increased risk of incident cognitive impairment in African-Americans consistently using H-2 receptor antagonists over a five-year observation period.Citation46 This warrants further study into the impact of chronic exposure to anticholinergic medications, the potential for this exposure to influence cognition over time, and the extent of exposure required to induce adverse cognitive outcomes.
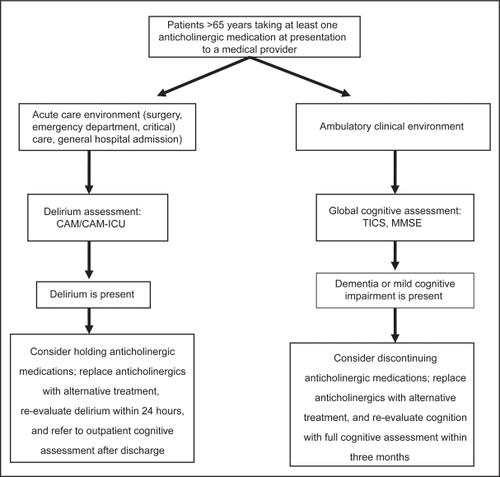
We suggest the logical management of anticholinergics use as described in Figure . As illustrated in this review, the elderly population, and specifically those experiencing an acute illness, is uniquely sensitive to the central anticholinergic adverse effects of medications and should be closely monitored for the development of unwanted adverse effects on cognition. Recognizing patients at risk due to exposure of anticholinergics should warrant cognitive evaluation not only in acute care environments, but also ambulatory environments when subjective complaints of cognitive impairment supplement clinical suspicion. In clinical practice situations where anticholinergic cognitive adverse effects are suspected, the course of action might be to consider the withdrawal of potentially offending medication(s). Although the expected clinical impact on cognitive deficits with a reduction in anticholinergic burden remains to be sufficiently studied, removal of potentially harmful medications in lieu of equally effective alternatives with lower anticholinergic activity might be a good practice.
Scenario resolution
During the hospitalization, the patient received a geriatrics consultation and her anticholinergics burden was reduced by discontinuing ranitidine, oxybutynin, diphenhydramine, and digoxin. Ranitidine was replaced with esomeprazole, Tylenol PM® was replaced with acetaminophen as needed, and no replacements were instituted for oxybutynin and digoxin. Other medications with notable systemic anticholinergic properties, warfarin and atenolol, were continued during the hospital stay and at discharge. The patient’s delirium resolved within 48 hours of hospital admission due to either resolving medical illness or a reduction of anticholinergic burden. Repeat MMSE was not performed, though follow-up for further cognitive testing was arranged within four weeks after discharge, where mild cognitive impairment was identified and the patient continues to follow in the geriatric clinic for appropriate monitoring of cognitive function.
Conclusion
In a world facing an exponential growth of older patients, high prevalence of multiple chronic disease and substantial use of numerous medications, the integration of a routine recognition of the anticholinergic cognitive effects of medications into the care of hospitalized older adults may have the potential to improve patient and health care-related outcomes.
Acknowledgements
Supported by Grant (K23 AG 26770-01) from the John A. Hartford Foundation, the Atlantic Philanthropies, the Starr Foundation, and the National Institute on Aging. The authors report no conflicts of interest in this work.
References
- United States Census BureauNation’s Population One-Third Minority5102009 Accessed on Feb 10, 2009. Available from: http://www.census.gov/Press-Release/www/releases/archives/population/006808.html.
- WolffJLPrevalence, expenditures, and complications of multiple chronic conditions of the elderlyArch Intern Med20021622269227612418941
- SchubertCCComorbidity profile of dementia patients in primary care: Are they sicker?JAGS2006541104109
- BoustaniMScreening for DementiaSystematic Evidence Review.Rockville, MDAgency for Healthcare Research and Quality2003 Available from: http://www.ahrq.gov/clinic/uspstfix.htm.
- TollefsonGDThe relationship of serum anti-cholinergic activity to mental status performance in an elderly nursing home populationJ Neuropsychiatry Clin Neurosci1991333143191821247
- MulsantBHSerum anticholinergic activity in a community-based sample of older adultsArch Gen Psychiatry20036019820312578438
- BlazerDG2ndThe risk of anticholinergic toxicity in the elderly: a study of prescribing practices in two populationsJ Gerontol19833831356129272
- WimoAAn estimate of the total worldwide societal costs of dementia in 2005Alzheimer and Dementia2007381921
- TuneLEAnticholinergic effects of medications in elderly patientsJ Clin Psychiatry20016221111411584981
- TuneLEAssociation of postoperative delirium with raised serum levels of anti-cholinergic drugsLancet1981282486516536116042
- MillerPSAssociation of low serum anticholinergic levels and cognitive impairment in elderly presurgical patientsAm J Psychiatry19881453423453344848
- AncelinMLNon-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort studyBMJ2006332753945545916452102
- BottigiKALong-term cognitive impact of anticholinergic medications in older adultsAm J Geriatr Psychiatry2006141198098417068321
- CaeiroLDelirium in acute stroke: a preliminary study of the role of anticholinergic medicationsEur J Neurol20041169970415469455
- ChewMLSerum anticholinergic activity and cognition in patients with moderate-to-severe dementiaAm J Geriatr Psychiatry2005135354538
- FlackerJMThe association of serum anticholinergic activity with delirium in elderly medical patientsAm J Geriatr Psychiatry1998631419469212
- FlackerJMSerum anticholinergic activity changes with acute illness in elderly medical patientsJ Gerontol A Biol Sci Med Sci1999541M12M1610026657
- GolingerRCAssociation of elevated plasma anticholinergic activity with delirium in surgical patientsAm J Psychiatry19871449121812203631323
- HanLUse of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatientsArch Intern Med200616110991105
- HilmerSNA Drug Burden Index to define the functional burden of medications in older peopleArch Intern Med200716778178717452540
- Lechevallier-MichelNDrugs with anticholinergic properties and cognitive performance in the elderly: results from the PAQUID studyBr J Clin Pharmacol2004592143151
- LuCJChronic exposure to anticholinergic medications adversely affects the course of Alzheimer diseaseAm J Geriatr Psychiatry200311445846112837675
- MachJRJrSerum anticholinergic activity in hospitalized older persons with delirium: a preliminary studyJ Am Geriatr Soc19954354914957730529
- MarcantonioERThe relationship of postoperative delirium with psychoactive medicationsJAMA199427219151815227966844
- MillerPSAssociation of low serum anticholinergic levels and cognitive impairment in elderly presurgical patientsAm J Psychiatry198814533423453344848
- MinzenbergMJAssociation of anticholinergic load with impairment of complex attention and memory in schizophreniaAm J Psychiatry200416111612414702259
- MondimoreFMPost-ECT confusional states associated with elevated serum anticholinergic levelsAm J Psychiatry198314079309316859321
- MussiCImportance of serum anticholinergic activity in the assessment of elderly patients with deliriumJ Geriatr Psychiatry Neurol1999122828610483930
- NebesRDLow-level serum anticholinergicity as a source of baseline cognitive heterogeneity in geriatric depressed patientsPsychopharmacol Bull19973347157209493484
- NebesRDSerum anticholinergic activity, white matter hyperintensities, and cognitive performanceNeurology2005651487148916275844
- PattenSBDelirium in psychiatric inpatients: a case-control studyCan J Psychiatry20014616216611280086
- PlaschkeKEEG changes and serum anti-cholinergic activity measured in patients with delirium in the intensive care unitAnaesthesia2007621217122317991256
- RoeCMUse of anticholinergic medications by older adults with dementiaJAGS200250836842
- RovnerBWSelf-care capacity and anticholinergic drug levels in nursing home patientsAm J Psychiatry198814511071093337276
- ThienhausOJAnticholinergic serum levels and cognitive performanceEur Arch Psychiatry Clin Neurosci1990240128332147899
- InouyeSKClarifying confusion: the confusion assessment method. A new method for detection of deliriumAnn Intern Med19901139419482240918
- AlbertMSThe Delirium Symptom Interview: an interview for the detection of delirium symptoms in hospitalized patientsJ Geriatr Psychiatry Neurol1992514211571069
- FolsteinMF“Mini-Mental State:” A practical method for grading the cognitive state of patients for the clinicianJ Psychiatry Res197512189198
- BrandtJSThe telephone interview for cognitive statusNeuropsychiatry Neuropsychol Behav Neurol198812111117
- DyerCBPostoperative delirium: a review of 80 primary data-collection studiesArch Intern Med199515554614657864702
- CarnahanRMThe Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: Association with Serum Anticholinergic ActivityJ Clin Pharmacol2006461481148617101747
- TrzepaczPTThe neuropathogenesis of deliriumPsychosomatics199443743917916159
- ThomasCSerum anticholinergic activity and cerebral cholinergic dysfunction: An EEG study in frail elderly with and without deliriumBMC Neuroscience200898611018171468
- DixonCETraumatic brain injury reduces hippocampal high-affinity [3H]choline uptake but not extracellular choline levels in ratsNeurosci Lett19941801271307700564
- DixonCEIncreased anticholinergic sensitivity following closed skull impact and controlled cortical impact traumatic brain injury in the ratJ Neurotrauma1994112752877996582
- BoustaniMThe association between cognition and histamine-2 receptor antagonists in African AmericansJ Am Geriatr Soc20075581248125317661965